1. Introduction
Short-term memory (STM) is a cognitive system in the brain that holds the function to store sensory events, movements, and mental information in a short accessible interval for a short period [1]. Previously, several studies showed that the retrieval of STM decreases as age increases [2].
Several studies have shown that STM supports information previously encountered as memory [3]. It can also act as a system with limited capacity that can represent information after the individual has no sensory input. However, it is essential to note the STM can be modified through selective attention [4].
Several studies in the past have shown that the deficit of STM can be caused by severe cognitive impairment, and the inhibition deficit theory proposes that STM damage arises from the difficulty in recalling information that is relevant to the access task and the difficulty in removing information which is no longer relevant during STM maintenance [5-7].
There are studies showing that as age increases, the STM of the individual decreases; however, there is no precise age division for when the decay of STM occurs [8, 9]. Therefore, in this study, the relationship between age and the ability to recall short-term memories is memorizing the sequence of items and numbers. Furthermore, this study will study the effect of age on people's STM and the period of the decay of STM. To examine the impact on STM, a random sequence of numbers will be used to test people's STM; the participants will be selected randomly through social media and volunteers to avoid selection bias and non-probability sampling.
This study will examine whether participants that belong to a younger age, under 18 overall, will perform better in recalling STM than other age groups and the period of decay of STM. This study will also find a correlation between different age groups and STM and determine whether the correlation is positive or negative. The adopted methodology will be used in this study. Items and numbers will be used instead of vocabulary to avoid cultural bias [2]. There will be no "yes" or "no" options; rather, digital numbers will be used to avoid these biases, where participants will inform the numbers shown verbally.
The study "Effects of Age upon Retrieval from STM" examined three groups of 10 adults with the mean ages of 20, 37.5, and 68.1 years old to present and provide a direct assessment of the possible age difference in retrieving information from STM and used numbers as the items to let the participants memorize the sequence of numbers appearing on the projectors with a total of 24 practice trials and another 48 actual trials including the breaking time; the interval between each test is around 30 seconds [2]. The effect of age on STM showed a negative correlation between age and the speed of searching contents of STM. Specifically, the study found that young subjects had a significantly higher search speed for items than older subjects. Young subjects searched at a rate of 25.6 items per second, and the middle and older subjects' search speed was at 15.9 and 14.1 items per second. Indicating that aging hurt STM; there are no significant differences between the search speeds of the middle and older subjects. However, when examining the errors made by the issues, listing in age, the error rates are 0.6%, 1.0%, and 1.4%; there is no significant difference in error rates between the chosen age subjects, which indicates that all ages can perform recalling items and listed materials successfully but at a slower pace as the issues get older. The experiment successfully covered a spectrum of ages from 19-21 years old, 33 to 43, and 58 to 85 years old. The Age groups varied widely in their education level, providing variety in sampling. During the experiment, the display of the digits and items on the projector is programmed and automated, minimizing human intervention with less distraction. The procedure and methodology adopted are straightforward, with only a "yes" or "no" selection simplified, minimizing the experiment's duration and alleviating the effort for the participants to respond.
However, with only two "yes" or "no" options, the participants can guess the solution with a 50% chance of receiving the correct answer. This is a systematic error that can potentially increase the type I error (false positive solution) in the experiment, therefore obtaining a potentially inaccurate result from the participant, as there are no options for not remembering the sequence of items and numbers or simply allowing the participant to skip the response.
The experiment took 2 hours to collect the data and run a full trial. The duration of the method chosen is extensive, neglecting the participants' physical conditions and the exhaustion and fatigue factors. Other studies have shown an overall decrease in the efficiency of STM when individuals are tired or under exhaustion [10].
Another inconsiderable factor in the investigation is there are no distinctions in considering the genders and the effect on STM. Although the young subjects were equal in gender, the older subjects were not, in fact, all men [2]. Since different individuals have different degrees of ability to store information, dividing the groups into gender categories can be a factor to be considered. A study conducted in India showed that females exhibit a greater STM than men in memorizing meaningful words [11].
