1. Introduction
Dyslexia is a specific learning disorder. Individuals who suffer this disorder show reading difficulties as either not being able to identify written words correctly or quickly as the others. Developmental dyslexia (DD) is the most commonly used term for children who experience severe difficulties in learning how to decode printed words. Dyslexics children have difficulty in identifying printed words, and have huge problems to recognize unfamiliar words, which makes them slow readers [1]. Dyslexics individuals show constant difficulty in learning to read. However, these problems are not determined by intellectual disability, sensory impairment, financial pressure, shortage of motivation or lack of sufficient educational opportunities [2].
Reading skill is regarded as one of the essential abilities in life, and lay the foundation for early education. Being literal is crucial in people development because reading provides not only the key for education, but also to mental health and well-being. Dyslexia interferes with personal, academic, social and emotional functioning, which happens in 5~17% of children [3]. Learning to read is one of the key outcomes for children in their early education. Dyslexics exhibit difficulty to how to manipulate and isolate phonemes and how signs (graphemes) are mapped with sounds (phonemes). Phonological awareness (PA) is the capability to distinguish and identify the sounds, which is regarded as a significant predicator to develop reading [4]. Though PA is crucial to develop accurate decoding ability, it is not enough to achieve reading abilities [5]. Grapheme to phoneme mapping and conversion is necessary for development of decoding abilities, which means the effect of PA should be strengthened at the same time of grapheme-to-phoneme training. Additionally, an important improvement of literacy skills in childhood might benefit from an early intervention for phonological skills [2].
Non-invasive brain stimulation (NIBS) is a modulatory technique with increasing popularity for drawing causal reasoning and exploring the interactions of task-specific networks. NIBS is an active and non-invasive way which allows to manipulate the function of brain in healthy individuals and study various cognitive functions to explore the relationships between brain and behavior [4]. NIBS explores the causal relationship between structure and function, and studies functional interactions by transiently changing neural activity.
Transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) are the methods that have widespread application in NIBS. Under the influence of a magnetic field, TMS generates electric fields in the brain through the electromagnetic induction. A coil with a strong pulse of electric current is placed over the head of participant to induce the magnetic field. The induced electric field evokes action potentials (AP)and changes brain’s neural activity. In a word, TMS make use of a rapidly changing, strong magnetic field to induce electric currents in the brain through coils, which then induce AP in cortical axons. The effects would differ with the stimulation place, the stimulation intensity, the pulses number and frequency. TMS can be transmitted as a single or repeated pulse (rTMS)before or during a task, which are offline TMS or online TMS, respectively [4].
tDCS is a non-invasive technique for modulating neuronal activity in the cerebral cortex. It consists a constant weak current (1~2mA) to the brain by electrodes placed in specific cortical areas of the scalp. The current passes between the positively charged anode and the negatively charged cathode, and provokes a sub-threshold regulation without depolarizing AP [5]. Anodal tDCS usually excites the local cerebral cortex, yet cathodal tDCS reduces the excitability of cerebral cortex. In addition, tDCS regulates brain activity by using weak direct current to alter the spontaneous firing of neurons and alter the concentration of neurotransmitters, such as γ-aminobutyric acid (GABA) and glutamate [6, 7]. After stimulation, the effects of tDCS can last from minutes to hours. In humans, neuromodulation effects of tDCS have been discovered on cognitive function, motor function, lower-order processing stages of specific sensations [8].
Compared with TMS, tDCS is cheaper and easier to be conducted on individuals. In addition, it shows little adverse side effects than TMS, such as skin tingling at the electrode, burning sensation, headache. Therefore, tDCS is more suitable for stimulation combining with behavioral intervention for training and therapy.
This review aims to investigate the function of various neuromodulation protocols on reading skills, and special consideration was given to variability in target cortical areas, number of sessions, and target population. The significance of the combination of NIBS with other cognitive trainings is also discussed.
2. Methods
2.1. Literature search
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analysis) guidelines were applied for the current review. Two literature databases, PubMed and Web of Science (webofknowledge.com) were searched in early June, 2022. The keyword strings “dyslexia” AND “tDCS”, “dyslexia” AND “transcranial direct current stimulation”, “dyslexia” AND “TMS”, and “dyslexia” AND “transcranial magnetic stimulation” were used for search and setting the year duration at 2012~2022.
2.2. Screening criteria
The inclusion criteria used to screen papers were as following:
-The paper is in English
-The paper is not a review or meta-analysis
-The paper is not a case study
2.3. Eligibility criteria
The eligibility criteria were used to screen the abstracts of each paper
-The research purpose is to explore modulation for developmental dyslexia
-The sample recruited is developmental dyslexics, either adults or children/adolescents
-Non-invasive brain stimulation applied is either tDCS or TMS
3. Results
The search criteria yielded 34 records in PubMed and 59 references in Web of Science. Totally, 64 records were remained for screening after 29 duplicates were removed. From these references, 1 record of Correction, 4 records of Case Study and 15 records of Review Paper were excluded at assessing the title. 44 references were assessed for eligibility. Among these studies, 5 records were removed because they did not aim for dyslexia research. Furthermore, 21 referenced were excluded because the recruited population were not developmental dyslexics. Finally, 6 studies were removed since the non-invasive stimulation were neither TMS nor tDCS.
Therefore, 12 studies were included in the current review (Figure 1, a graphical overview of the selection process).
3.1. Research on adults with DD
The study by Costanzo et al was the only research to apply TMS to adults with DD. This study replicated the previous study by the same authors with the same design to healthy adults [9,10]. A sample of 10 dyslexic adults aged 19 to 51 years received 6 TMS treatments over 2 days, with TMS intervention in the left and right inferior parietal lobules (IPL) and superior temporal gyrus (STG), the vertex as a control condition as well as sham. Combined with reading trainings which contained 30 words, 30 non-words and 600 syllables, the intervention included 10 rTMS sequences, 50 stimuli, and a frequency of 5 Hz with a 30-s interval between each training. Reading abilities of word, non-word and text were assessed before and immediately after each stimulation for 6 min. The result showed that L-STG stimulation improved the accuracy of text reading and the speed of word reading, which supported that the posterior L-STG played significant function in the whole-word representation of words and in processing complex linguistic representations [11,12]. An ameliorate effect on the accuracy of non-word was observed after both L-IPL and R-IPL. Compared with the documented data in the previous study to healthy adults by the same authors [10], only the stimulation of L-IPL had a specific effect on non-word reading. The results also found that R-STG improved text reading accuracy, which was unexpected in comparison with their previous studies among typical readers [10], i.e. an increasement of errors after R-STG intervention. The result of improved text reading ability after the intervention of L-STG and R-STG could indicate the possible existence of complex compensatory mechanisms in the brains of dyslexic patients, which is consistent with evidence of reverse asymmetry in temporoparietal regions (R>L) in dyslexic patients. Evidence for increased right STG activation in dyslexics during reading and comprehension of words and sentences [13,14]. Overall, the study indicated that L-STG and L-IPL of dyslexics had important and differentiated functions in word, non-word and text reading.
