1. Introduction
Antibiotics, according to MedlinePlus [1], are medicinal agents used to counter bacterial infections in humans and animals. This field of medicine encompasses a wide range of antimicrobial approaches. Antibiotics primarily function by disrupting specific components within cells, leading to the malfunction of cellular structures and possibly resulting in cell death [2]. This includes two mechanisms: bacteriostatic, which inhibits the growth of bacteria, and bactericidal, which kills bacteria. This review delves into antibiotics that hinder protein and DNA synthesis.
Protein synthesis is the cellular process of creating proteins, involving two main phases: Transcription and Translation [3]. On the other hand, as described by Blanco et al.[4], DNA synthesis involves the unwinding of the DNA double helix followed by the generation of a complementary DNA strand, using the parental DNA as a leading template [5]. Despite both processes using double-stranded and single-stranded DNA as initial templates [6], it’s crucial to differentiate between them, as they serve different purposes. Consequently, inhibitors targeting protein and DNA synthesis adopt significantly different working mechanisms.
Over the last century, scientists have faced the formidable challenge of developing antibiotics to combat bacterial strains responsible for various diseases. Their goal has been to optimize the safety and effectiveness of antibiotics for medical use. Through innovation and the discovery of antibacterial agents, there is promising potential to elevate healthcare standards and quality of life. By assessing and comparing all the factors, this review aims to present a precise comparison of the similarities and differences between the protein and DNA synthesis inhibitors and evaluate the effectiveness of two kinds of inhibitors against bacterial infections, along with their safety for regular medication use.
2. Properties of Antibiotics
The extent of absorption of a medication intake by the human body strongly relies on the lipophilicity and hydrophilicity of the substance. Lipophilicity is a vital factor to consider in drug discovery, as it links to pharmacokinetics and is crucial for crossing the brain and blood barrier.
The more lipophilic a drug is, the easier it is to diffuse across lipid-based membranes and, therefore can be absorbed to surrounding tissues more efficiently. Most of the hydrophilic drug compounds enter the renal system and are excreted out of the body [7]. Ultimately, antibiotics with high lipophilic properties would be favored as oral intake drugs in pharmaceutical use, while hydrophilic drugs would be best injected intravenously.
For inhibitors of the Anti-30S ribosomal unit, an overwhelming majority of antibiotics pose a hydrophilic property. An example is the aminoglycosides, a bactericidal antibiotic consisting of gentamicin, amikacin, tobramycin, neomycin, and streptomycin [8], are mostly hydrophilic, which in turn suggests that they have a high solubility in water. One aminoglycoside drug gentamicin has a solubility of 115 mg/ml at 25° C in water [9]; another antibiotic named neomycin is soluble in water with a solubility of 50 mg/ml [10]. Due to this hydrophilic behavior, most of the drug only gets transported in the extracellular fluid, leaving the body through glomerular filtration. Hence, this indicates that the use of gentamicin for elderly patients with low weight or reduced renal function could be less effective [11]. Anti-50S ribosomal unit inhibiting antibiotics present a variety: Macrolides, Chloramphenicol, and Lincosamide are all lipophilic [12-14], while Linezolid is moderately lipophilic and hydrophilic [15]. Concluding from the statements above, amongst all protein synthesis-inhibiting antibiotics, the number of lipophilic and hydrophilic drugs is approximately even. Most particularly, inhibitors of Anti-30S ribosomal unit tend to be hydrophilic, whereas most Anti-50S ribosomal unit inhibitors are lipophilic. Out of the two different mechanism pathways, Anti-50S ribosomal unit inhibitors have a greater potential to develop into effective medicines.
As for DNA Synthesis Inhibitors, there are two types of antibiotics: Fluoroquinolone and Metronidazole. Fluoroquinolone consists of three groups: hydrophilic compounds, intermediate lipophilic substances, and lipophilic compounds [16]. Nalidixic acid is one of the lipophilic antibiotics and is considered insoluble in water, with a solubility of 0.1 mg/L in water [17]. Two examples of intermediate lipophilic antibiotics would be ciprofloxacin, which has a relatively low solubility of 35mg/ml in water [18], and moxifloxacin, with a solubility of 24mg/ml in water [19]. Lastly, hydrophilic fluoroquinolones include norfloxacin and lomefloxacin [16]. Overall, the lipophilicity in drugs inhibiting DNA synthesis is a spectrum, with limited visible trends. This raises the level of difficulty in comparing DNA synthesis inhibitors with protein synthesis inhibitors.