In the experiment, the young subjects are all staff from the Massachusetts General Hospital, and the older issues are participants of a longitudinal study conducted by the Veterans Administration Outpatient Clinic in Boston, Massachusetts. This is a form of convenient sampling, finding participants based on geographical proximity, as shown in the young subjects. This is a selection bias when choosing the participants in this experiment [2]. This results in a potential lack of representation of the entire population to conclude the findings because there are other unknown proportions of the whole population that are not included in the sample group [12]
2. Methods
2.1. Participants
According to the American Medical Association's age designations, in this study, the participants will be divided into four age groups: Ages 1-12, 13-17, 18-64, and 65 and older. In Groups 1-12 and 13-17, participants were recruited at random from 3 different schools in British Columbia, Canada. The other two groups, 18-64 and 65 or older, are selected from the parents and their relatives of groups 1-12 and 13-17. The mean of the 80 participants in different age groups is 1-12 is 9.25, 13-17 is 14.65, 18-64 is 41.5; and 65 and older is 68.6. The total age mean value is 33.5 years old. Participants are informed about this investigation beforehand and consented to participate.
2.2. Material and procedure
Participants were to attempt to recall the number of digits remembered. The software used displayed digits ranging from 0-9 randomly. A 20-digit number was generated for each participant using the software; the maximum for the software is 10, and twice was done to obtain a 20-digit number. The participants were to complete the trial while the number on the display screen was displayed sequentially in front of the participants. A digit number was shown at a time, starting with 1 digit first, and the number of digits displayed will follow the sequence of cumulation on the basis of the previous digits. An example of the series will be 1, 13, 135, and 1357; the maximum number of digits will depend on how many digits the participant can remember. Each digit was displayed for 1 second, and the participant informed the practitioner of the digits on the screen. For the first four digits, the participant was required to notify the practitioner no more than 3 seconds after seeing the numbers. After four digits, participants were to inform the practitioner of the digits within 30 seconds. If the participants recalled any digit wrong during the informing interval, the practitioner should only record the previous number of digits remembered by the participants. For example, if a participant recalled a 6-digit number incorrectly, the data recorded should be the 5-digit number identified successfully. Each participant was to complete only one trial, with a total of 20 participants in each age group, 80 participants in total.
A 13-inch Macbook Pro 2021 with MacOS 13.3 operating system will be used as a screen to display the numbers, using the software "The Random Number Generator" developed by Nicholas Dean.
2.3. Statistical Analysis
A descriptive analysis shows the mean, median, mode, and standard deviation of the number of digits recalled among all age groups (1-12, 13-17, 18-64, 65 and older). One-way ANOVA shows the statistical significance of the number of digits recalled between all age groups. The participants' ages and the number of digits recalled are plotted in a scatter plot, and a linear regression is done to obtain a correlation between age and the number of digits identified, which is shown as unfavorable. A post hoc analysis is done subsequently to show the significance in the number of digits recalled between each individual age group, as the results of ANOVA have established importance in the number of digits identified between the age groups.
3. Results
The four age groups ranging from one to 12, 13 to 17, 18 to 65, and above 65 were investigated in terms of their memory ability, which is evaluated through the number of digits the participants can recall during the numbers remember tasks (as described in Methods - Materials and Procedure).
There was a significant difference (p<0.001) in the mean number of digits recalled between the age groups (F(3, 76) =4.235, p<0.001). With the increase of age in the four groups, a decrease in the number of digits that the participants can recall was found in Table 1, from the age group 1-12 (M = 8.1, SD = 1.83) to the age group 65 and above (M = 5.25, SD = 1.12), indicating that getting older could result in a decline of people’s memory functions.
Table 1: Descriptive Analysis of all age groups (1-12, 13-17, 18-64, 65 and older)
Age Group: | 1-12 | 13-17 | 18-65 | 65 and above |
Mean ±SD of number digits recalled | 8.1 ± 1.83 | 7.85 ± 2.01 | 7.40 ± 2.82 | 5.25 ± 1.12 |
Median of number digits recalled | 9 | 7.5 | 7 | 5 |
Mode of number digits recalled | 9 | 7 | 7 | 5 |
Range of number digits recalled | 6 | 8 | 10 | 4 |
Minimum of number digits recalled | 5 | 5 | 3 | 4 |
Maximum of number digits recalled | 11 | 13 | 13 | 8 |
SD for Standard Deviation
Table 2: One-way ANOVA for all age groups (1-12, 13-17, 18-64, 65 and older)
df1 | df2 | F | p |
3 | 76 | 4.235 | 0.000004 |
df1: degree of freedom of rows; df2: degree of freedom of columns;
The average number of digits ages 1-12 groups can recall is 8.10 digits; ages 13-17 are 7.85 digits; ages 18-64 have a mean of 7.40, and ages 65 and older have a standard of 5.65. There is a decrease in the mean number of digits overall as the age groups increase, indicating a negative correlation between age and STM (Figure 1). The mode for the number of digits recalled for age groups 1-12 is 9, both 13-17 and 18-64 are 7, and 65 and older is 5.
