1. Introduction
Urinary Tract Infection- (UTI) is one of the most prevalent bacterial infections in the world, with at least half of women gets the infection once in their life time [1]. Scholars estimated that in 2019 alone, there were 404.61 million cases with 236790 deaths, and it was being predicted that the situation would deteriorate even more as the disease becomes even more prevalent [2]. Most notably, the uropathogenic Escherichia coli (UPEC) is the most common UTI-causing pathogen, covering 80% of total UTI cases [3].
In a typical UPEC-causing urinary tract infection, the process begins from bacterial contamination of the urethra, where the intruding uropathogens start to ascend up toward the bladder. The key for bacteria to colonize the bladder is bacterial adhesins; bacteria construct adhesins into appendages such as type 1 pili that enables them to attach to host uroepithelium cells and ascend upward [4]. Due to the nutrient-deficient nature of the bladder, the uropathogens would start to obtain resources from the host cells, either through releasing toxins to damage host cell membranes or invading host cells and forming intracellular bacterial communities (IBC). The damage triggers immune response, and the inflammation of uroepithelium cells expresses the infection. For UPEC bacteria, if they manage to enter uroepithelium cytoplasm and establish successful IBC without being expelled by Toll-like Receptor 4 (TLR 4), they could evade neutrophils from the immune system and have the potential to resettle to the kidney, which is lethal [5].
Amoxicillin and trimethoprim are among the most commonly prescribed antibiotics to combat urinary tract infection. Interestingly, although both drugs are being used in treatment of Urinary Tract Infection caused by uropathogenic Escherichia coli (UPEC), two drugs are completely different both in their molecular structures and their antibacterial mechanism. Recently, scientists reported that the wide-spread usage of first-line antibiotics has triggered antibiotic resistance of UPEC against amoxicillin and trimethoprim [6]. Multiple antibacterial strains are forming in UTI-causing pathogen groups such as UPEC, imposing a severe health threat to the public health [5]. Therefore, the prevalence of antibiotic resistance in UPEC has prompted the writing of this paper; the paper summarizes the overall similarities and differences of amoxicillin and trimethoprim in treating UPEC-causing UTI to provide a detailed review on the contemporary performances of these two drugs.
2. About The Targeted Bacteria
Escherichia coli is the most common UTI-causing pathogen. These bacteria are gram-negative, rod-shaped, and facultative anaerobic (Figure 1). Belonging to the genus Escherichia, they reside in animal intestine and the environment; most of their strains are harmless, but uropathogenic Escherichia coli (UPEC) could cause urinary tract infection, while certain E coli strains could cause other diseases such as diarrhea and respiratory illness [7]. Recall its involvement in UTI, the species is characterized by their unique formation of Intracellular Bacterial Communities (IBC) within uroepithelium cells. Infected uroepithelium cells would be exfoliated, while UPEC either get exfoliated with their current hosts or migrate to search their next host [8].
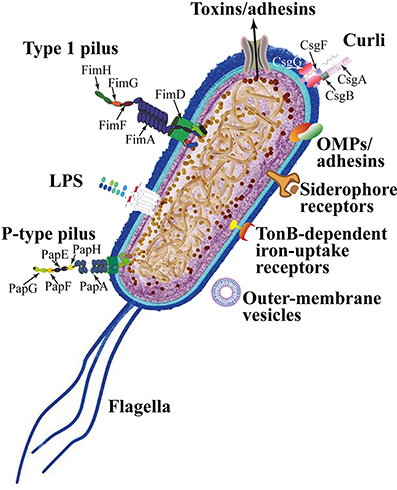
Figure 1. The cellular structure of Escherichia coli. Notice that the bacteria releases toxins via its protein channels, and the species has a thin layer of peptidoglycan (Characteristic of gram-negative bacterium). The picture also lists out structures of Escherichia coli that are potential targets for future antibiotics [8].
3. Amoxicillin
3.1. Core Structure and Disease-fighting Mechanism
Amoxicillin is a penicillin-derivative drug, belonging to the class of beta-lactam antibiotics. Same as its parental drug penicillin, its core is a 6-aminopenicillanic acid (6-APA), the critical structural requirement for antibiotic activities of beta-lactam drugs (Figure 2) [9]. 6-APA is an important intermediate for synthetic antibiotics because it allows different side chains to be attached to its core, generating various beta-lactam antibiotics with variations on their side chains [10].
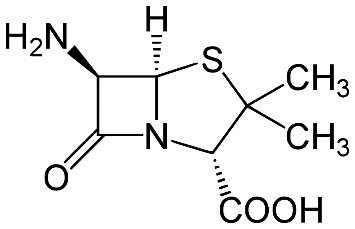
Figure 2. The chemical structure of 6-aminopeniciliac acid (6-APA). It is crucial to the antibiotic mechanism of beta-lactam antibiotics, and it is recognizable in all variations of beta-lactam class [11].
Amoxicillin, like all beta-lactam antibiotics, interrupts the production of bacterial peptidoglycan operated by transpeptidase. Transpeptidase is responsible of assembling amino acids that compose the bacterial cell wall, known as peptidoglycan. The peptidoglycan serves the important role of providing protection to bacterial cells. The chemical structure of beta-lactams strongly resembles transpeptidase’s amino acid substrate, D-Ala-D-Ala; beta lactam is a cyclic resembling chemical of D-Ala-D-Ala terminus, tricking transpeptidase to misrecognize the beta-lactam for its amino acid substrate (Figure 3) [12].
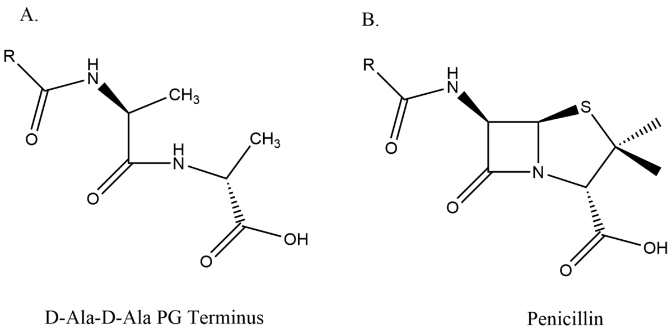
Figure 3. The chemical structures of substrate D-Ala-D-Ala terminus and penicillin (beta-lactam molecules). The beta-lactam is the cyclic resemblance of D-Ala-D-Ala [13].
Normally, E. coli transpeptidase would hydrolyze and break off from the peptide chain in milliseconds after it crosslinks the meso-diaminopimelic acid (m-DAP) to connect the peptidoglycan [14]. However, once the carboxyl group of amoxicillin reacts with the serine amino acid on transpeptidase, a stable acyl-enzyme intermediate is formed and will persist for several hours [15]. Amoxicillin serves the role of a competitive inhibitor, which inhibits transpeptidase from its peptidoglycan synthesis. Since bacterial species like Escherichia coli do not require the formation of new cell wall during spore phase, amoxicillin is most effective when treating bacteria that are rapidly multiplying; the inability of transpeptidase would leave pores on the bacterial cell wall, causing the bacteria to lyse and die (Figure 4) [16].