Helth and Lavidor have carried the only tDCS research on dyslexic adults [15]. The study conducted five tDCS interventions over a 2-week period in which 1.5mA of anodic stimulation was applied to the V5 region for 20 minutes, and the right orbitofrontal cortex was used as a reference site. 19 adults whose native language is Hebrew were ever diagnosed as DD and aged at 19~35 years old, and were assigned randomly to an active anodal or a sham group. The accuracy and speed of text reading, rapid automatized naming (RAN) letter and number, as well as symbol search were assessed before the stimulation, and immediately after end of the last stimulation. The final text reading was assessed after a week. The results showed that the speed of text reading significantly improved in the active tDCS group, as well as the speed of letter-naming and number-naming comparing with those in the sham group. However, the increase in speed of text reading didn’t decrease the accuracy of reading. In the final evaluation after one week of stimulation, the accuracy of reading was sustained or even improved. As RAN is commonly used as a test of reading fluency for reading ability identification standardized in many languages [16], the significant improvement of RAN represented the enhanced reading fluency. The result indicated V5 was involved in text reading based on the fact that the reading ability significantly improved after V5 anode stimulation. In this research, the stimulation montage was effective in promoting visual-orthographic process for swift reading, which demonstrated the effects of tDCS on the fluency of text reading in patients with reading disabilities. The reason why the authors chose tDCS rather than TMS used by previous study [10] was they considered TMS of 500 pulses with a frequency and intensity of 100% of the movement threshold for 7 minutes may not be appropriate for dyslexia, as many participants have reported pain and discomfort while using a similar regimen.
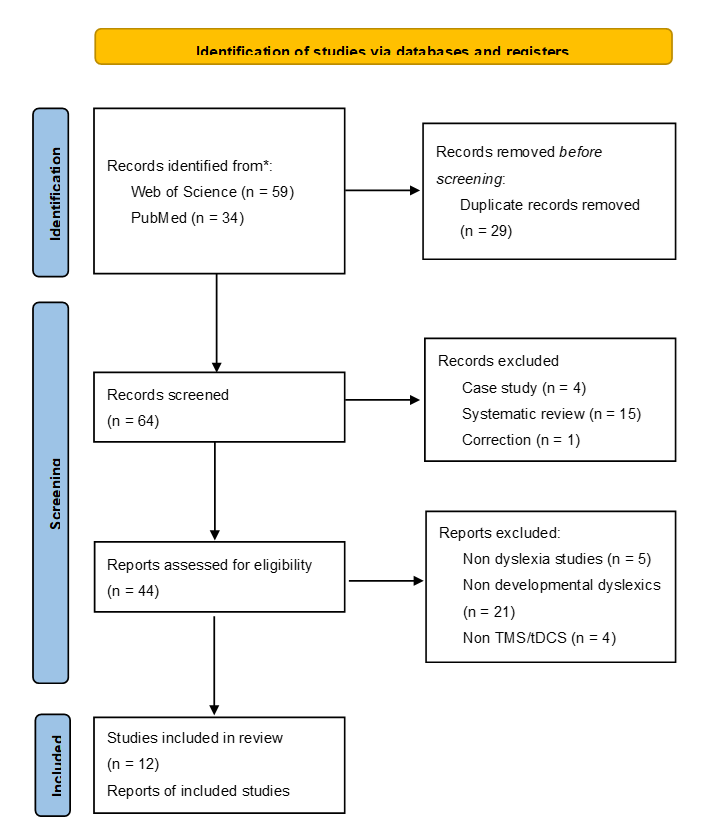
Figure 1. PRISMA flowchart detailing the search strategy employed for database exploration
12 eligible papers are all summarized in Figure 2.