3. Mechanisms of Action
The protein synthesis of prokaryotes undergoes four different stages: activation of amino acids, initiation, extension, and termination of peptide chain synthesis.
1) Activation of amino acids: Free amino acids are required to be activated to obtain energy to participate in protein synthesis. The activation reaction is catalyzed by aminoacyl tRNA synthase, and the final amino acid is connected to tRNA3 ˊ End AMP 3 ˊ- On OH, synthesis of aminoacyl tRNA.
2) The initiation of peptide chain synthesis: Firstly, IF1 and IF3 bind to the 30S subunit to prevent the binding of large subunits; Next, IF2 and GTP bind to small subunits to facilitate subsequent binding of initial tRNA; The small subunit complex formed is attached to mRNA through ribosome binding sites, where the starting tRNA and AUG start codon pair and release IF3, forming a 30S start complex. The large subunit combines with a 30S starting complex to replace IF1 and IF2+GDP, forming a 70S starting complex. In this way, complete ribosomes are assembled at the correct location of mRNA.
3) Extension of peptide chains: The extension is carried out in three steps
a) Carry: The complex formed by loading tRNA with EF-Tu and GTP is transported to the ribosome, where GTP is hydrolyzed and EF-TuGDP is released. Under the action of EF-Ts and GTP, EF-TuGDP can be reused.
b) Peptide transfer: Peptidyl transferase connects two adjacent amino acids to form peptide bonds, which do not require energy input.
c) Displacement: The transferase (EF-G) utilizes the energy released by GTP hydrolysis to move the ribosome along a codon of mRNA, releasing empty tRNA and transporting the new peptide chain to the P site.
4) Termination and release of peptide chains: Release factors (RF1 or RP2) recognize the termination codon and, under the action of RP3, promote peptide acyltransferase transformation, causing the ester bonds between the peptide chains carried by tRNA and tRNA to be hydrolyzed and cut off. The peptide chains are released from the ribosome and tRNA, and finally, the ribosome and mRNA are separated.
Protein biosynthesis information in bacterial DNA is used to synthesize RNA molecules called messenger RNA, a process known as transcription. Then, the macromolecular structure called ribosomes synthesizes the proteins present in mRNA, a process known as translation. The catalyzation of protein synthesis by completed by the contribution of ribosomes and cytoplasmic factors. The bacterial 70S ribosome is composed of two ribonucleoprotein subunits, namely the 30S and 50S subunits. Antibiotics inhibit protein biosynthesis by targeting the 30S or 50S subunits of bacterial ribosomes.
Tetracycline drugs, such as tetracycline, doxycycline, aureomycin, and minocycline, act on the conserved sequence of 16S r-RNA of the 30S ribosomal subunit to prevent t-RNA from binding to the A site, which is presented in Figure 1. Chloramphenicol, a 50S subunit inhibitor interacts with the conserved sequence of the peptide transferase cavity of the 23S r-RNA of the 50S subunit. Therefore, it disrupts protein synthesis by inhibiting the binding of t-RNA to the ribosomal A site.
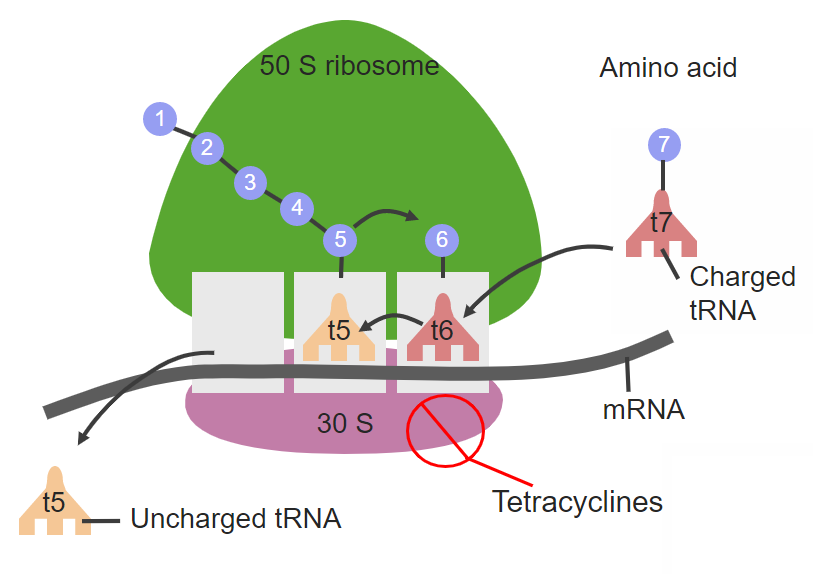
Figure 1. Tetracycline site of action on 30S ribosomal subunit.