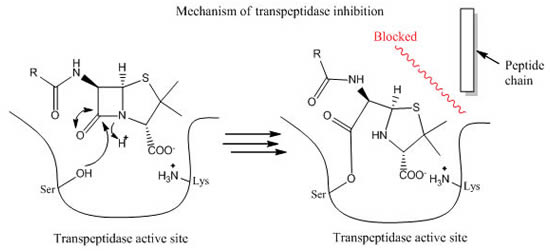
Figure 4. The mechanism of beta-lactam antibiotics. The binding of beta-lactam to transpeptidase active site would create a stable acyl-enzyme intermediate; it would block the synthesis process of transpeptidase for hours [17].
3.2. Features of Amoxicillin
Penicillin depends on the carbonyl bond on the beta lactam ring to perform its antimicrobial activities, but the reactive nature also makes the antibiotic more susceptible to breakdown under acidic environments [16]. Subsequently, series of semisynthetic beta-lactam antibiotics was synthesized to further stabilize their core structure. Among them, Amoxicillin is an extension of ampicillin: same as ampicillin, a benzene ring is added on the side chain of 6-APA to enhance the drug’s resistance to penicillin-breaking beta lactamases produced by E coli. Also, both drugs include an additional amino group on the benzene side chain to better penetrate the complex outer membrane of gram-negative bacteria such as E coli [18]. However, ampicillin has difficulty sustaining its structures under acidic conditions of stomach. But amoxicillin appends a hydroxyl electron withdrawing group on its benzene ring, which greatly enhances its stability in stomach compared to ampicillin (Figure 5) [16,19,20].
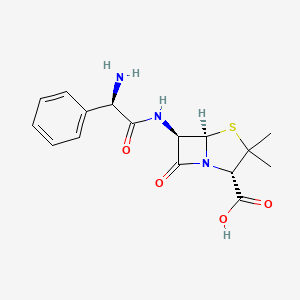
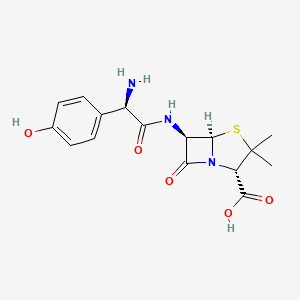
Figure 5. The 2D chemical structure of Ampicillin (Left) and Amoxicillin (Right). Both includes an amide bond to penetrate the protein complex on the outer membrane of gram-negative E coli. Amoxicillin also has a hydroxyl group at the end of the benzene ring to enhance its acid stability [19,21].
3.3. Current Performance
Amoxicillin is a strong candidate for prescription of Urinary Tract Infection. The addition of hydroxyl group at its side chain has made this drug well-tolerated [20]. Unless allergic to amoxicillin or with severe renal damage, amoxicillin does not showcase severe adverse effect, and it is considered to be safe for pregnant women, surpassing most of antibiotics in terms of its safety for patients [22].
However, amoxicillin and other beta-lactam antibiotics have been widely used for decades, exerting strong selective pressure over UTI-causing pathogens, especially virulent Escherichia coli [23]. Studies have shown that the overuse of amoxicillin has caused the prevalence of amoxicillin-resistant UTI-causing E. coli (UPEC) with the percentage to reach over 20% in several regions. In particular, the resistance rate in Aveiro, Portugal is 42.4% between 2001-2009,[24]. followed by Europe’s 28% between 2007 to 2008 [25], 63.6% in South Korea between 2010-2011 [26]. and 35.5% in 2012 France [27]. Although these studies investigated different patients, the consistently high percentage of amoxicillin resistance is indeed alarming.
The primary antibiotic resistance of UTI-causing Escherichia coli (UPEC) is the application of beta-lactamases. These enzymes resemble the shape of beta-lactam antibiotics’ target, DD-transpeptidase, and are secreted into the surrounding environment of bacterial cells in which antibiotics are present [28]. A serine beta-lactamase, for instance, attacks the carbonyl carbon on the beta-lactam ring after binding to a beta-lactam antibiotic, namely amoxicillin. This process is known as acylation and it leads to the collapse of the beta-lactam ring, forming a stable acyl enzyme composed of the beta-lactamase and the drug. Deacylation ensues, which hydrolyzes the beta-lactamase’s oxygen and the carbonyl carbon of the broken ring. It releases an antibiotic molecule with its beta-lactam ring open, rendering it completely useless (Figure 6) [29,30].
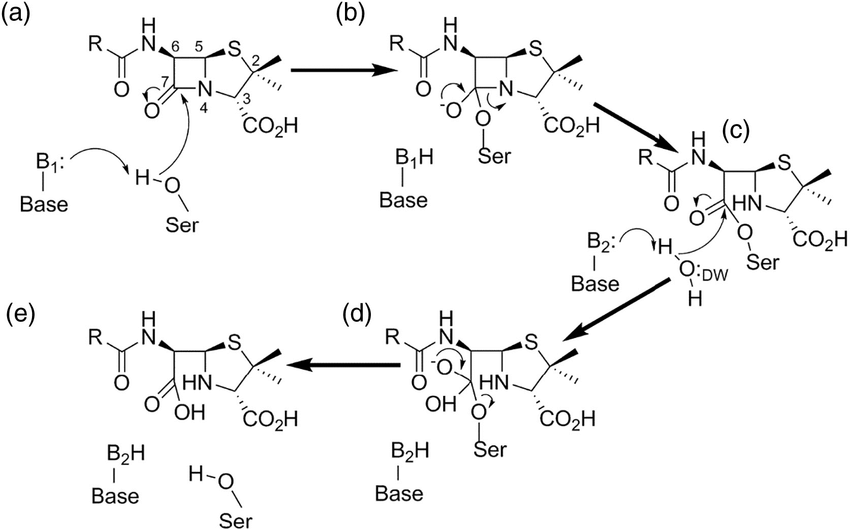
Figure 6. The detailed illustration of serine beta-lactamase undergoing the process of breaking down beta-lactam drug. After the activation of the serine, the enzyme attacks the carbonyl carbon on the beta-lactam ring, forming a stable intermediate. The enzyme then hydrolyzes the intermediate and releases the now useless drug. Notice that after the process the carbonyl group is replaced by a carboxyl group, destroying the beta-lactam ring structure that is crucial to the antibiotic process [30].
In response, pharmacologists have developed combination therapies, pairing amoxicillin with beta-lactamase inhibitors. These inhibitors are not effective antibiotics when used alone, but instead they mainly serve to inactivate beta-lactamase in combination treatments. An amoxicillin combinational drug is essentially a mixture of amoxicillin and inhibitor molecules, which they carry out their separate duties (inhibitors shut down beta-lactamase while amoxicillin halts bacterial peptidoglycan synthesis) [31]. Studies has confirmed that combination therapies like amoxicillin-clavulanate (AMC) and amoxicillin-sulbactam (AMX-SUL) are more effective in treating amoxicillin-sensitive (or resistant) pathogens than amoxicillin alone [32,33]. Despite the recent discovery of several E. coli strains resistant to the beta-lactamase inhibitor clavulanic acid, the most prevalent inhibitor-resistant strains such as TEM and OXA-1 are found to have less virulent factors than AMC-susceptible strains [34]. Therefore, treating UPEC population that is increasingly amoxicillin-resistant, amoxicillin is still widely used as a first-line antibiotic today, exists mostly in the form of combination therapies.