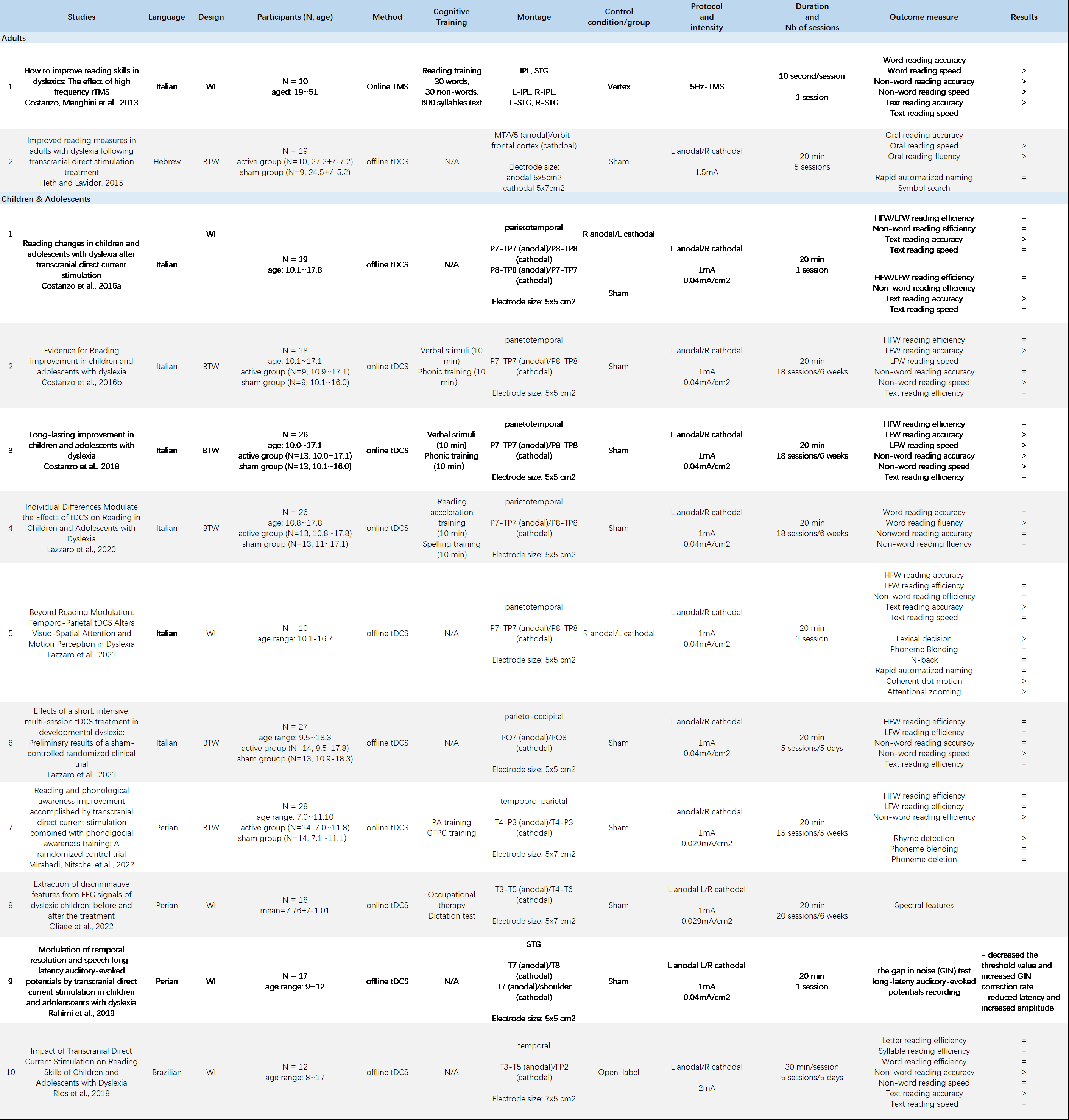
Figure 2. Brain stimulation study on reading ability of Developmental Dyslexia
3.2. Research on children and adolescents with DD
3.2.1. Reading ability modulation. Some researchers including Costanzo, Varuzza et al. and Costanzo, Varuzza et al. have aimed to investigate the effect of tDCS on pediatric populations with DD [17,18]. A few researchers called for caution to apply NIBS to young populations, uncovered the uncertain risks as well as potential side effects of stimulation on the developmental brain [19, 20]. The main concern was improvements in specific learning abilities may be detrimental to certain skills. Costanzo et al. emphasized t the significance of exploring potential effectiveness of DD in developing age, which were crucial to promoting learning in school, and broadening future professional possibilities consequentially.
In Costanzo et al.’s study, nineteen children and adolescents with DD (10~18 years old) were exposed to four experimental conditions, two active tDCS stimulations over parieto-temporal region, L anodal/R cathodal, R anodal/L cathodal, and no tDCS stimulation as sham condition [17]. The reading and reading-related tests were taken before and without delay after 20 min at 1mA stimulation. The single-session results showed that the left anodal/right cathodal condition markedly improved the accuracy of text reading accuracy by decreasing the number of mistakes in comparison to sham condition, but the right anodal/left cathodal condition significantly reduced the accuracy of text reading by rising the number of mistakes with sham condition and baseline. No effects were found in other reading tasks of word, non-word reading, as well as reading-related tasks of lexical decision-making, phonetic n-back, phoneme blending and RAN for color and letter. The study revealed that lateralization of the parietotemporal region was enhanced after left anode and right cathode tDCS, which contributed to the improvement of reading ability in DD. Conversely, the enhancement of right lateralization of the parieto-temporal region worsened reading performance. A positive effect was observed only in the accuracy of text reading in this study aligned with the evidence in adults with DD in the previous studies [9, 15]. However, no improvement found in non-word reading which was expected based on the findings from the results of [9]. The authors speculated that the lack of focality of tDCS might be a possible reason compared with TMS.
A more specific examination of the influence of tDCS in reading abilities was conducted by the same authors [18]. The same paradigm (same montage over the same brain region with the same protocol) was used for 18 stimulus sessions combined with 6 weeks of cognitive reading training. 18 children and adolescents with DD at age range from 10 to 17 years old were divided into either active tDCS group or sham group randomly. Both active and sham stimulation were delivered with a cognitive training, including 10 minutes of speech stimulus speedometer training to increase the reading speed, and 10 minutes of phonetic training, with a focus on letter pronunciation rules to promote the accuracy of reading. The reading skills, the same as the previous study [17], were assessed before, after, and once month after treatment. The same as previous research, the active tDCS group markedly improved the accuracy of low-frequency word reading, as well as the speed of non-word reading. This result was suggested by Rios et al., which is a one-group pretest-retest study to exam the reading abilities changes after a stand-alone tDCS treatment for 5 consecutive days with no cognitive reading training [21]. An intensive stimulation at 2mA for 30 min over temporal region implemented was applied to 12 participants to investigate the effects and evaluate the impact on children and adolescents with DD’s reading abilities. The research tasks tested the reading performance of letters, words, syllables, non-words and text before and after treatment. The accuracy of non-word reading also increased significantly, which was consisted with study Costanzo et al. [17]. Rios et al. thought the reason why the significant improvement in the accuracy and speed of non-word reading observed in this study were not found in Costanzo et al. was due to a single stimulation session which could not enough to cause changes of several reading tests [17].
In addition, Costanzo et al. showed the long-lasting influence in low-frequency-word and in non-word reading within one month after treatment [18]. This was reinforced by the same group of authors in 2018 on another group at 26 participants of children and adolescents with DD with the same intervention paradigm and reading ability measures [22]. A follow-up reading assessment was taken at 6-month after intervention to check its long-term efficacy. The results suggested that after multiple left/right vaginal tDCS in the parietal temporal region combined with cognitive reading training, this improvement was found to last 1 month and 6 months after treatment. Besides the improvement found in accuracy of low-frequency word reading and speed of non-word reading after treatment, low-frequency reading speed and accuracy of non-word reading were also found to be enhanced, which were not found in the previous study [18]. One of the possible explanations was nine participants out of 13 in every group (positive group and sham group) had taken part in the previous research, who might be familiar with the training and reading measures and achieve a better performance [18]. This research provided the proof of long-term enhancements after tDCS jointed with reading training in pediatric populations with DD, which was emphasized phonics instruction or sub-lexical abilities and correspondence of grapheme/phoneme, as well as phonemic awareness. The long-term improvement in non-word and low-frequency word reading was identified as a causal link between the “assembled phonology” and the plasticity of the parieto-temporal area, which was regulated by tDCS combined with cognitive reading training. Conversely, they thought the shortage of influence in high-frequency word and text reading tests may be due to the inability of its specific schema to evoke plasticity in the middle temporal gyrus, thus affecting the “addressed phonology” accordingly.