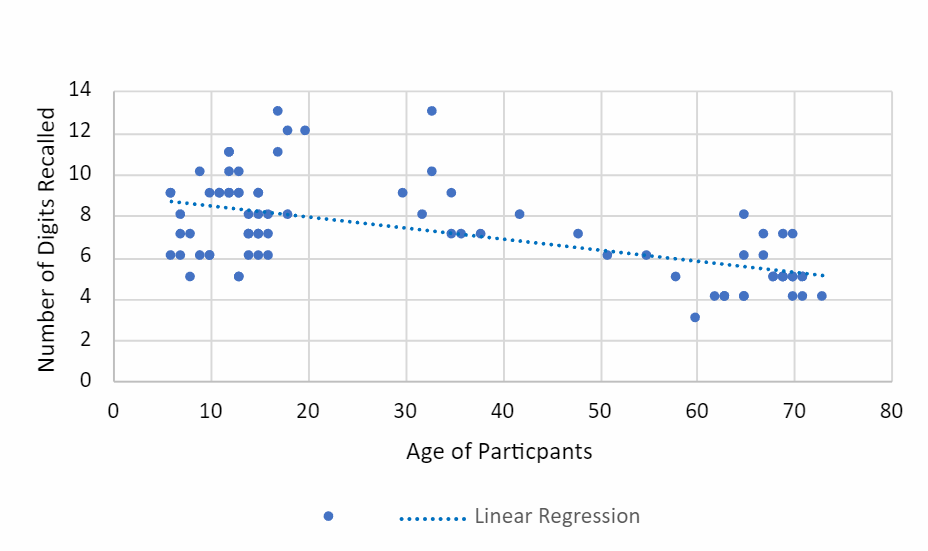
Figure 1: Age of Participants
Scatter-plot of the number of digits participants in different age groups, 1-12, 13-17, 18-64, and 65 and older can recall. Showed a negative linear regression of the number of digits identified over the age of A post-hoc analysis is done to show the significance in the number of digits recalled between each age group. The LSD analysis has shown effectiveness between age groups 1-12 and 65 and older, 13-17, and 65 and older(p<0.001). Age groups 18-64 and 65 and older also showed significance (p=0.001). The Turkey HSD test has shown importance between age groups 1-12 and 65 and older (p<0.001), and 13-17 and 65 and older (p<0.001), and there is no significance shown between 18-64 and 65 and older. The Bonferroni correction showed two importance between age groups 1-12 and 65 and older (p<0.001) and 13-17 and 65 and older (p=0.001). With this evidence, age does correlate with STM negatively when comparing the results of the two age groups 1-12 and 65 and older and 13-17 and 65 and older.
4. Discussion
There is no significant decay in the number of digits recalled between the ages 6-33, and this holds the range of digits remembered between 5-13. Data in the post hoc analysis implied age groups 1-12, 13-17, and 18-64 show no significant difference. This offers participants a strong visual STM in these age groups, with the ability to store and recall numbers within seconds [13]. Several studies have shown that as age increases, the brain's processing speed and recognition of objects decrease due to brain-related mechanisms [6, 9, 14, 15]. Consistent with the findings in this study, after the age of 33, the number of digits recalled decreases with no number of digits placed higher than 9, and ages 60 and older showed a significant decrease when compared to other generations.
Our study showed a significant decrease in the number of digits remembered after age 60 (Figure 1). In studies about age and its effect on hippocampal volume, researchers investigated 142 individuals older than 55 years old, and the results show a linear decrease in both right and left hippocampal volume [16]. The hippocampal contributes to the STM of humans, especially in the regulations of learning, memory, and emotion; therefore, if the importance of the hippocampal decreases, there will be an impact on the individual's cognition and concentration [17, 18]. This is consistent with the findings in this study.