4. Trimethoprim, In Comparison
4.1. Core Structure and Disease Fighting Mechanism
Trimethoprim is a broad-spectrum dihydropyrimidine antimicrobial that is used to treat a variety of aerobic gram-positive and gram-negative bacterial species, including pathogenic E coli [35]. Compared to amoxicillin, trimethoprim has a different aim. While beta-lactam antibiotics impede the peptidoglycan synthesis, causing bacterial cells to lyse, trimethoprim attacks the bacterial dihydrofolic acid reductase (DHFR) and prevents DNA synthesis [36]. Both trimethoprim and amoxicillin have similar mechanism in which they serve as enzyme inhibitors that prevent crucial biosynthesis, creating bactericidal effects. trimethoprim is a structural analog of DHFR’s supposed substrate, dihydrofolic acid (Figure 7) [37].
In detail, DHFR is responsible of reducing dihydrofolates into tetrahydrofolates (THF), crucial cofactors of purine and thymidylate synthesis. Since DHFR is the sole source of tetrahydrofolates, it becomes a well-established target for antibiotics to treat bacteria proliferation [38]. Trimethoprim diffuses itself into bacterial cell. Trimethoprim binds itself to DHFR via its aminopyrimidine group; this group is also found on the structure of dihydrofolates. The amino groups on aminopyrimidine forms intermolecular bonds with carboxylate group on DHFR, securing the inhibition (Figure 7) [39].
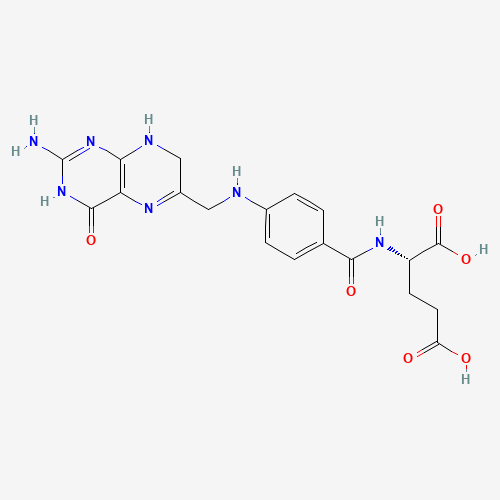
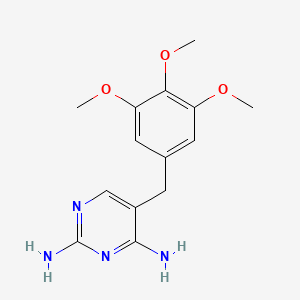
Figure 7. The molecular structures of dihydrofolates (left) and trimethoprim (right). Notice are aminopyrimidine groups are present on both molecules, the crux to secure bonding to carboxylate group on dihydrofolate reductase [40,41].
Similar to amoxicillin, trimethoprim is largely time-dependent. With a half-life of 8-10 hours, trimethoprim as a reversible inhibitor would eventually disassociate from DHFR and be excreted via urine [42].
4.2. Features of Trimethoprim: Adverse Effects
Recall that trimethoprim is an antifolate drug: it inhibits the production of folic acids. Although trimethoprim is highly selective in attacking bacterial DHFR-given that it cannot bind to mammal DHFR- and interferes only with bacterial DNA synthesis [43], its structural resemblance to folic acids also prompts it to disrupt human folic acid absorption. Human also relies on folic acid for DNA synthesis, but instead of making it human absorb folic acids via dietary pathways [44]. In detail, several studies have used cryo-electron microscopy in their investigation on the molecular structure of folic acid transporter, SLC19A1. Through these studies, scientists unveiled that the structural similarities between folic acids and antifolate drugs has granted antifolates access to bind with SLC19A1, interrupting human foliate absorption [45,46]. Further studies conducted by Meidahl Petersen, Kasper et al. in 2016 has confirmed on a statistic level that the use of trimethoprim is closely associated with low amount of folic acid in serum concentration [47].
Consequently, trimethoprim is contraindicated for folate-deficient patients, and excessive use of trimethoprim could be detrimental to kidney functions, especially for elder patients. Amoxicillin is classified as a class B drug, which indicates no proven risk for pregnant or breastfeeding patients. Trimethoprim, on the contrary, has proven to be dangerous for pregnancy. Dr. Hernández-Díaz and her team have testified that the use of antifolates, trimethoprim included, during pregnancy induce the risks of underdeveloped infants with possible neutral-tube and cardiovascular defects [48]. Trimethoprim is a class C drug, and it would not be recommended to pregnant patients unless the curing benefit outweighs the risks of fetus [49].
4.3. Current Performance
Just as amoxicillin, trimethoprim has been widely used in antibacterial treatments for decades, and as a result catalyzes various E coli. strains resistant to it. UPEC groups mitigate their susceptibility to trimethoprim by modifying their DHFR structure, making it resistant to trimethoprim binding. One such case is L28R mutant DHFR, which several point mutations occurring on UPEC’s DHFR promoter region increase the enzyme’s susceptibility to dihydrofolic acid while decreasing its susceptibility to trimethoprim [50]. This is done by the mutant enzyme’s newly formed interaction with the glutamate tail of dihydrofolic acids, while altering its structure to decrease trimethoprim affinity. Although the researchers quickly introduced 4’-desmethyltrimethoprim that drastically declines L28R E coli. activities (Figure 8), they have found that various E coli mutations have alarmingly evolved to a sophisticated level, with some strains acquiring up to 5 different point mutations in their promoter genes [50]. This complexity demonstrates that the situation regarding trimethoprim resistance is in peril.
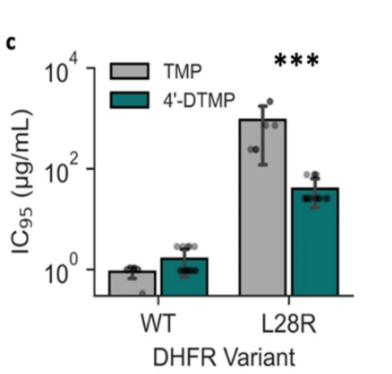
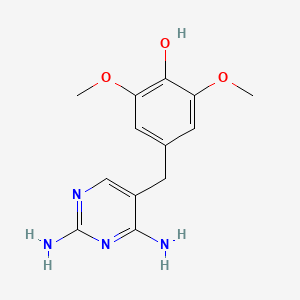
Figure 8. A graph showing the experimental result of 4’-DTMP and trimethoprim (TMP) against wild type E coli. and L28R mutant E coli. (Left) 4’-DTMP appears weak to impede wild type bacterial growth, but its antimicrobial ability is approximately 30 folds compared to TMP when combating the mutant bacteria population. On the right, it is a 2D molecular structure of 4’-DTMP. Recall that L28R mutation modifies DHFR interaction with dihydrofolic acids, 4’-DTMP is a L28R-specific antibiotic in which one of its methyl group on the glutamate tail of TMP is being replaced by a hydroxyl group, enhancing its affinity with L28R-mutant DHFR [50,51].