Lastly, Costanzo et al. also provided preliminary evidence that a combined tDCS with cognitive training could have positive influence in certain aspects of reading ability, in comparison to the individual cognitive training [18]. This evidence was further supported by Lazzaro et al., who conducted a study to check the effect of a short duration, intensive and multi-session tDCS scheme and seek the appropriate number of stimulation session as well as the most useful intensity, and verify the potential additional function of a reading training [23]. The results demonstrated that the improvements in non-word reading speed were found only in the positive group compared to baseline immediately after intervention, as well as 1 week later. However, tDCS efficacy found in this study on non-word reading speed was only one-third of that in the study of Costanzo [18]. Average speed increase relative to baseline was 5 seconds vs 15 seconds. A potential reason for the heightened impact could derive from the combined reading training. The combined effect of reading training and tDCS synergistically enhanced both the intrinsic excitability related to reading ability through cognitive training and the extrinsic neuromodulation provided by tDCS, thereby reinforcing the synaptic connections within the neural network responsible for reading skills. These findings suggested tDCS stimulation coupled with cognitive training because these protocols could result in more significant improvements in reading skills compared to tDCS used on its own.
Lazzaro et al. offered an additional potential explanation for the limited impact on non-word reading observed in this research [23], which could be more tDCS treatment sessions in the study of Costanzo, 5 vs 18 [18]. They suggested that a lengthier yet less concentrated tDCS procedure for pediatric populations since that protocol appeared to yield better results in terms of enhancing reading skills compared to a shorter and more intensive alternative. This finding aligns with neurobiological research indicating that conducting more than 10 sessions of tDCS in a multi-session approach increases the likelihood of accumulating biological effectiveness over an extended period [6].
The last alternative explanation for the reduced efficacy provided by Lazzaro et al. was the stimulation montage [23]. The electrodes placement in this study targeted parieto-occipital regions, which in the previous studies was parieto-temporal areas [17, 18, 21, 22]. Based on this result, they suggested that stimulating the parietal-temporal regions may exert a more pronounced impact on reading proficiency in contrast to stimulating the parietal-occipital region.
In the study of Rios et al., there were no notable variances for letter and syllable tasks, but a trend of enhancement among the younger population was observed at the individual level analysis [21]. It’s speculated that older individuals had already required competence and proficiency, especially for the letter task. Big age and grade range in the study hindered the ability to interpret the developmental differences in reading skills. Lazzaro et al. designed a study conducted with a double-blind control to investigate the influence of individual variations in tDCS results and tDCS effectiveness as determined by reading assessments in clinical settings [24]. The same experimental design was applied as a previous study [22]. The differential parts were the coupled reading training and reading outcome measures. Instead of using 10 min verbal stimuli plus 10 min phonic training for cognitive stimuli, 10 min reading acceleration plus 10 min spelling training were used in this study. Norm-referenced clinical measures were used in reading outcome assessment to assess the relevance of tDCS outcomes in clinical practice. As considering the contribution of individual differences, the result of study showed that word reading fluency experienced enhancements in both the active and sham tDCS groups at every assessment time, with more pronounced enhancements observed in participants who had lower levels of fluency initially. Nevertheless, six months following the intervention, only less fluent subjects in active group exhibited greater improvement compared to the individuals in the sham group. The result also demonstrated that the active group’s older children displayed enhanced word reading fluency compared to their younger counterparts at each follow-up within the group of participants who had lower fluency levels, whereas a similar pattern of word reading fluency improvement based on age only became apparent in the sham group after six months of treatment. The author suggested that the effect of age could be due to the neurodevelopmental disparities presenting in the parietal-temporal regions of children with DD. Concerning IQ, the study showed that more improvement in proficiency in reading words was found as IQ scores increased over each assessment period among group the less fluent individuals within the active treatment group. Among the sham group, reading word proficiency remained stable from the start of the treatment until the end, and reverted to baseline levels when IQ scores increased during the follow-up assessments. Further, it was supposed that the presence of elevated cognitive abilities prolonged the advantageous impact of tDCS stimulation and reading training exercises, facilitating a more rapid and substantial enhancement in response to the combined interventions. In addition, Lazzaro [23] also provided preliminary evidence of the impact of tDCS on standardized reading assessments and an investigation into the factors influencing improvements after tDCS intervention [24]. The findings indicated a greater proportion of individuals who exhibited improvements in word reading fluency in the active group compared to the sham group. In the active group, about 50% of participants obtained a non-clinical score (>-2SD) in word reading fluency immediately following the completion of the treatment, whereas only 8% of participants in the sham group reached this level. The result about the influence on word reading fluency, altered by tDCS, has expanded the beneficial interaction between tDCS intervention and cognitive reading training, which was initially observed in terms of informal reading assessments, to encompass standardized reading measures typically utilized in clinical settings [17, 18, 22].
3.2.2. Neurocognitive ability improvement. Neurocognitive abilities were measured in Costanzo and Varuzza et al., which included lexical decision, verbal n-back, phoneme blending, and rapid automatized naming for color and letter [17]. They found that compared to the baseline, reduced phonemic blending times in stimulation involving anodal placement on the left and cathodal placement on the right, as well as anodal placement on the right and cathodal placement on the left., phonemic blending accuracy increased with the left anodal and right cathodal, verbal n-back task enhanced after right anodal/left cathodal intervention, but not to sham condition for all. One possible reason for no definitive efficacy on neurocognitive ability of rapid automatized naming could be observed as in the previous study on adults with DD was probably due to only a single stimulation session applied [15]. In the study of Heth and Lavior, 5 sessions of stimulation with 20 min at 1.5mA was used [15].
