1. Introduction
New technologies refer to innovations, advancements, or practical technologies in a certain field or industry that offer progress or advantages over existing technologies. Despite a series of breakthroughs in new technologies in recent years, many Chinese research institutions still face difficulties in the transformation of innovative results, and the profitability of enterprises remains to be improved.
The biopharmaceutical industry is a representative sector of the 21st century equipped with new technologies. This industry is composed of the biotechnology sector and the pharmaceutical sector, primarily combining modern biotechnologies with the research and development, production, and various forms of new drugs, as well as integrating them with the diagnosis, prevention, and treatment of various diseases. However, there are high market risks during the R&D process in the biopharmaceutical industry, such as the uncertainty of product market acceptance and sales performance. How to identify consumption growth points in the biopharmaceutical industry under the context of new technology development and thereby stimulate the market vitality of the biopharmaceutical sector has become a pressing issue.
In view of this, the research conducted a social survey from June to September 2024 on residents’ willingness to consume biopharmaceutical products. First-hand sample data from China was collected, and residents’ information on their knowledge reserves, usage experiences, and willingness to pay for biopharmaceuticals was analyzed. The regression model was used to identify the key factors influencing residents’ willingness to consume biopharmaceuticals.
2. Literature Review
A portion of the literature relevant to this study focuses on the development of the biopharmaceutical industry. Mao Jie et al. [1] found that tax incentives have had a significant positive effect on the technological innovation activities of biopharmaceutical companies. Shen Jiawen [2] pointed out that China needs to build a globally competitive biopharmaceutical innovation ecosystem. Zheng Haoyuan et al. [3] aimed to help China stay on the right track in the development of its biopharmaceutical industry in Asia. Jin Xia et al. [4] constructed the Biopharmaceutical Development Index for China, which fully takes into account the strategic, high-tech, growth potential, and high-risk characteristics of the biopharmaceutical industry. Sheng Tianyi et al. [5] summarized the current status of policy formulation for high-quality development in China’s biopharmaceutical industry. Wang Liwei et al. [6] assessed the technological innovation capacity of the biopharmaceutical industry in several regions of China. Zhang Lili and Su Jun [7] proposed corresponding policy suggestions for China’s strategic emerging biopharmaceutical industry.
Another portion of the literature related to this study explores consumption points in China. Fang Fuqian [8] pointed out that the main driving force behind the growth in consumption among Chinese residents will include increased demand for medical services. Lu Zhaoyang and Zheng Zhongwei [9] suggested that for China to cultivate new consumption growth points, it must strengthen the transformation and upgrading of its industrial structure and work to reduce various income gaps. Wang Yuguo [10] argued that to promote the rapid development of China’s new technology consumption sector, it is necessary to deepen the study of new technology consumption economics.
Based on the existing research mentioned above, this study may make the following four contributions: First, in terms of topic selection, few studies focus on the consumption growth points of biopharmaceuticals, especially at the micro-individual level. This research fills this gap by assessing and analyzing the basic characteristics of micro-individuals and their willingness to consume biopharmaceuticals, identifying the key factors influencing their willingness to consume. Second, in terms of research methodology, this study organizes and analyzes first-hand survey data using Stata, and uses a linear regression model to analyze the factors influencing micro-individuals’ willingness to consume biopharmaceuticals. The study follows a logically rigorous approach, progressing step by step from analyzing the basic characteristics of micro-individuals to their consumption willingness and the factors influencing it. Third, in terms of the research sample, this study uses 244 valid samples obtained from a social practice survey. The data is comprehensive, new, and closely aligned with real-world conditions, which is beneficial for reflecting current consumption willingness toward biopharmaceuticals in China, and contributes to more accurate and timely economic conclusions in the study of consumption growth points in high-tech sectors.
3. Research Design
3.1. Questionnaire Design
The variables in this survey include basic personal information, new technology knowledge reserves, and willingness to pay for new technologies. Specific variables are shown in Tables 1-4. The questionnaire consists of single-choice questions, matrix single-choice questions, and multiple-choice questions. For matrix single-choice questions involving strength measurement, a 5-point scale was used, with options ranging from 1 to 5 to represent varying degrees of intensity. The higher the number, the stronger the intensity.