The number of digits participants recalled successfully ranges significantly between the age groups 1-12, 13-17, and 18-64. The age group 18-64 has the most fantastic range of 3-13. However, the ages that performed the smallest range with stable results were participants older than 60. Although there is a significant decrease in the number of digits recalled by participants after the age of 60 compared with other periods, it is essential to note that after the age of 60, the number of digits placed by the participants is more or less in a linear trend. The majority of the participants who are older than the age of 60 identified four and five digits most frequently. Several studies have shown that aging increases discriminal dispersion and visual and spatial STM. The advancement of age limits memory capacity, impairs visual STM performance and diminishes the attentional resources of the individual [19-22]. The result of this study consists of these findings showing a negative correlation between age and the number of digits recalled. However, for participants older than 60, there are no significant changes in the number of digits identified, which contradicts these studies. Several studies have shown a potential biological mechanism: individuals who carry APOE ε2, a genetic allele of the APOE gene, offer an advantage in memory storage regardless of STM task difficulty and across all ages. The effect of this genetic allele can occur throughout an individual's life, which can explain how individuals aged 65, 67, 69, and 70 have a higher number of digits recalled and a great range between age groups [23-25].
5. Conclusion
Some studies suggest that gender does influence STM in individuals. Gender is worth considering in this study because with a generalization on the age of the participants without considering the factors of gender, the results and findings of this study cannot apply and generalize both genders [11, 26-28]. Global participants can be enrolled because environmental factors, geographical factors, and health conditions can affect an individual's STM [7, 28-30].
In summary, the results obtained throughout this study show that participants in age groups 1-12 and 13-17 perform better in recalling STM than other older age groups. Specifically, from ages 6-33, participants did not show a significant decline in the number of digits remembered, and after period 55, participants did not recall digits that were higher than 8, with a considerable decrease at age 60; only three numbers were placed. With the data collected, this study has provided a generalization of the negative correlation between age and STM; as an individual's age increases, the STM and the ability to recall short items decrease.
References
[1]. Aben, B., Stapert, S., & Arjan Blokland. (2012). About the Distinction between Working Memory and Short-Term Memory. 3. https://doi.org/10.3389/fpsyg.2012.00301
[2]. Anders, T. R., Fozard, J. L., & Lillyquist, T. D. (1972). Effects of age upon retrieval from short-term memory. Department of Psychology, 6(2), 214–217. https://doi.org/10.1037/h0032103
[3]. Norris, D. (2017). Short-term memory and long-term memory are still different. 143(9), 992–1009. https://doi.org/10.1037/bul0000108
[4]. Posner, M. I. (1980). Orienting of Attention - Michael I. Posner, 1980. Quarterly Journal of Experimental Psychology. https://journals.sagepub.com/doi/abs/10.1080/00335558008248231
[5]. Rozek, E., Kemper, S., & McDowd, J. (2012). Learning to ignore distracters. Psychology and aging, 27(1), 61–66. https://doi.org/10.1037/a0025578
[6]. Alodie Rey-Mermet, & Gade, M. (2017). Inhibition in aging: What is preserved? What declines? A meta-analysis. 25(5), 1695–1716. https://doi.org/10.3758/s13423-017-1384-7
[7]. Möllers, T., Stocker, H., Perna, L. W., Rujescu, D., Bernd Holleczek, Schöttker, B., & Brenner, H. (2022). OUP accepted the manuscript. 51(6). https://doi.org/10.1093/ageing/afac113