Similar to amoxicillin, the most prevalent trimethoprim-related treatment regarding UPEC-causing UTI is a combinational therapy. Trimethoprim/sulfamethoxazole, or TMP-SMX, is drug mixture of trimethoprim and sulfamethoxazole. They are essentially different drugs that perform their inhibitions on the same process, the bacterial tetrahydrofolic acid synthesis. Sulfamethoxazole, a sulfonamide, is a competitive inhibitor to para-aminobenzoic acid that inhibits the dihydropteroate synthetase from producing dihydropteroic acid, the precursor for dihydrofolic acid [42]. The addition of sulfamethoxazole onto trimethoprim therapy helps the treatment to deplete the excess dihydrofolic acids that accumulate under an inactive DHFR, effectively terminating the tetrahydrofolic acid synthesis (Figure 9) [52]. The combination therapy also reduces the chance of bacterial resistance to trimethoprim that is produced from passage techniques, alternations to the tetrahydrofolic acid synthesis pathway [52].
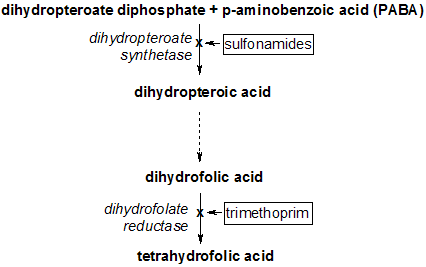
Figure 9. The role of TMP/SMX in tetrahydrofolic acid synthesis pathway. Recall that TMP is a competitive inhibitor, so the accumulation of dihydrofolic acids as a result of inactive DHFR reduces TMP efficiency. The addition of sulfonamides that disrupts the synthesis of the dihydrofolic acid’s precursor helps TMP by reducing the excess dihydrofolic acid [53].
Therefore, despite that sulfamethoxazole does not directly address the mutation occurred in the structure of DHFR, its addition onto trimethoprim creates a combinational drug that is more effective against UPEC that are sensitive to trimethoprim or sulfamethoxazole alone. In fact, in a study conducted by Knottnerus, Bart J. et al in 2012 the experiment concluded that based on statistical results, the effects of TMP/SMX are stronger than our beta-lactam counterpart, amoxicillin clavulanate, in terms of short-term clinical and bacteriological outcomes [54]. This is partly due to the beta-lactam nature of amoxicillin, since it inhibits the synthesis of peptidoglycan and is more successful in treating gram-positive bacterial species rather than gram-negative Escherichia coli [20].
However, the pervasive usage of TMP/SMX for decades also prompts the emergence of TMP/SMX resistant UPEC population. TMP/SMX resistant UPEC have developed insensitive target enzymes, acquiring the resistance through either naturally developed mutation or spreading of resistance genes [55]. In beforementioned studies, the investigated percentage of E coli. strains resistant to TMP/SMX is 25.4% in Aveiro Portugal between 2001-2009 [24], 16.1% in 2007 Europe [25], 35.9% in 2010 South Korea [26], and 12.2% in France [27]. Regardless, TMP/SMX is still available for most UTI-causing Escherichia coli.
5. Conclusion
Uropathogenic Escherichia coli are the dominant pathogens behind the prevalent urinary tract infection. They are adaptive to immune response, coercing patients to strongly rely on antibiotic or other clinical treatments. The spread of antibiotic-resistant strains further deteriorates the situation. Amoxicillin and trimethoprim are derived from chemicals disparate to each other. Hence, they undermine different bacterial activities. However, both of them inhibit bacterial enzymes to impede metabolic processes and have shown in vitro decent antibacterial performance against Escherichia coli. From statistical perspective, studies have shown that trimethoprim sulfamethoxazole is more effective against UTI caused by gram-negative bacteria than amoxicillin clavulanate. But after decades of excessive use, both drugs have experienced considerable bacterial resistance against them. Nevertheless, it is likely that both trimethoprim and amoxicillin would continue to be used, in the form of combination therapies, as first-line antibiotics against UPEC-causing UTI in the future.
References
[1]. Rahn, David D. “Urinary Tract Infections: Contemporary Management.” Urologic Nursing, Oct. 2008, https://www.researchgate.net/profile/David-Rahn/publication/23447519_Urinary_tract _infections_contemporary_management/links/0046351d574f5c1874000000/Urinary-tract-infections-contemporary-management.pdf
[2]. Yang, Xiaorong, et al. “Disease Burden and Long-Term Trends of Urinary Tract Infections: A Worldwide Report.” Frontiers in Public Health, vol. 10, 27 July 2022, https://doi.org/10.3389/fpubh.2022.888205.
[3]. Mazzulli, Tony, et al. “Susceptibility of Community Gram-Negative Urinary Tract Isolates to Mecillinam and Other Oral Agents.” Canadian Journal of Infectious Diseases, vol. 12, no. 5, Sept. 2001, pp. 289–92, https://doi.org/10.1155/2001/601743.
[4]. Sauer, Maximilian M., et al. “Binding of the Bacterial Adhesin FimH to Its Natural, Multivalent High-Mannose Type Glycan Targets.” Journal of the American Chemical Society, vol. 141, no. 2, 13 Dec. 2018, pp. 936–944, https://doi.org/10.1021/jacs.8b10736.
[5]. Flores-Mireles, Ana L., et al. “Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options.” Nature Reviews Microbiology, vol. 13, no. 5, 8 Apr. 2015, pp. 269–284, https://doi.org/10.1038/nrmicro3432.
[6]. Gupta, Kalpana. “Increasing Prevalence of Antimicrobial Resistance among Uropathogens Causing Acute Uncomplicated Cystitis in Women.” JAMA, vol. 281, no. 8, Feb. 1999, p. 736, https://doi.org/10.1001/jama.281.8.736.
[7]. “E. Coli (Escherichia Coli) | E. Coli | CDC.” Www.cdc.gov, US Department of Health and Human Services, 26 Feb. 2020, www.cdc.gov/ecoli/index.html/.
[8]. Terlizzi, Maria E., et al. “UroPathogenic Escherichia Coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-Antibiotic Antimicrobial Strategies.” Frontiers in Microbiology, vol. 8, no. 1566, Aug. 2017, https://doi.org/10.3389/fmicb.2017.01566.
[9]. Patrick, Graham L. An Introduction to Medicinal Chemistry. 1995. 6th ed., Oxford University Press, 2017, p. 434, www.google.com.hk/books/edition/An_Introduction_to_Medicinal_ Chemistry/bBqeDgAAQBAJ?hl=zh-CN&gbpv=0.