An addition thorough examination on neurocognitive abilities was conducted by Lazzaro and Beroni et al. on 10 children and adolescent with DD [25]. Besides lexicon decision, verbal n-back, phoneme blending, and rapid automatized naming, coherent dot motion and attentional zooming were included. The reading abilities of word, non-word and text were measured by reading tasks. They found that participants showed fewer errors in text reading after the left anodal/right cathodal stimulation compared to right anodal/left cathodal condition, which was aligned with previous findings on text reading accuracy in the study of Costanzo and Varuzza et al. [17]. In addition to reading tasks improvement, some neurocognitive abilities showed significant changes. They found the individuals showed decreased response time for word recognition in the lexical decision task after the left anodal/right cathodal stimulation, which meant reading fluency enhanced. In the CDM task, the study demonstrated that sensitivity to motion was greater during left anodal/right cathodal stimulation compared to right anodal/left cathodal condition and the form of the corresponding curves showed the difference remarkably, which indicated that the stimuli underwent processed in a markedly distinct manner from than in right anodal/left cathodal treatment. Their findings revealed that reading and general neurocognitive functions, including visuo-spatial attention and functioning in the MD stream, were influenced by the tDCS intervention, and these alterations depended on the polarity of stimulation. In order to investigate whether the combined tDCS intervention had positive impacts on PA skills, in addition to its effects on reading proficiency Mirahadi and collaborators conducted a randomized, double-controlled trial on 28 children and adolescents with DD (7~11 years old) [26]. The findings demonstrated that the behavioral intervention (PA+GTPC) led to enhancements in all outcome measures over the course of time for both active and sham groups by within-group comparison. The authors thought it could be inferred that the PA intervention proved to be an effective approach for enhancing both reading proficiency and PA skills Besides the effectiveness of a phonological-based behavioral intervention enhanced the excitability of the left inferior parieto-temporal regions and frontal regions. Group comparison analysis demonstrated that applied to the left parietal-temporal junction resulted in enhanced non-word reading, but did not affect high-frequency or low-frequency word reading This result aligned with the phonological deficit theory posits that the dorsal reading pathway, including the left parietal-temporal junction, which is associated with grapheme-to-phoneme conversion, plays a role in non-word reading. However, The ventral reading pathway, encompassing the left occipito-temporal junction, which is associated with whole word reading [27]. For secondary outcomes, the significant improvements of the capacity to identify rhymes and perform phoneme deletions were observed in the study. This result suggested the left parietal-temporal junction plays a crucial role in PA performance. The critical contribution of this study is to reveal that the positive impact of tDCS treatment when combined with PA and GTPC training interventions on nonword reading abilities, as well as certain PA tasks such as rhyme detection and phoneme deletion showed a greater magnitude of improvement compared to the effect of the behavioral intervention (PA and GTPC training) on its own.
3.2.3. tDCS on relevant visual and auditory processing. Some theories, proposed that impairments either auditory temporal processing or rapid auditory processing could potentially underlie DD. The significance of central processing of auditory information stands out in individuals with dyslexia. Reports indicated that children diagnosed with DD encountered challenges to process rapid changing or transient acoustic events [28]. In addition, the ability of processing rapid successive information is critical to develop the phonological system. Poor language skills in individuals with dyslexia could be a result of a broader deficiency in processing rapid temporal information [28, 29]. At the functional viewpoint, some dyslexic children exhibited impairment in rapid auditory processing at both the upper brainstem and auditory cortex levelsRahimi and Mohamadkhani conducted a study to investigate the effect impact of tDCS on auditory-evoked potentials and temporal resolution an in children and adolescents with DD [30]. The gap in noise (GIN) test was applied for behavioral assessment because GIN was utilized as a due to its validity in assessing temporal resolution, particularly in individuals experiencing central auditory processing disorder [31]. Furthermore, GIN could index primary cortical processing. Long-latency auditory-evoked potentials (LLAEP) test was implemented as a promising measure in central auditory processing research, which signifies cortical activity encompassing auditory skills, from the simplest to the most intricate, following tDCS treatment in children with DD [32].
The authors found that noticeable reductions in threshold values and improvements in the percentage of correct responses were observed in the GIN test as part of the behavioral assessment. The findings indicated that enhanced activation of the left superior temporal region due to left anodal and right cathodal tDCS intervention proved beneficial in facilitating improvements in central auditory processing among pediatric individuals. The authors also found that the electrophysiological test in the study exhibited shortened latency and amplified wave amplitude for the P1, N1, and P2 waves after both anodal tDCS on the left STG and cathodal tDCS on the right STG, and anodal tDCS on the left STG and cathodal tDCS on the right shoulder, when compared to baseline and sham conditions. These results implyed that tDCS had the potential to bring about changes in auditory temporal resolution and speech LLAEP in children and adolescents with DD. In addition, elevated excitability solely in the left STG led to alterations in both temporal resolution and LLAEP.
A lack of PA, which results in difficulties with reading and spelling, can be a cause of dyslexia [33, 34]. Functional magnetic resonance imaging (fMRI) research discovered a decrease in grey matter volume within parieto-temporal and temporo-occipital areas in patients with DD [35, 36]. In addition, other irregularities in structure were discovered in the cerebellum and lingual gyrus [36]. These abnormalities in structure showed a direct relationship between phonological processing and spelling proficiency [37, 38].
Since EEG (electroencephalogram) recordings do not involve radiation risks and are significantly more economical, it is a reliable and safe method to acquire data in children and adolescents. Oliaee et al. [39] designed a study to investigate discriminating characteristics of EEG signals on dyslexic children associated with a particular tDCS therapy with occupational therapy. It is the study that tested a wealth of EEG features and found discriminative features for 16 children by recording EEG signals before and after the combined intervention. All participants aged around 8 years old were implemented 20 sessions in 6 weeks with a 2-day interval as minimum between sessions. The occupational therapy including 5 elements as working memory for visual spatial information, sustained attention to visual stimuli, selective attention to visual cues, perception of visual figure-ground relationships, processing speed for visual tasks, and the integration of visual and motor skills. EEG was recorded for each participant with resting-state eye-closed mode in a specialized environment before and after the intervention.