[8]. Brown, L. J., & Brockmole, J. R. (2010). The Role of Attention in Binding Visual Features in Working Memory: Evidence from Cognitive Ageing. 63(10), 2067–2079. https://doi.org/10.1080/17470211003721675
[9]. Zhu, Z., Deng, J., Li, M., Qin, Y., Jingyi Jessica Li, & Yang, Y. (2022). Processing speed mediates the relationship between brain structure and semantic fluency in aging. 788, 136838–136838. https://doi.org/10.1016/j.neulet.2022.136838
[10]. Asuman Şahana, Alparslan Ermana, & Sebahat Meneka. (2015). The effect of physical fatigue on short-term memory. Procedia - Social and Behavioral Sciences, 174, 2425–2429. https://doi.org/10.1016/j.sbspro.2015.01.912
[11]. Adyalkar, S. (2019). Relationship between Short-Term Memory and Gender of 18-20 Years. The International Journal of Indian Psychology, 7(3). https://doi.org/10.25215/0703.004
[12]. Wolf, C., Joye, D., Smith, T. J., & Fu, Y. (2016). The SAGE Handbook of Survey Methodology. https://doi.org/10.4135/9781473957893
[13]. Hollingworth, A., Richard, A. M., & Luck, S. J. (2008). Understanding the function of visual short-term memory: Transsaccadic memory, object correspondence, and gaze correction. 137(1), 163–181. https://doi.org/10.1037/0096-3445.137.1.163
[14]. Verhaeghen, P. (2013). The elements of cognitive aging: Meta-analyses of age-related differences in processing speed and their consequences. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780195368697.001.0001
[15]. Fjell, A. M., & Walhovd, K. B. (2010). Structural Brain Changes in Aging: Courses, Causes and Cognitive Consequences. 21(3). https://doi.org/10.1515/revneuro.2010.21.3.187
[16]. Erickson, K. I., Ruchika Shaurya Prakash, Voss, M. W., Chaddock, L., Heo, S., McLaren, M. E., Pence, B. D., Martin, S. F., Vieira, V. J., Woods, J. A., McAuley, E., & Kramer, A. F. (2010). Brain-Derived Neurotrophic Factor Is Associated with Age-Related Decline in Hippocampal Volume. 30(15), 5368–5375. https://doi.org/10.1523/jneurosci.6251-09.2010
[17]. Hartley, T. T., Bird, C. M., Chan, D., Cipolotti, L., Husain, M., Faraneh Vargha-Khadem, & Burgess, N. (2006). The hippocampus is required for short-term topographical memory in humans. 17(1), 34–48. https://doi.org/10.1002/hipo.20240
[18]. Frodl. (2014). Reduced hippocampal volume correlates with executive dysfunctioning in major depression. Journal of Psychiatry & Neuroscience : JPN, 31(5). https://pubmed.ncbi.nlm.nih.gov/16951734/
[19]. Noack, H., Lövdén, M., & Lindenberger, U. (2012). Normal aging increases discriminal dispersion in visuospatial short-term memory. 27(3), 627–637. https://doi.org/10.1037/a0027251
[20]. Bastin, C. (2017). Differential age-related effects on conjunctive and relational visual short-term memory binding. 26(9), 1181–1190. https://doi.org/10.1080/09658211.2017.1421228
[21]. Lugtmeijer, S., Geerligs, L., Tsvetanov, K. A., Mitchell, D. J., None Cam-CAN, & Campbell, K. L. (2023). Lifespan differences in visual short-term memory load-modulated functional connectivity. 270, 119982–119982. https://doi.org/10.1016/j.neuroimage.2023.119982
[22]. Luszcz, M. A., & Bryan, J. (1998). Toward Understanding Age-Related Memory Loss in Late Adulthood. 45(1), 2–9. https://doi.org/10.1159/000022048
[23]. Nahid Zokaei, Board, A. G., Slavkova, E., Mackay, C. E., Nobre, A. C., & Husain, M. (2021). Superior short-term memory in APOE ε2 carriers across the age range. 397, 112918–112918. https://doi.org/10.1016/j.bbr.2020.112918
[24]. Chen, J., Shu, H., Wang, Z., Liu, D., Shi, Y., Xu, L., & Zhang, Z. (2016). Protective effect of APOE epsilon 2 on intrinsic functional connectivity of the entorhinal cortex is associated with better episodic memory in elderly individuals with risk factors for Alzheimer’s disease. 7(37), 58789–58801. https://doi.org/10.18632/oncotarget.11289
[25]. Alexopoulos, P., Richter-Schmidinger, T., Horn, M., Maus, S., Reichel, M., Sidiropoulos, C., Rhein, C., Piotr Lewczuk, Doerfler, A., & Johannes Kornhuber. (2011). Hippocampal Volume Differences Between Healthy Young Apolipoprotein E ε2 and ε4 Carriers. 26(2), 207–210. https://doi.org/10.3233/jad-2011-110356