[10]. Peter, Doyle Frank, et al. “Recovery of Solid 6-Aminopenicillanic Acid.” Beecham Research Laboratories Ltd, June 1960, https://patents.google.com/patent/US2941995A/en.
[11]. “6-APA.” Wikipedia, 14 Apr. 2022, https://en.wikipedia.org/wiki/6-APA.
[12]. Nguyen-Disteche, Martine, et al. “Isolation of the Membrane-Bound 26000-Mrpenicillin-Binding Protein of Streptomyces Strain K15 in the Form of a Penicillin-Sensitive D-Alanyl-D-Alanine-Cleaving Transpeptidase.” The Biochemical Society, vol. 207, June 1982, https://doi.org/10.1042%2Fbj2070109.
[13]. Omargs10. “English: Structural Similarity between the Two Structures.” Wikimedia Commons, 1 Mar. 2016, https://commons.wikimedia.org/wiki/File:Penicillin_vs_PG_terminus_structure. png.
[14]. Lupoli, Tania J., et al. “Transpeptidase-Mediated Incorporation Ofd-Amino Acids into Bacterial Peptidoglycan.” Journal of the American Chemical Society, vol. 133, no. 28, July 2011, pp. 10748–51, https://doi.org/10.1021/ja2040656.
[15]. Cohen, Jonathan, et al. Infectious Diseases. 2010. 3rd ed., Elsevier/Mosby, 2010, pp. 1288–89.
[16]. I Edward Alcamo. Microbes and Society: An Introduction to Microbiology. Jones And Bartlett, 2003, p. 198, https://books.google.com.hk/books?id=oRAZd3AlTlkC&hl=zh-CN&source=gbs_navlinks_s
[17]. “Medicinal Chemistry of Beta-Lactam Antibiotics.” PharmaFactz, https://pharmafactz.com/ medicinal-chemistry-of-beta-lactam-antibiotics/#.
[18]. Sharma, S., et al. “Comparative Study between Penicillin and Ampicillin.” Scholars Journal of Applied Medical Sciences (SJAMS) Sch. J. App. Med. Sci, vol. 1, no. 4, 2013, pp. 291–94, https://doi.org/10.36347/sjams.2013.v01i04.019.
[19]. PubChem. “Amoxicillin.” Pubchem.ncbi.nlm.nih.gov, 24 June 2005, https://pubchem.ncbi.nlm. nih.gov/compound/33613.
[20]. Kaur, Simar, et al. “AMOXICILLIN: A BROAD SPECTRUM ANTIBIOTIC.” International Journal of Pharmacy and Pharmaceutical Science, International Journal of Pharmacy and Pharmaceutical Science, 7 May 2011, https://web.archive.org/web/20180412213455id_/ https://ijppsjournal.com/Vol3Issue3/2249.pdf.
[21]. Pubchem. “Ampicillin.” Nih.gov, PubChem, 2019, https://pubchem.ncbi.nlm.nih.gov/ compound/6249.
[22]. Damkier, Per, et al. “In Utero Exposure to Antibiotics and Risk of Congenital Malformations: A Population-Based Study.” American Journal of Obstetrics and Gynecology, vol. 221, no. 6, Dec. 2019, pp. 648.e1–15, https://doi.org/10.1016/j.ajog.2019.06.050.
[23]. Jaana Harmoinen, et al. “Orally Administered Targeted Recombinant Beta-Lactamase Prevents Ampicillin-Induced Selective Pressure on the Gut Microbiota: A Novel Approach to Reducing Antimicrobial Resistance.” Antimicrobial Agents and Chemotherapy, vol. 48, no. 1, American Society for Microbiology, Jan. 2004, pp. 75–79, https://doi.org/10.1128/aac.48.1.75-79.2004.
[24]. Linhares, Inês, et al. “Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections: A Ten-Year Surveillance Study (2000–2009).” BMC Infectious Diseases, vol. 13, no. 1, Jan. 2013, https://doi.org/10.1186/1471-2334-13-19.
[25]. Kahlmeter, Gunnar, and Hanna Odén Poulsen. “Antimicrobial Susceptibility of Escherichia Coli from Community-Acquired Urinary Tract Infections in Europe: The ECO·SENS Study Revisited.” International Journal of Antimicrobial Agents, vol. 39, no. 1, Jan. 2012, pp. 45–51, https://doi.org/10.1016/j.ijantimicag.2011.09.013.
[26]. Dong Sup Lee, et al. “Antimicrobial Susceptibility Pattern and Epidemiology of Female Urinary Tract Infections in South Korea, 2010-2011.” EuropePMC, vol. 57, no. 11, Nov. 2013, pp. 5384–93, https://doi.org/10.1128/aac.00065-13.
[27]. Neuzillet, Yann, et al. “French Results of the ARESC Study: Clinical Aspects and Epidemiology of Antimicrobial Resistance in Female Patients with Cystitis. Implications for Empiric Therapy.” Médecine et Maladies Infectieuses, vol. 42, no. 2, Feb. 2012, pp. 66–75, https://doi.org/10.1016/j.medmal.2011.07.005.
[28]. Neu, Harold C. “Effect of β-Lactamase Location in Escherichia Coli on Penicillin Synergy.” Applied Microbiology, vol. 17, no. 6, Mar. 1969, pp. 783–86, https://doi.org/10.1128/aem.17. 6.783-786.1969.
[29]. Bonomo, Robert A. “β-Lactamases: A Focus on Current Challenges.” Cold Spring Harbor Perspectives in Medicine, vol. 7, no. 1, Jan. 2017, https://doi.org/10.1101/cshperspect. a025239.
[30]. Tooke, Catherine L., et al. “β-Lactamases and β-Lactamase Inhibitors in the 21st Century.” Journal of Molecular Biology, vol. 431, no. 18, Aug. 2019, pp. 3472–500, https://doi.org/10.1016/j.jmb.2019.04.002.
[31]. Evans, Justin, et al. “Amoxicillin Clavulanate.” Europepmc.org, Europe PMC, 9 Mar. 2019, https://europepmc.org/article/nbk/nbk538164#article-17475.r14.
[32]. Brogden, R. N., et al. “Amoxycillin/Clavulanic Acid.” Drugs, vol. 22, no. 5, Nov. 1981, pp. 337–62, https://doi.org/10.2165/00003495-198122050-00001.
[33]. Bantar, C., et al. “Intravenous Amoxicillin-Sulbactam AgainstEscherichia Coli: Optimizing the Dose, Component Ratio and Infusion Time Using a Human Pharmacodynamic Model.” Journal of Chemotherapy, vol. 21, no. 3, June 2009, pp. 296–301, https://doi.org/10.1179/joc.2009.21.3.296.
[34]. Oteo, Jesús, et al. “Inhibitor-Resistant TEM- and OXA-1-Producing Escherichia Coli Isolates Resistant to Amoxicillin-Clavulanate Are More Clonal and Possess Lower Virulence Gene Content than Susceptible Clinical Isolates.” Antimicrobial Agents and Chemotherapy, vol. 58, no. 7, July 2014, pp. 3874–81, https://doi.org/10.1128/AAC.02738-13.