3.2. Questionnaire Distribution and Collection
The questionnaire was distributed online through the Wenjuanxing official website, collecting a total of 246 responses. Figure 1 shows the time distribution of responses from the surveyed group. Samples numbered 107 and 112 had response times exceeding 500 seconds, which were considered outliers and thus marked as invalid. Therefore, the final survey contained 244 valid samples, with an exceptionally high response rate of 99.18%.
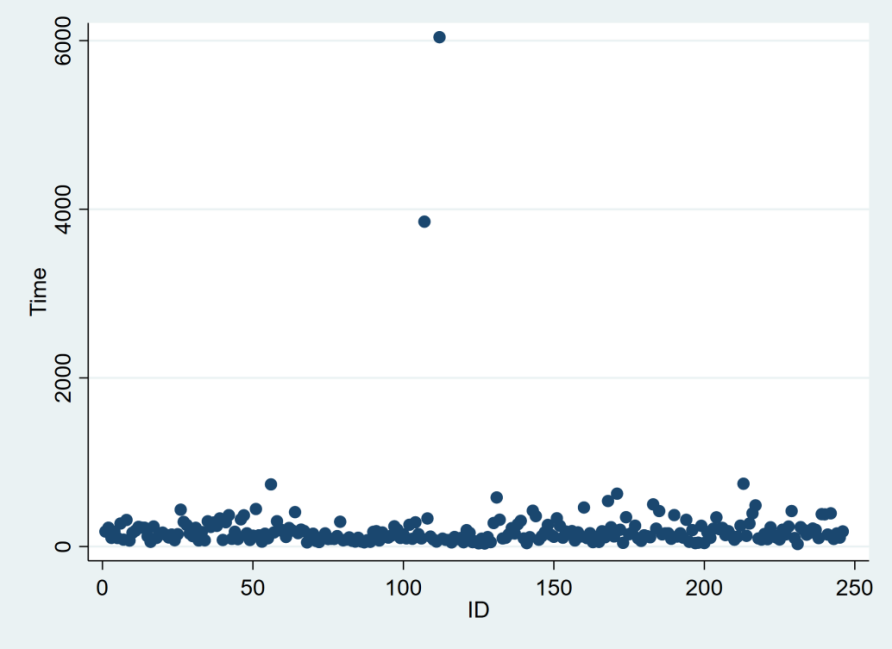
Figure 1: Time Distribution of Questionnaire Responses
Data Source: Survey data plotted using Stata
4. Characteristics of Consumption Points in the Context of New Technologies in China
4.1. Descriptive Statistical Analysis
As shown in Table 1, the majority of the survey respondents were female (68.7%), aged between 46 and 60 years (46.75%), and employed in corporate organizations (35.77%). In conclusion, the survey sample balanced gender representation, focused on middle-aged and young adults, and involved individuals with relatively stable employment, thus ensuring that the research data holds some significance.
Table 1: Descriptive Statistical Analysis
Option | Type | Proportion |
Gender | Male | 31.3% |
Female | 68.7% | |
Age | 18 years and below | 2.85% |
18 to 30 years (inclusive) | 22.76% | |
31 to 45 years (inclusive) | 25.2% | |
46 to 60 years (inclusive) | 46.75% | |
Over 60 years | 2.44% | |
Occupation | Freelancer | 22.36% |
Corporate employee | 35.77% | |
Student | 21.54% | |
Researcher | 4.47% | |
Healthcare worker | 6.5% | |
Other | 9.35% |
4.2. Analysis of Knowledge Reserve on New Technologies
As seen in Table 2, most respondents had heard of but were not familiar with the specifics of genetic testing (68.29%), held a relatively optimistic view on the future development of new technologies in the medical field (45.93%), and hoped to improve suboptimal health conditions and prevent diseases in advance (67.89%). Additionally, all respondents acknowledged the potential drawbacks of artificial intelligence in healthcare (with all options showing some proportion), but the biggest challenge they identified for the development of new technologies in healthcare was technical maturity (78.86%).