This study demonstrated that the power spectrum increased following the treatment, the behavior of EEG features became more aligned with the expected normal behavior exhibited by a typical individual. Moreover, enhanced synchronization between channels P3 and F8 was found, and PLI exhibited an elevation in the P3 and F8 channels as well. These findings supported the idea that the combined application of tDCS treatment and occupational therapy intervention has the potential to enhance the well-being of children with DD. These results revealed the significance of information derived from EMD-based, spectral, and phase-related features, particularly in the brain’s partial and posterior areas. The combined tDCS treatment and occupational therapy employed in this study revealed insights into the distinguishing EEG characteristics that could contribute to evaluate the effectiveness of a treatment plan for dyslexia and other relevant applications.
References
[1]. Hulme C, Snowling MJ. Reading disorders and dyslexia. Curr Opin Pediatr. 2016 Dec;28(6):731-735. doi: 10.1097/MOP.0000000000000411.
[2]. Snowling, M. J., & Hulme, C. (2012). Annual research review: The nature and classification of reading disorders–a commentary on proposals for DSM-5. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(5), 593–607.
[3]. Ferrer, E., Shaywitz, B. A., Holahan, J. M., Marchione, K., & Shaywitz, S. E. (2010). Uncoupling of reading and IQ over time: Empirical evidence for a definition of dyslexia. Psychological Science, 21(1), 93–101.
[4]. Valero-Cabre, A., Amengual, J. L., Stengel, C., Pascual-Leone, A., & Coubard, O. A. (2017). Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neuroscience and Biobehavioral Reviews, 83(December), 381e404.
[5]. Nitsche, M. A., and Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527 (Pt 3), 633–639
[6]. Nitsche, M. A., Nitsche, M. S., Klein, C. C., Tergau, F., Rothwell, J. C., & Paulus, W. (2003). Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clinical Neurophysiology, 114(4), 600–604.
[7]. Nitsche, M.A., Cohen, L.G., Wassermann, E.M., Priori, A., Lang, N., Antal, A., Paulus, W., Pascual-Leone, A., 2008. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 1 (3), 206–223.
[8]. Zaehle T, Beretta M, Jäncke L, Herrmann CS, Sandmann P (2011) Excitability changes induced in the human auditory cortex by transcranial direct current stimulation: direct electrophysiological evidence. Exp Brain Res 215(2):135–140.
[9]. Costanzo, F., Menghini, D., Caltagirone, C., Oliveri, M., Vicari, S., 2013. How to improve reading skills in dyslexics: the effect of high frequency rTMS. Neuropsychologia 51 (14), 2953–2959
[10]. Costanzo, F., Menghini, D., Caltagirone, C., Oliveri, M., & Vicari, S. (2012). High frequency rTMS over the left parietal lobule increases non-word reading accuracy. Neuropsychologia, 50(11), 2645–2651.
[11]. Graves, W. W., Grabowski, T. J., Mehta, S., & Gupta, P. (2008). The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. Journal of Cognitive Neuroscience, 20(9), 1698–1710.
[12]. Jobard, G., Vigneau, M., Mazoyer, B., & Tzourio-Mazoyera, N. (2007). Impact of modality and linguistic complexity during reading and listening tasks. NeuroImage, 34, 784–800.
[13]. Leonard CM, Eckert MA. Asymmetry and dyslexia. Dev Neuropsychol. 2008;33(6):663–681.
[14]. Rimrodt, S. L., Clements-Stephens, A. M., Pugh, K. R., Courtney, S. M., Gaur, P., Pekar, J. J., et al. (2009). Functional MRI of sentence comprehension in children with dyslexia: Beyond word recognition. Cerebral Cortex, 19(2), 402–413.
[15]. Heth I, Lavidor M. Improved reading measures in adults with dyslexia following transcranial direct current stimulation treatment. Neuropsychologia 2015; 70:107–113.
[16]. Ben-Dror, I., Shany, M., 2002. Assessing Developmental Dyslexia in the Hebrew Language: Theoretical and Practical Aspects and a Case Study. Perspectiva. vol. 23. Orton Dyslexia, Israel, pp. 69–80.
[17]. Costanzo, F., Varuzza, C., Rossi, S., Sdoia, S., Varvara, P., Oliveri, M., et al., 2016a. Reading changes in children and adolescents with dyslexia after transcranial direct current stimulation. Neuroreport. 23, 295–300.
[18]. Costanzo, F., Varuzza, C., Rossi, S., Sdoia, S., Varvara, P., Oliveri, M., et al. 2016b. Evidence for reading improvement following tDCS treatment in children and adolescents with Dyslexia. Restor. Neurol. Neurosci. 34, 215–226.
[19]. Kadosh, R. C., Levy, N., O’Shea, J., Shea, N., and Savulescu, J. (2012). The neuroethics of non-invasive brain stimulation. Curr. Biol. 22, R108–R111.
[20]. Krause B, Cohen Kadosh R (2013) Can transcranial electrical stimulation improve learning difficulties in atypical brain development? A future possibility for cognitive training. Dev Cogn Neurosci 6:176–194.
[21]. Rios, D. M., Rios, C. M., Bandeira, I. D., Queiros Campbell, F., de Carvalho Vaz, D., & Lucena, R. (2018). Impact of transcranial direct current stimulation on reading skills of children and adolescents with dyslexia. Child Neurology Open, 5, 2329048 × 1879825.
[22]. Costanzo, F., Rossi, S., Varuzza, C., Varvara, P., Vicari, S., Menghini, D., 2018. Long-lasting improvement following tDCS treatment combined with a training for reading in children and adolescents with dyslexia. Neuropsychologia 130, 38–43.
[23]. Lazzaroa, G., Costanzoa F., Varuzzaa C., Rossia S., Vicaria, S., & Menghinia, D. (2021). Effects of a short, intensive, multi-sess.ion tDCS treatment in developmental dyslexia: Preliminary results of a sham-controlled randomized clinical trial. Progress in Brain Research, Volume 264.
[24]. Lazzaro, G., Bertoni, S., Menghini, D., Costanzo, F., Franceschini, S., Varuzza, C., Ronconi, L., Battisti, A., Gori, S., Facoetti, A., & Vicari, S. (2021). Beyond reading modulation: Temporo-parietal tDCS alters visuo-spatial attention and motion perception in dyslexia. Brain Sciences, 11(2), 263.