[35]. Scholar, Eric. “Trimethoprim.” XPharm: The Comprehensive Pharmacology Reference, 2007, pp. 1–6, https://doi.org/10.1016/b978-008055232-3.62804-1.
[36]. DrugBank. “Trimethoprim.” Go.drugbank.com, DrugBank, 23 Jan. 2005, https://go.drugbank.com/drugs/DB00440.
[37]. Saniya Arfin, et al. Autophagy and Metabolism: Potential Target for Cancer Therapy. 2022. Academic Press, 2022, pp. 1–39, https://doi.org/10.1016/b978-0-323-99879-6.00008-0.
[38]. Schnell, Jason R., et al. “Structure, Dynamics, and Catalytic Function of Dihydrofolate Reductase.” Annual Review of Biophysics and Biomolecular Structure, vol. 33, no. 1, June 2004, pp. 119–40, https://doi.org/10.1146/annurev.biophys.33.110502.133613.
[39]. Wróbel, Agnieszka, et al. “Trimethoprim and Other Nonclassical Antifolates an Excellent Template for Searching Modifications of Dihydrofolate Reductase Enzyme Inhibitors.” The Journal of Antibiotics, vol. 73, no. 1, Oct. 2019, pp. 5–27, https://doi.org/10.1038/s41429-019-0240-6.
[40]. Pubchem. “Trimethoprim.” Pubchem.ncbi.nlm.nih.gov, https://pubchem.ncbi.nlm.nih.gov/ compound/5578.
[41]. Pubchem. “Dihydrofolic Acid.” Pubchem.ncbi.nlm.nih.gov, https://pubchem.ncbi.nlm.nih.gov/ compound/Dihydrofolic-acid.
[42]. Kemnic, Tyler R., and Meghan Coleman. “Trimethoprim Sulfamethoxazole.” Nih.gov, StatPearls Publishing, 2019, www.ncbi.nlm.nih.gov/books/NBK513232/.
[43]. Boyer, Zachary W., et al. “Synthesis and Characterization of Functionalized Amino Dihydropyrimidines toward the Analysis of Their Antibacterial Structure–Activity Relationships and Mechanism of Action.” ACS Omega, vol. 7, no. 42, American Chemical Society, Oct. 2022, pp. 37907–16, https://doi.org/10.1021/acsomega.2c05071.
[44]. Thaler, C. J. “Folate Metabolism and Human Reproduction.” Geburtshilfe Und Frauenheilkunde, vol. 74, no. 9, Sept. 2014, pp. 845–51, https://doi.org/10.1055/s-0034-1383058.
[45]. Zhang, Qixiang, et al. “Recognition of Cyclic Dinucleotides and Folates by Human SLC19A1.” Nature, Oct. 2022, pp. 1–3, https://doi.org/10.1038/s41586-022-05452-z.
[46]. Wright, Nicholas J., et al. “Methotrexate Recognition by the Human Reduced Folate Carrier SLC19A1.” Nature, vol. 609, no. 7929, Sept. 2022, pp. 1056–62, https://doi.org/10.1038/s41586-022-05168-0.
[47]. Meidahl Petersen, Kasper, et al. “The Effect of Trimethoprim on Serum Folate Levels in Humans.” American Journal of Therapeutics, vol. 23, no. 2, Mar. 2016, pp. e382–87, https://doi.org/10.1097/mjt.0000000000000372.
[48]. Hernández-Díaz, Sonia, et al. “Folic Acid Antagonists during Pregnancy and the Risk of Birth Defects.” New England Journal of Medicine, vol. 343, no. 22, Nov. 2000, pp. 1608–14, https://doi.org/10.1056/nejm200011303432204.
[49]. “Trimethoprim (Trimethoprim Tablet): Uses, Dosage, Side Effects, Interactions, Warning.” RxList, 1 Mar. 2021, www.rxlist.com/trimethoprim-drug.htm.
[50]. Manna, Madhu Sudan, et al. “A Trimethoprim Derivative Impedes Antibiotic Resistance Evolution.” Nature Communications, vol. 12, no. 1, May 2021, p. 2949, https://doi.org/10.1038/s41467-021-23191-z.
[51]. PubChem. “4-((2,4-Diaminopyrimidin-5-Yl)Methyl)-2,6-Dimethoxyphenol.” Pubchem.ncbi.nlm. nih.gov, https://pubchem.ncbi.nlm.nih.gov/compound/10423570.
[52]. Wormser, Gary P., et al. “Co-Trimoxazole (Trimethoprim-Sulfamethoxazole) an Updated Review of Its Antibacterial Activity and Clinical Efficacy.” Drugs, vol. 24, no. 6, Dec. 1982, pp. 459–518, https://doi.org/10.2165/00003495-198224060-00002.
[53]. “English: Tetrahydrofolate Synthesis Pathway.” Wikimedia Commons, https://commons. wikimedia.org/wiki/File:THFsynthesispathway.png.
[54]. Knottnerus, B. J., et al. “Comparative Effectiveness of Antibiotics for Uncomplicated Urinary Tract Infections: Network Meta-Analysis of Randomized Trials.” Family Practice, vol. 29, no. 6, Apr. 2012, pp. 659–70, https://doi.org/10.1093/fampra/cms029.
[55]. Eliopoulos, G. M., and P. Huovinen. “Resistance to Trimethoprim-Sulfamethoxazole.” Clinical Infectious Diseases, vol. 32, no. 11, June 2001, pp. 1608–14, https://doi.org/10.1086/320532.
Cite this article
Teng,Y. (2024). Comparing amoxicillin and trimethoprim: Overall performance in combating Escherichia coli-causing urinary tract infection. Theoretical and Natural Science,44,70-80.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Rahn, David D. “Urinary Tract Infections: Contemporary Management.” Urologic Nursing, Oct. 2008, https://www.researchgate.net/profile/David-Rahn/publication/23447519_Urinary_tract _infections_contemporary_management/links/0046351d574f5c1874000000/Urinary-tract-infections-contemporary-management.pdf
[2]. Yang, Xiaorong, et al. “Disease Burden and Long-Term Trends of Urinary Tract Infections: A Worldwide Report.” Frontiers in Public Health, vol. 10, 27 July 2022, https://doi.org/10.3389/fpubh.2022.888205.
[3]. Mazzulli, Tony, et al. “Susceptibility of Community Gram-Negative Urinary Tract Isolates to Mecillinam and Other Oral Agents.” Canadian Journal of Infectious Diseases, vol. 12, no. 5, Sept. 2001, pp. 289–92, https://doi.org/10.1155/2001/601743.
[4]. Sauer, Maximilian M., et al. “Binding of the Bacterial Adhesin FimH to Its Natural, Multivalent High-Mannose Type Glycan Targets.” Journal of the American Chemical Society, vol. 141, no. 2, 13 Dec. 2018, pp. 936–944, https://doi.org/10.1021/jacs.8b10736.