Table 2: Knowledge Reserve Information on Biopharmaceutical New Technologies
Option | Type | Proportion |
Understanding of Genetic Testing | Haven’t heard of it | 6.5% |
Have heard of it but don’t understand the specific items | 68.29% | |
Have heard of and understand the specific items | 20.33% | |
Have done some genetic testing items | 4.88% | |
Views on the Future Development of New Technologies in Healthcare | Very optimistic | 24.39% |
Relatively optimistic | 45.93% | |
Neutral | 26.83% | |
Relatively pessimistic | 2.44% | |
Very pessimistic | 0.41% | |
Benefits of Improving Medical Technology | Improve suboptimal health conditions and prevent diseases | 67.89% |
Improve immunity against epidemics | 36.59% | |
Develop more targeted drugs for infectious diseases, shortening disease duration and reducing pain | 20.73% | |
Cure chronic diseases or make treatment simpler | 22.76% | |
Better prevent cardiovascular diseases in the elderly | 21.14% | |
Achieve progress in major diseases | 29.67% | |
Other | 1.22% | |
Drawbacks of Artificial Intelligence in Healthcare Services | Patient privacy leaks | 54.88% |
Instrument risks | 49.19% | |
Impact on employment in the healthcare sector | 30.89% | |
Lack of trust in AI, more reliance on traditional healthcare | 55.69% | |
Ethical issues, such as responsibility division in medical accidents | 45.93% | |
Other | 3.25% | |
Challenges in the Development of New Technologies in Healthcare | Technical maturity | 78.86% |
Legal, regulatory, and ethical issues | 61.79% | |
Public acceptance and trust issues | 63.82% | |
Funding and return issues | 35.37% | |
Cross-sector cooperation and integration issues | 33.74% | |
Other | 2.03% |
4.3. Experience with New Technology Usage
As shown in Table 3, the majority of the surveyed residents had not used any medical AI products or services (73.12%). Among those who had used such products or services, most had used AI-assisted diagnostic tools (62.22%).
Table 3: Experience with Biopharmaceutical New Technologies
Option | Type | Proportion |
Have you used any medical AI products or services? | Yes | 26.83% |
No | 73.17% | |
List the medical AI products or services you have usedAI-assisted diagnostics62.22% | AI-assisted diagnostics | 62.22% |
AI medical imaging analysis | 42.22% | |
Virtual nursing assistant | 24.44% | |
Smart medical devices | 26.67% |
4.4. Willingness to Pay for New Technologies
As seen in Table 4, the majority of the surveyed group was willing, albeit with some reservations, to try AI-assisted medical products or services (64.63%). They were more inclined to increase spending on AI healthcare to pursue better health (71.95%) and tended to introduce digital health monitoring and management into daily life to prevent potential health issues (78.86%). They were willing, but with some reservations, to try medical products or services based on large language models (61.38%), and believed that insufficient understanding of certain high-tech products (63.01%) and the high prices and poor cost-effectiveness of some products (61.79%) were the main obstacles to further consumption of high-tech products.
Table 4: Willingness to Pay for Biopharmaceutical New Technologies
Option | Type | Proportion |
Willingness to try AI-assisted medical products or services | Very willing | 23.58% |
Willing, but with reservations | 64.63% | |
Not very willing | 9.35% | |
Not willing at all | 2.44% | |
Willingness to increase spending on AI healthcare to pursue better health | Yes | 71.95% |
No | 28.05% | |
Tendency to consume AI medical technology in specific situations | In daily life, introduce digital health monitoring and management to prevent issues | 78.86% |
After diagnosis, use AI technology to assist in treatment, such as smart medication monitoring | 21.14% | |
Willingness to try medical products or services based on large language models | Very willing | 25.61% |
Willing, but with reservations | 61.38% | |
Not very willing | 10.16% | |
Not willing at all | 2.85% | |
Reasons hindering further consumption of high-tech products | Some products are overpriced, low cost-effectiveness | 61.79% |
Products lack a sense of technology and experiential value | 38.62% | |
Insufficient understanding of certain high-tech products | 63.01% | |
Concerns over privacy leakage and other ethical issues | 42.28% |
5. Research Design
5.1. Selection of Variables and Data Sources
The dependent variable in this study is the biopharmaceutical consumption points in the context of new technologies. Proxy variables include “Willingness to try AI-assisted medical products or services,” “Willingness to increase spending on AI healthcare to pursue further health,” and “Willingness to try medical products or services based on large language models.” The core independent variables are the knowledge reserves and usage experience of biopharmaceutical new technologies in China, with “Perception of the future development of new technologies, such as AI biopharmaceutical large language models in the medical field” being used to measure knowledge reserves, and “Have you ever used any medical AI products or services?” being used to measure usage experience. The data for this study comes from 244 valid samples obtained through a nationwide questionnaire survey.