[26]. Silvia Erika Kober, Johanna Louise Reichert, Neuper, C., & Wood, G. (2016). Interactive effects of age and gender on EEG power and coherence during a short-term memory task in middle-aged adults. 40, 127–137. https://doi.org/10.1016/j.neurobiolaging.2016.01.015
[27]. Kunimi, M. (2016). Effects of age, gender, and stimulus presentation period on visual short-term memory. Journal of Women & Aging. https://www.tandfonline.com/doi/full/10.1080/08952841.2014.950499
[28]. Pauls, F., Petermann, F., & Lepach, A. C. (2013). Gender differences in episodic memory and visual working memory including the effects of age. 21(7), 857–874. https://doi.org/10.1080/09658211.2013.765892
[29]. Meir, N., & Armon-Lotem, S. (2017). Independent and Combined Effects of Socioeconomic Status (SES) and Bilingualism on Children’s Vocabulary and Verbal Short-Term Memory. 8. https://doi.org/10.3389/fpsyg.2017.01442
[30]. Ogata, S., Tanaka, H., Omura, K., Honda, C., & Hayakawa, K. (2016). Association between intake of dairy products and short-term memory with and without adjustment for genetic and family environmental factors: A twin study. 35(2), 507–513. https://doi.org/10.1016/j.clnu.2015.03.023
Cite this article
Zhu,H.D. (2024). The Impact of Age on Short-term Memory. Lecture Notes in Education Psychology and Public Media,45,6-15.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the International Conference on Global Politics and Socio-Humanities
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Aben, B., Stapert, S., & Arjan Blokland. (2012). About the Distinction between Working Memory and Short-Term Memory. 3. https://doi.org/10.3389/fpsyg.2012.00301
[2]. Anders, T. R., Fozard, J. L., & Lillyquist, T. D. (1972). Effects of age upon retrieval from short-term memory. Department of Psychology, 6(2), 214–217. https://doi.org/10.1037/h0032103
[3]. Norris, D. (2017). Short-term memory and long-term memory are still different. 143(9), 992–1009. https://doi.org/10.1037/bul0000108
[4]. Posner, M. I. (1980). Orienting of Attention - Michael I. Posner, 1980. Quarterly Journal of Experimental Psychology. https://journals.sagepub.com/doi/abs/10.1080/00335558008248231
[5]. Rozek, E., Kemper, S., & McDowd, J. (2012). Learning to ignore distracters. Psychology and aging, 27(1), 61–66. https://doi.org/10.1037/a0025578
[6]. Alodie Rey-Mermet, & Gade, M. (2017). Inhibition in aging: What is preserved? What declines? A meta-analysis. 25(5), 1695–1716. https://doi.org/10.3758/s13423-017-1384-7
[7]. Möllers, T., Stocker, H., Perna, L. W., Rujescu, D., Bernd Holleczek, Schöttker, B., & Brenner, H. (2022). OUP accepted the manuscript. 51(6). https://doi.org/10.1093/ageing/afac113
[8]. Brown, L. J., & Brockmole, J. R. (2010). The Role of Attention in Binding Visual Features in Working Memory: Evidence from Cognitive Ageing. 63(10), 2067–2079. https://doi.org/10.1080/17470211003721675
[9]. Zhu, Z., Deng, J., Li, M., Qin, Y., Jingyi Jessica Li, & Yang, Y. (2022). Processing speed mediates the relationship between brain structure and semantic fluency in aging. 788, 136838–136838. https://doi.org/10.1016/j.neulet.2022.136838
[10]. Asuman Şahana, Alparslan Ermana, & Sebahat Meneka. (2015). The effect of physical fatigue on short-term memory. Procedia - Social and Behavioral Sciences, 174, 2425–2429. https://doi.org/10.1016/j.sbspro.2015.01.912
[11]. Adyalkar, S. (2019). Relationship between Short-Term Memory and Gender of 18-20 Years. The International Journal of Indian Psychology, 7(3). https://doi.org/10.25215/0703.004
[12]. Wolf, C., Joye, D., Smith, T. J., & Fu, Y. (2016). The SAGE Handbook of Survey Methodology. https://doi.org/10.4135/9781473957893
[13]. Hollingworth, A., Richard, A. M., & Luck, S. J. (2008). Understanding the function of visual short-term memory: Transsaccadic memory, object correspondence, and gaze correction. 137(1), 163–181. https://doi.org/10.1037/0096-3445.137.1.163