[25]. Lazzaro, G., Costanzo, F., Varuzza, C., Rossi, S., De Matteis, M. E., Vicari, S., & Menghini, D. (2021). Individual differences modulate the effects of tDCS on reading in children and adolescents with dyslexia. Scientific Studies of Reading, 25(6), 1–17.
[26]. Mirahadi, S. S., Nitsche, M. A., Pahlavanzadeh B., Mohamadi R., Ashayeri, H. & Abolghasemi, J. (2022). Reading and phonological awareness improvement accomplished by transcranial direct current stimulation combined with phonological awareness training: A randomized controlled trial. APPLIED NEUROPSYCHOLOGY: CHILD
[27]. Vandermosten, M., Hoeft, F., & Norton, E. S. (2016). Integrating MRI brain imaging studies of pre-reading children with current theories of developmental dyslexia: A review and quantitative meta-analysis. Current Opinion in Behavioral Sciences, 10, 155–161.
[28]. Boets B, Wouters J, van Wieringen A, Ghesquière P (2007) Auditory processing, speech perception and phonological ability in pre-school children at high-risk for dyslexia: a longitudinal study of the auditory temporal processing theory. Neuropsychologia 45(8):1608–1620.
[29]. Raschle NM, Stering PL, Meissner SN, Gaab N (2014) Altered neuronal response during rapid auditory processing and its relation to phonological processing in pre reading children at familial risk for dyslexia. Cereb Cortex 24:2489–2501.
[30]. Rahimi, V., Mohamadkhani, G., Alaghband-Rad, J. Kermani, F., Hikfarjad, H., & Marofizade, S. (2018). Modulation of temporal resolution and speech long-latency auditoryevoked potentials by transcranial direct current stimulation in children and adolescents with dyslexia. Experimental Brain Research.
[31]. Musiek FE, Shinn JB, Jirsa R, Bamiou DE, Baran JA, Zaida E (2005) GIN (gaps-in-noise) test performance in subjects with confirmed central auditory nervous system involvement. Ear Hear 26(6):608–618
[32]. Regaçone SF, Gução ACB, Giacheti CM, Romero AC, Frizzo ACF (2014) Long latency auditory evoked potentials in students with specific learning disorders. Audiol Commun Res 1:8–13.
[33]. Ziegler JC, Perry C, Zorzi M (2020) Learning to read and dyslexia: from theory to intervention through personalized computational models. Curr Direct Psychol Sci 29(3):293–300.
[34]. Share DL (2021) Common misconceptions about the phonological deficit theory of dyslexia. Brain Sci 11(11):1510.
[35]. Ulrike K, Neef NE, Kraft I, Schaadt G, Do¨rr L, Brauer J, Czepezauer I, et al (2020) The emergence of dyslexia in the developing brain. Neuroimage 211.
[36]. Sihvonen AJ, Virtala P, Thiede A, Laasonen M, Kujala T (2021) Structural white matter connectometry of reading and dyslexia. Neuroimage 241:118411.
[37]. Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G (2008). Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Hum Brain Map 29(5):613–625.
[38]. Pernet CR, Poline JB, Demonet JF, Rousselet GA (2009) Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neurosci 10(1):1–19.
[39]. Oliaee, A., Mohebbi, M., Shirani, S., & Rostami, R. (2022). Extraction of discriminative features from EEG signals of dyslexic children; before and after the treatment. Cognitive Neurodynamics
Cite this article
MU,Y. (2024). A systematic review of transcranial magnetic and direct current stimulation application on the improvement of reading ability of developmental dyslexia. Theoretical and Natural Science,32,143-154.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Hulme C, Snowling MJ. Reading disorders and dyslexia. Curr Opin Pediatr. 2016 Dec;28(6):731-735. doi: 10.1097/MOP.0000000000000411.
[2]. Snowling, M. J., & Hulme, C. (2012). Annual research review: The nature and classification of reading disorders–a commentary on proposals for DSM-5. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(5), 593–607.
[3]. Ferrer, E., Shaywitz, B. A., Holahan, J. M., Marchione, K., & Shaywitz, S. E. (2010). Uncoupling of reading and IQ over time: Empirical evidence for a definition of dyslexia. Psychological Science, 21(1), 93–101.
[4]. Valero-Cabre, A., Amengual, J. L., Stengel, C., Pascual-Leone, A., & Coubard, O. A. (2017). Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neuroscience and Biobehavioral Reviews, 83(December), 381e404.
[5]. Nitsche, M. A., and Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527 (Pt 3), 633–639
[6]. Nitsche, M. A., Nitsche, M. S., Klein, C. C., Tergau, F., Rothwell, J. C., & Paulus, W. (2003). Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clinical Neurophysiology, 114(4), 600–604.
[7]. Nitsche, M.A., Cohen, L.G., Wassermann, E.M., Priori, A., Lang, N., Antal, A., Paulus, W., Pascual-Leone, A., 2008. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 1 (3), 206–223.
[8]. Zaehle T, Beretta M, Jäncke L, Herrmann CS, Sandmann P (2011) Excitability changes induced in the human auditory cortex by transcranial direct current stimulation: direct electrophysiological evidence. Exp Brain Res 215(2):135–140.
[9]. Costanzo, F., Menghini, D., Caltagirone, C., Oliveri, M., Vicari, S., 2013. How to improve reading skills in dyslexics: the effect of high frequency rTMS. Neuropsychologia 51 (14), 2953–2959
[10]. Costanzo, F., Menghini, D., Caltagirone, C., Oliveri, M., & Vicari, S. (2012). High frequency rTMS over the left parietal lobule increases non-word reading accuracy. Neuropsychologia, 50(11), 2645–2651.
[11]. Graves, W. W., Grabowski, T. J., Mehta, S., & Gupta, P. (2008). The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. Journal of Cognitive Neuroscience, 20(9), 1698–1710.
[12]. Jobard, G., Vigneau, M., Mazoyer, B., & Tzourio-Mazoyera, N. (2007). Impact of modality and linguistic complexity during reading and listening tasks. NeuroImage, 34, 784–800.
[13]. Leonard CM, Eckert MA. Asymmetry and dyslexia. Dev Neuropsychol. 2008;33(6):663–681.
[14]. Rimrodt, S. L., Clements-Stephens, A. M., Pugh, K. R., Courtney, S. M., Gaur, P., Pekar, J. J., et al. (2009). Functional MRI of sentence comprehension in children with dyslexia: Beyond word recognition. Cerebral Cortex, 19(2), 402–413.