5.2. Model Design
The research model for this study is a univariate linear regression, as shown below:
\( Y=αX+β+ε \) (1)
Where, \( Y \) is the dependent variable (biopharmaceutical consumption points in the context of new technologies), \( X \) represents the core independent variables (knowledge reserves and usage experience of biopharmaceutical new technologies in China), and \( ε \) is the random error term.
5.3. Regression Analysis
Table 2 reports the regression results for Models (1), (2), and (3), which examine the impact of biopharmaceutical usage experience on the willingness to try medical products or services based on large language models, the willingness to increase spending on AI healthcare, and the willingness to try AI-assisted medical products or services, respectively. Table 2 also reports the results for Models (4), (5), and (6), which examine the impact of biopharmaceutical knowledge reserves on these same willingness factors. The estimated coefficients for the explanatory variables in all models are significantly positive at the 1% significance level. The regression results preliminarily suggest that, for biopharmaceutical consumption points in the context of new technologies, the overall impact of the usage experience and knowledge reserves of biopharmaceutical new technologies in China is positive and encouraging.
Table 5: Baseline Regression
(1) | (2) | (3) | (4) | (5) | (6) | |
Y | Y | Y | Y | Y | Y | |
X | 0.2598 *** | 0.2410*** | 0.2806*** | 0.4063*** | 0.2001*** | 0.4386*** |
( 2.83 ) | ( 3.83 ) | ( 2.89) | ( 9.03 ) | ( 5.9000) | ( 9.29 ) | |
_cons | 3.0281 *** | 0.6517*** | 3.0225*** | 9.0300*** | 0.5342*** | 2.6975*** |
( 63.36) | (19.80) | (59.85 ) | ( 49.97 ) | ( 12.98) | (47.08) | |
N | 244 | 244 | 244 | 244 | 244 | 244 |
R2 | 0.0320 | 0.0571 | 0.0333 | 0.2521 | 0.1256 | 0.2627 |
z statistics in parentheses
* p < 0.1, ** p < 0.05, *** p < 0.01
6. Conclusion
The core conclusion of this study is that both the biopharmaceutical usage experience and knowledge reserves of residents have a positive and significant impact on biopharmaceutical consumption points. Based on these conclusions and the characteristics of the survey samples, the following policy recommendations are proposed:
Establish a biopharmaceutical feedback mechanism to create a comprehensive product feedback system. Consumers should be encouraged to share their experiences so that products can be promptly adjusted and optimized. Additionally, a quick-response mechanism should be set up to address and provide feedback on issues raised by consumers.
Strengthen public education on new biopharmaceutical technologies to enhance public awareness and trust in these technologies. Furthermore, biopharmaceutical technology exhibition and experience centers could be established to allow the public to personally experience the advantages and application effects of new technologies.
References
[1]. Mao, J., Zhang, L., & Huang, S. (2024). The impact of tax incentives on technological innovation in biopharmaceutical companies: Empirical evidence from listed companies in the biopharmaceutical industry. Tax Research, 7, 128-135. https://doi.org/10.19376/j.cnki.cn11-1011/f.2024.07.022
[2]. Shen, J. (2024). Accelerating the improvement of China’s biopharmaceutical technological innovation policy system. China’s National Conditions and Strength, 2, 14-18. https://doi.org/10.13561/j.cnki.zggqgl.2024.02.004
[3]. Zheng, H., Li, S., Yang, Y., Xu, M., Shen, Y., & Wan, X. (2024). Current development trends and countermeasures of the biopharmaceutical application industry. Journal of Zhangzhou Vocational and Technical College, 1, 85-90. https://doi.org/10.13908/j.cnki.issn1673-1417.2024.01.0014
[4]. Jin, X., Yao, S., Feng, L., Li, W., Miao, X., He, W., & Yang, H. (2024). Comprehensive evaluation of the development of China’s biopharmaceutical industry and regional disparity analysis. Chinese Journal of Biotechnology, 5, 134-146. https://doi.org/10.13523/j.cb.2311057
[5]. Sheng, T., Jiang, R., & Shao, R. (2024). Policy text analysis of high-quality development of biopharmaceutical industries at the provincial (municipal) level from the perspective of policy tools. China New Drugs, 12, 1194-1200.