[5]. Flores-Mireles, Ana L., et al. “Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options.” Nature Reviews Microbiology, vol. 13, no. 5, 8 Apr. 2015, pp. 269–284, https://doi.org/10.1038/nrmicro3432.
[6]. Gupta, Kalpana. “Increasing Prevalence of Antimicrobial Resistance among Uropathogens Causing Acute Uncomplicated Cystitis in Women.” JAMA, vol. 281, no. 8, Feb. 1999, p. 736, https://doi.org/10.1001/jama.281.8.736.
[7]. “E. Coli (Escherichia Coli) | E. Coli | CDC.” Www.cdc.gov, US Department of Health and Human Services, 26 Feb. 2020, www.cdc.gov/ecoli/index.html/.
[8]. Terlizzi, Maria E., et al. “UroPathogenic Escherichia Coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-Antibiotic Antimicrobial Strategies.” Frontiers in Microbiology, vol. 8, no. 1566, Aug. 2017, https://doi.org/10.3389/fmicb.2017.01566.
[9]. Patrick, Graham L. An Introduction to Medicinal Chemistry. 1995. 6th ed., Oxford University Press, 2017, p. 434, www.google.com.hk/books/edition/An_Introduction_to_Medicinal_ Chemistry/bBqeDgAAQBAJ?hl=zh-CN&gbpv=0.
[10]. Peter, Doyle Frank, et al. “Recovery of Solid 6-Aminopenicillanic Acid.” Beecham Research Laboratories Ltd, June 1960, https://patents.google.com/patent/US2941995A/en.
[11]. “6-APA.” Wikipedia, 14 Apr. 2022, https://en.wikipedia.org/wiki/6-APA.
[12]. Nguyen-Disteche, Martine, et al. “Isolation of the Membrane-Bound 26000-Mrpenicillin-Binding Protein of Streptomyces Strain K15 in the Form of a Penicillin-Sensitive D-Alanyl-D-Alanine-Cleaving Transpeptidase.” The Biochemical Society, vol. 207, June 1982, https://doi.org/10.1042%2Fbj2070109.
[13]. Omargs10. “English: Structural Similarity between the Two Structures.” Wikimedia Commons, 1 Mar. 2016, https://commons.wikimedia.org/wiki/File:Penicillin_vs_PG_terminus_structure. png.
[14]. Lupoli, Tania J., et al. “Transpeptidase-Mediated Incorporation Ofd-Amino Acids into Bacterial Peptidoglycan.” Journal of the American Chemical Society, vol. 133, no. 28, July 2011, pp. 10748–51, https://doi.org/10.1021/ja2040656.
[15]. Cohen, Jonathan, et al. Infectious Diseases. 2010. 3rd ed., Elsevier/Mosby, 2010, pp. 1288–89.
[16]. I Edward Alcamo. Microbes and Society: An Introduction to Microbiology. Jones And Bartlett, 2003, p. 198, https://books.google.com.hk/books?id=oRAZd3AlTlkC&hl=zh-CN&source=gbs_navlinks_s
[17]. “Medicinal Chemistry of Beta-Lactam Antibiotics.” PharmaFactz, https://pharmafactz.com/ medicinal-chemistry-of-beta-lactam-antibiotics/#.
[18]. Sharma, S., et al. “Comparative Study between Penicillin and Ampicillin.” Scholars Journal of Applied Medical Sciences (SJAMS) Sch. J. App. Med. Sci, vol. 1, no. 4, 2013, pp. 291–94, https://doi.org/10.36347/sjams.2013.v01i04.019.
[19]. PubChem. “Amoxicillin.” Pubchem.ncbi.nlm.nih.gov, 24 June 2005, https://pubchem.ncbi.nlm. nih.gov/compound/33613.
[20]. Kaur, Simar, et al. “AMOXICILLIN: A BROAD SPECTRUM ANTIBIOTIC.” International Journal of Pharmacy and Pharmaceutical Science, International Journal of Pharmacy and Pharmaceutical Science, 7 May 2011, https://web.archive.org/web/20180412213455id_/ https://ijppsjournal.com/Vol3Issue3/2249.pdf.
[21]. Pubchem. “Ampicillin.” Nih.gov, PubChem, 2019, https://pubchem.ncbi.nlm.nih.gov/ compound/6249.
[22]. Damkier, Per, et al. “In Utero Exposure to Antibiotics and Risk of Congenital Malformations: A Population-Based Study.” American Journal of Obstetrics and Gynecology, vol. 221, no. 6, Dec. 2019, pp. 648.e1–15, https://doi.org/10.1016/j.ajog.2019.06.050.
[23]. Jaana Harmoinen, et al. “Orally Administered Targeted Recombinant Beta-Lactamase Prevents Ampicillin-Induced Selective Pressure on the Gut Microbiota: A Novel Approach to Reducing Antimicrobial Resistance.” Antimicrobial Agents and Chemotherapy, vol. 48, no. 1, American Society for Microbiology, Jan. 2004, pp. 75–79, https://doi.org/10.1128/aac.48.1.75-79.2004.
[24]. Linhares, Inês, et al. “Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections: A Ten-Year Surveillance Study (2000–2009).” BMC Infectious Diseases, vol. 13, no. 1, Jan. 2013, https://doi.org/10.1186/1471-2334-13-19.
[25]. Kahlmeter, Gunnar, and Hanna Odén Poulsen. “Antimicrobial Susceptibility of Escherichia Coli from Community-Acquired Urinary Tract Infections in Europe: The ECO·SENS Study Revisited.” International Journal of Antimicrobial Agents, vol. 39, no. 1, Jan. 2012, pp. 45–51, https://doi.org/10.1016/j.ijantimicag.2011.09.013.
[26]. Dong Sup Lee, et al. “Antimicrobial Susceptibility Pattern and Epidemiology of Female Urinary Tract Infections in South Korea, 2010-2011.” EuropePMC, vol. 57, no. 11, Nov. 2013, pp. 5384–93, https://doi.org/10.1128/aac.00065-13.
[27]. Neuzillet, Yann, et al. “French Results of the ARESC Study: Clinical Aspects and Epidemiology of Antimicrobial Resistance in Female Patients with Cystitis. Implications for Empiric Therapy.” Médecine et Maladies Infectieuses, vol. 42, no. 2, Feb. 2012, pp. 66–75, https://doi.org/10.1016/j.medmal.2011.07.005.
[28]. Neu, Harold C. “Effect of β-Lactamase Location in Escherichia Coli on Penicillin Synergy.” Applied Microbiology, vol. 17, no. 6, Mar. 1969, pp. 783–86, https://doi.org/10.1128/aem.17. 6.783-786.1969.
[29]. Bonomo, Robert A. “β-Lactamases: A Focus on Current Challenges.” Cold Spring Harbor Perspectives in Medicine, vol. 7, no. 1, Jan. 2017, https://doi.org/10.1101/cshperspect. a025239.