[15]. Heth I, Lavidor M. Improved reading measures in adults with dyslexia following transcranial direct current stimulation treatment. Neuropsychologia 2015; 70:107–113.
[16]. Ben-Dror, I., Shany, M., 2002. Assessing Developmental Dyslexia in the Hebrew Language: Theoretical and Practical Aspects and a Case Study. Perspectiva. vol. 23. Orton Dyslexia, Israel, pp. 69–80.
[17]. Costanzo, F., Varuzza, C., Rossi, S., Sdoia, S., Varvara, P., Oliveri, M., et al., 2016a. Reading changes in children and adolescents with dyslexia after transcranial direct current stimulation. Neuroreport. 23, 295–300.
[18]. Costanzo, F., Varuzza, C., Rossi, S., Sdoia, S., Varvara, P., Oliveri, M., et al. 2016b. Evidence for reading improvement following tDCS treatment in children and adolescents with Dyslexia. Restor. Neurol. Neurosci. 34, 215–226.
[19]. Kadosh, R. C., Levy, N., O’Shea, J., Shea, N., and Savulescu, J. (2012). The neuroethics of non-invasive brain stimulation. Curr. Biol. 22, R108–R111.
[20]. Krause B, Cohen Kadosh R (2013) Can transcranial electrical stimulation improve learning difficulties in atypical brain development? A future possibility for cognitive training. Dev Cogn Neurosci 6:176–194.
[21]. Rios, D. M., Rios, C. M., Bandeira, I. D., Queiros Campbell, F., de Carvalho Vaz, D., & Lucena, R. (2018). Impact of transcranial direct current stimulation on reading skills of children and adolescents with dyslexia. Child Neurology Open, 5, 2329048 × 1879825.
[22]. Costanzo, F., Rossi, S., Varuzza, C., Varvara, P., Vicari, S., Menghini, D., 2018. Long-lasting improvement following tDCS treatment combined with a training for reading in children and adolescents with dyslexia. Neuropsychologia 130, 38–43.
[23]. Lazzaroa, G., Costanzoa F., Varuzzaa C., Rossia S., Vicaria, S., & Menghinia, D. (2021). Effects of a short, intensive, multi-sess.ion tDCS treatment in developmental dyslexia: Preliminary results of a sham-controlled randomized clinical trial. Progress in Brain Research, Volume 264.
[24]. Lazzaro, G., Bertoni, S., Menghini, D., Costanzo, F., Franceschini, S., Varuzza, C., Ronconi, L., Battisti, A., Gori, S., Facoetti, A., & Vicari, S. (2021). Beyond reading modulation: Temporo-parietal tDCS alters visuo-spatial attention and motion perception in dyslexia. Brain Sciences, 11(2), 263.
[25]. Lazzaro, G., Costanzo, F., Varuzza, C., Rossi, S., De Matteis, M. E., Vicari, S., & Menghini, D. (2021). Individual differences modulate the effects of tDCS on reading in children and adolescents with dyslexia. Scientific Studies of Reading, 25(6), 1–17.
[26]. Mirahadi, S. S., Nitsche, M. A., Pahlavanzadeh B., Mohamadi R., Ashayeri, H. & Abolghasemi, J. (2022). Reading and phonological awareness improvement accomplished by transcranial direct current stimulation combined with phonological awareness training: A randomized controlled trial. APPLIED NEUROPSYCHOLOGY: CHILD
[27]. Vandermosten, M., Hoeft, F., & Norton, E. S. (2016). Integrating MRI brain imaging studies of pre-reading children with current theories of developmental dyslexia: A review and quantitative meta-analysis. Current Opinion in Behavioral Sciences, 10, 155–161.
[28]. Boets B, Wouters J, van Wieringen A, Ghesquière P (2007) Auditory processing, speech perception and phonological ability in pre-school children at high-risk for dyslexia: a longitudinal study of the auditory temporal processing theory. Neuropsychologia 45(8):1608–1620.
[29]. Raschle NM, Stering PL, Meissner SN, Gaab N (2014) Altered neuronal response during rapid auditory processing and its relation to phonological processing in pre reading children at familial risk for dyslexia. Cereb Cortex 24:2489–2501.
[30]. Rahimi, V., Mohamadkhani, G., Alaghband-Rad, J. Kermani, F., Hikfarjad, H., & Marofizade, S. (2018). Modulation of temporal resolution and speech long-latency auditoryevoked potentials by transcranial direct current stimulation in children and adolescents with dyslexia. Experimental Brain Research.
[31]. Musiek FE, Shinn JB, Jirsa R, Bamiou DE, Baran JA, Zaida E (2005) GIN (gaps-in-noise) test performance in subjects with confirmed central auditory nervous system involvement. Ear Hear 26(6):608–618
[32]. Regaçone SF, Gução ACB, Giacheti CM, Romero AC, Frizzo ACF (2014) Long latency auditory evoked potentials in students with specific learning disorders. Audiol Commun Res 1:8–13.
[33]. Ziegler JC, Perry C, Zorzi M (2020) Learning to read and dyslexia: from theory to intervention through personalized computational models. Curr Direct Psychol Sci 29(3):293–300.
[34]. Share DL (2021) Common misconceptions about the phonological deficit theory of dyslexia. Brain Sci 11(11):1510.
[35]. Ulrike K, Neef NE, Kraft I, Schaadt G, Do¨rr L, Brauer J, Czepezauer I, et al (2020) The emergence of dyslexia in the developing brain. Neuroimage 211.
[36]. Sihvonen AJ, Virtala P, Thiede A, Laasonen M, Kujala T (2021) Structural white matter connectometry of reading and dyslexia. Neuroimage 241:118411.
[37]. Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G (2008). Developmental dyslexia: gray matter abnormalities in the occipitotemporal cortex. Hum Brain Map 29(5):613–625.
[38]. Pernet CR, Poline JB, Demonet JF, Rousselet GA (2009) Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neurosci 10(1):1–19.
[39]. Oliaee, A., Mohebbi, M., Shirani, S., & Rostami, R. (2022). Extraction of discriminative features from EEG signals of dyslexic children; before and after the treatment. Cognitive Neurodynamics