[6]. Wang, L., Yuan, Y., Mao, K., & Lu, J. (2023). Research on the evaluation of technological innovation capacity in the biopharmaceutical industry based on high-value patents. Chinese Journal of Biotechnology, 10, 120-128. https://doi.org/10.13523/j.cb.2303037
[7]. Zhang, L., & Su, J. (2021). Exploration of policy changes and governance characteristics in China’s biopharmaceutical industry. Chinese Health Economics, 6, 62-65.
[8]. Fang, F. (2021). Analysis of the consumption potential and growth points of Chinese residents: Based on the goal of realizing socialist modernization by 2035. Economic Dynamics, 2, 50-64.
[9]. Lu, Z., & Zheng, Z. (2016). The logical starting point, constraints, and path choices for cultivating new consumption growth points under the new economic normal. Economic Issues Exploration, 1, 1-6.
[10]. Wang, Y. (2020). Thoughts on advancing the development of new technology consumption in China. Economics and Management, 2, 1-6.
Cite this article
Chai,Y. (2025). Research on Consumption Points under the Background of New Technologies: An Industry Survey Analysis Based on the Biopharmaceutical Sector. Advances in Economics, Management and Political Sciences,172,41-48.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Business and Policy Studies
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Mao, J., Zhang, L., & Huang, S. (2024). The impact of tax incentives on technological innovation in biopharmaceutical companies: Empirical evidence from listed companies in the biopharmaceutical industry. Tax Research, 7, 128-135. https://doi.org/10.19376/j.cnki.cn11-1011/f.2024.07.022
[2]. Shen, J. (2024). Accelerating the improvement of China’s biopharmaceutical technological innovation policy system. China’s National Conditions and Strength, 2, 14-18. https://doi.org/10.13561/j.cnki.zggqgl.2024.02.004
[3]. Zheng, H., Li, S., Yang, Y., Xu, M., Shen, Y., & Wan, X. (2024). Current development trends and countermeasures of the biopharmaceutical application industry. Journal of Zhangzhou Vocational and Technical College, 1, 85-90. https://doi.org/10.13908/j.cnki.issn1673-1417.2024.01.0014
[4]. Jin, X., Yao, S., Feng, L., Li, W., Miao, X., He, W., & Yang, H. (2024). Comprehensive evaluation of the development of China’s biopharmaceutical industry and regional disparity analysis. Chinese Journal of Biotechnology, 5, 134-146. https://doi.org/10.13523/j.cb.2311057
[5]. Sheng, T., Jiang, R., & Shao, R. (2024). Policy text analysis of high-quality development of biopharmaceutical industries at the provincial (municipal) level from the perspective of policy tools. China New Drugs, 12, 1194-1200.
[6]. Wang, L., Yuan, Y., Mao, K., & Lu, J. (2023). Research on the evaluation of technological innovation capacity in the biopharmaceutical industry based on high-value patents. Chinese Journal of Biotechnology, 10, 120-128. https://doi.org/10.13523/j.cb.2303037
[7]. Zhang, L., & Su, J. (2021). Exploration of policy changes and governance characteristics in China’s biopharmaceutical industry. Chinese Health Economics, 6, 62-65.
[8]. Fang, F. (2021). Analysis of the consumption potential and growth points of Chinese residents: Based on the goal of realizing socialist modernization by 2035. Economic Dynamics, 2, 50-64.
[9]. Lu, Z., & Zheng, Z. (2016). The logical starting point, constraints, and path choices for cultivating new consumption growth points under the new economic normal. Economic Issues Exploration, 1, 1-6.
[10]. Wang, Y. (2020). Thoughts on advancing the development of new technology consumption in China. Economics and Management, 2, 1-6.