[14]. Verhaeghen, P. (2013). The elements of cognitive aging: Meta-analyses of age-related differences in processing speed and their consequences. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780195368697.001.0001
[15]. Fjell, A. M., & Walhovd, K. B. (2010). Structural Brain Changes in Aging: Courses, Causes and Cognitive Consequences. 21(3). https://doi.org/10.1515/revneuro.2010.21.3.187
[16]. Erickson, K. I., Ruchika Shaurya Prakash, Voss, M. W., Chaddock, L., Heo, S., McLaren, M. E., Pence, B. D., Martin, S. F., Vieira, V. J., Woods, J. A., McAuley, E., & Kramer, A. F. (2010). Brain-Derived Neurotrophic Factor Is Associated with Age-Related Decline in Hippocampal Volume. 30(15), 5368–5375. https://doi.org/10.1523/jneurosci.6251-09.2010
[17]. Hartley, T. T., Bird, C. M., Chan, D., Cipolotti, L., Husain, M., Faraneh Vargha-Khadem, & Burgess, N. (2006). The hippocampus is required for short-term topographical memory in humans. 17(1), 34–48. https://doi.org/10.1002/hipo.20240
[18]. Frodl. (2014). Reduced hippocampal volume correlates with executive dysfunctioning in major depression. Journal of Psychiatry & Neuroscience : JPN, 31(5). https://pubmed.ncbi.nlm.nih.gov/16951734/
[19]. Noack, H., Lövdén, M., & Lindenberger, U. (2012). Normal aging increases discriminal dispersion in visuospatial short-term memory. 27(3), 627–637. https://doi.org/10.1037/a0027251
[20]. Bastin, C. (2017). Differential age-related effects on conjunctive and relational visual short-term memory binding. 26(9), 1181–1190. https://doi.org/10.1080/09658211.2017.1421228
[21]. Lugtmeijer, S., Geerligs, L., Tsvetanov, K. A., Mitchell, D. J., None Cam-CAN, & Campbell, K. L. (2023). Lifespan differences in visual short-term memory load-modulated functional connectivity. 270, 119982–119982. https://doi.org/10.1016/j.neuroimage.2023.119982
[22]. Luszcz, M. A., & Bryan, J. (1998). Toward Understanding Age-Related Memory Loss in Late Adulthood. 45(1), 2–9. https://doi.org/10.1159/000022048
[23]. Nahid Zokaei, Board, A. G., Slavkova, E., Mackay, C. E., Nobre, A. C., & Husain, M. (2021). Superior short-term memory in APOE ε2 carriers across the age range. 397, 112918–112918. https://doi.org/10.1016/j.bbr.2020.112918
[24]. Chen, J., Shu, H., Wang, Z., Liu, D., Shi, Y., Xu, L., & Zhang, Z. (2016). Protective effect of APOE epsilon 2 on intrinsic functional connectivity of the entorhinal cortex is associated with better episodic memory in elderly individuals with risk factors for Alzheimer’s disease. 7(37), 58789–58801. https://doi.org/10.18632/oncotarget.11289
[25]. Alexopoulos, P., Richter-Schmidinger, T., Horn, M., Maus, S., Reichel, M., Sidiropoulos, C., Rhein, C., Piotr Lewczuk, Doerfler, A., & Johannes Kornhuber. (2011). Hippocampal Volume Differences Between Healthy Young Apolipoprotein E ε2 and ε4 Carriers. 26(2), 207–210. https://doi.org/10.3233/jad-2011-110356
[26]. Silvia Erika Kober, Johanna Louise Reichert, Neuper, C., & Wood, G. (2016). Interactive effects of age and gender on EEG power and coherence during a short-term memory task in middle-aged adults. 40, 127–137. https://doi.org/10.1016/j.neurobiolaging.2016.01.015
[27]. Kunimi, M. (2016). Effects of age, gender, and stimulus presentation period on visual short-term memory. Journal of Women & Aging. https://www.tandfonline.com/doi/full/10.1080/08952841.2014.950499
[28]. Pauls, F., Petermann, F., & Lepach, A. C. (2013). Gender differences in episodic memory and visual working memory including the effects of age. 21(7), 857–874. https://doi.org/10.1080/09658211.2013.765892
[29]. Meir, N., & Armon-Lotem, S. (2017). Independent and Combined Effects of Socioeconomic Status (SES) and Bilingualism on Children’s Vocabulary and Verbal Short-Term Memory. 8. https://doi.org/10.3389/fpsyg.2017.01442
[30]. Ogata, S., Tanaka, H., Omura, K., Honda, C., & Hayakawa, K. (2016). Association between intake of dairy products and short-term memory with and without adjustment for genetic and family environmental factors: A twin study. 35(2), 507–513. https://doi.org/10.1016/j.clnu.2015.03.023