[30]. Tooke, Catherine L., et al. “β-Lactamases and β-Lactamase Inhibitors in the 21st Century.” Journal of Molecular Biology, vol. 431, no. 18, Aug. 2019, pp. 3472–500, https://doi.org/10.1016/j.jmb.2019.04.002.
[31]. Evans, Justin, et al. “Amoxicillin Clavulanate.” Europepmc.org, Europe PMC, 9 Mar. 2019, https://europepmc.org/article/nbk/nbk538164#article-17475.r14.
[32]. Brogden, R. N., et al. “Amoxycillin/Clavulanic Acid.” Drugs, vol. 22, no. 5, Nov. 1981, pp. 337–62, https://doi.org/10.2165/00003495-198122050-00001.
[33]. Bantar, C., et al. “Intravenous Amoxicillin-Sulbactam AgainstEscherichia Coli: Optimizing the Dose, Component Ratio and Infusion Time Using a Human Pharmacodynamic Model.” Journal of Chemotherapy, vol. 21, no. 3, June 2009, pp. 296–301, https://doi.org/10.1179/joc.2009.21.3.296.
[34]. Oteo, Jesús, et al. “Inhibitor-Resistant TEM- and OXA-1-Producing Escherichia Coli Isolates Resistant to Amoxicillin-Clavulanate Are More Clonal and Possess Lower Virulence Gene Content than Susceptible Clinical Isolates.” Antimicrobial Agents and Chemotherapy, vol. 58, no. 7, July 2014, pp. 3874–81, https://doi.org/10.1128/AAC.02738-13.
[35]. Scholar, Eric. “Trimethoprim.” XPharm: The Comprehensive Pharmacology Reference, 2007, pp. 1–6, https://doi.org/10.1016/b978-008055232-3.62804-1.
[36]. DrugBank. “Trimethoprim.” Go.drugbank.com, DrugBank, 23 Jan. 2005, https://go.drugbank.com/drugs/DB00440.
[37]. Saniya Arfin, et al. Autophagy and Metabolism: Potential Target for Cancer Therapy. 2022. Academic Press, 2022, pp. 1–39, https://doi.org/10.1016/b978-0-323-99879-6.00008-0.
[38]. Schnell, Jason R., et al. “Structure, Dynamics, and Catalytic Function of Dihydrofolate Reductase.” Annual Review of Biophysics and Biomolecular Structure, vol. 33, no. 1, June 2004, pp. 119–40, https://doi.org/10.1146/annurev.biophys.33.110502.133613.
[39]. Wróbel, Agnieszka, et al. “Trimethoprim and Other Nonclassical Antifolates an Excellent Template for Searching Modifications of Dihydrofolate Reductase Enzyme Inhibitors.” The Journal of Antibiotics, vol. 73, no. 1, Oct. 2019, pp. 5–27, https://doi.org/10.1038/s41429-019-0240-6.
[40]. Pubchem. “Trimethoprim.” Pubchem.ncbi.nlm.nih.gov, https://pubchem.ncbi.nlm.nih.gov/ compound/5578.
[41]. Pubchem. “Dihydrofolic Acid.” Pubchem.ncbi.nlm.nih.gov, https://pubchem.ncbi.nlm.nih.gov/ compound/Dihydrofolic-acid.
[42]. Kemnic, Tyler R., and Meghan Coleman. “Trimethoprim Sulfamethoxazole.” Nih.gov, StatPearls Publishing, 2019, www.ncbi.nlm.nih.gov/books/NBK513232/.
[43]. Boyer, Zachary W., et al. “Synthesis and Characterization of Functionalized Amino Dihydropyrimidines toward the Analysis of Their Antibacterial Structure–Activity Relationships and Mechanism of Action.” ACS Omega, vol. 7, no. 42, American Chemical Society, Oct. 2022, pp. 37907–16, https://doi.org/10.1021/acsomega.2c05071.
[44]. Thaler, C. J. “Folate Metabolism and Human Reproduction.” Geburtshilfe Und Frauenheilkunde, vol. 74, no. 9, Sept. 2014, pp. 845–51, https://doi.org/10.1055/s-0034-1383058.
[45]. Zhang, Qixiang, et al. “Recognition of Cyclic Dinucleotides and Folates by Human SLC19A1.” Nature, Oct. 2022, pp. 1–3, https://doi.org/10.1038/s41586-022-05452-z.
[46]. Wright, Nicholas J., et al. “Methotrexate Recognition by the Human Reduced Folate Carrier SLC19A1.” Nature, vol. 609, no. 7929, Sept. 2022, pp. 1056–62, https://doi.org/10.1038/s41586-022-05168-0.
[47]. Meidahl Petersen, Kasper, et al. “The Effect of Trimethoprim on Serum Folate Levels in Humans.” American Journal of Therapeutics, vol. 23, no. 2, Mar. 2016, pp. e382–87, https://doi.org/10.1097/mjt.0000000000000372.
[48]. Hernández-Díaz, Sonia, et al. “Folic Acid Antagonists during Pregnancy and the Risk of Birth Defects.” New England Journal of Medicine, vol. 343, no. 22, Nov. 2000, pp. 1608–14, https://doi.org/10.1056/nejm200011303432204.
[49]. “Trimethoprim (Trimethoprim Tablet): Uses, Dosage, Side Effects, Interactions, Warning.” RxList, 1 Mar. 2021, www.rxlist.com/trimethoprim-drug.htm.
[50]. Manna, Madhu Sudan, et al. “A Trimethoprim Derivative Impedes Antibiotic Resistance Evolution.” Nature Communications, vol. 12, no. 1, May 2021, p. 2949, https://doi.org/10.1038/s41467-021-23191-z.
[51]. PubChem. “4-((2,4-Diaminopyrimidin-5-Yl)Methyl)-2,6-Dimethoxyphenol.” Pubchem.ncbi.nlm. nih.gov, https://pubchem.ncbi.nlm.nih.gov/compound/10423570.
[52]. Wormser, Gary P., et al. “Co-Trimoxazole (Trimethoprim-Sulfamethoxazole) an Updated Review of Its Antibacterial Activity and Clinical Efficacy.” Drugs, vol. 24, no. 6, Dec. 1982, pp. 459–518, https://doi.org/10.2165/00003495-198224060-00002.
[53]. “English: Tetrahydrofolate Synthesis Pathway.” Wikimedia Commons, https://commons. wikimedia.org/wiki/File:THFsynthesispathway.png.
[54]. Knottnerus, B. J., et al. “Comparative Effectiveness of Antibiotics for Uncomplicated Urinary Tract Infections: Network Meta-Analysis of Randomized Trials.” Family Practice, vol. 29, no. 6, Apr. 2012, pp. 659–70, https://doi.org/10.1093/fampra/cms029.
[55]. Eliopoulos, G. M., and P. Huovinen. “Resistance to Trimethoprim-Sulfamethoxazole.” Clinical Infectious Diseases, vol. 32, no. 11, June 2001, pp. 1608–14, https://doi.org/10.1086/320532.