As shown in the diagram, tetracycline binds reversely to the 30S ribosomal subunit and inhibits the active site of aminoacyl-tRNA from binding to the mRNA-ribosomal complex. This results in the death of bacteria, due to being unable to perform protein synthesis. [20]
On the other hand, Fluoroquinolones, a bactericidal DNA synthesis inhibitor antibiotic, has a mechanism of inhibiting the functions of two enzymes, one named DNA gyrase and the other being topoisomerase IV, shown in Figure 2. This prevents them from supercoiling in the cell, therefore inhibiting DNA synthesis [21]. Having fluoroquinolones that bind to enzymes would result in ligase inactivity, and reduced ability of DNA cleavage for DNA repair, leading to death in bacterial cells [22].
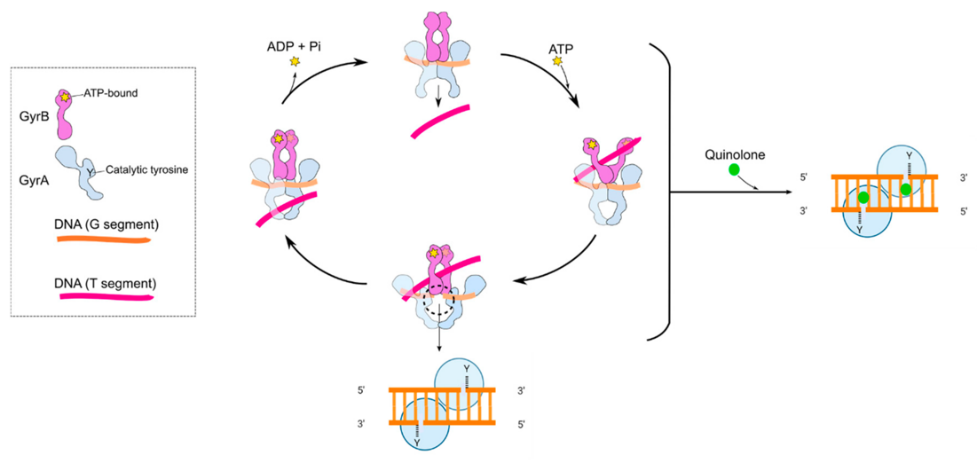
Figure 2. Mechanism of quinolones antibiotics in a cleverage complex.
Diagram shows the binding of GyrA (purple in colour, ATP-bound shown as a star) and GyrB (blue in colour, with catalytic tyrosine active site present) for DNA gyrase. [23]
Metronidazole, another DNA synthesis inhibitor, enters the body by passive diffusion [24] and produces free radicals inside bacterial cells and protists [25], which results in the damage of the DNA’s helix patterned structure [26], and death. The mechanism of metronidazole requires an anaerobic environment, as it reduces metabolic reaction in cells. After administration of the drug, the amount of metronidazole decreases in body due to the interference of intracellular electron transport proteins, which effectively keeps a high concentration gradient that helps in the transport of the drug molecules within the body. The free radicals that form in the body would react in cells, which leads to bacteria death [27].
4. Clinical Safety Profiles
Aminoglycosides are a class of antibiotics commonly used to treat bacterial infections [28]. Examples include gentamicin, amikacin, and kanamycin. These medications are effective against a wide range of bacteria but come with potential risks. Aminoglycosides can cause side effects such as kidney damage (nephrotoxicity,+1+1+1) and damage to the inner ear (ototoxicity+1+1+1). To minimize these risks, healthcare providers carefully monitor kidney function and use therapeutic (Dynamic) drug monitoring platform to ensure appropriate dosing [29].
Neomycin is an aminoglycoside antibiotic often found in topical preparations and used as a bowel preparation agent. When used topically, neomycin is generally safe. However, caution is required when using neomycin orally or systemically due to potential side effects such as kidney damage and inner ear damage. Frequent monitoring is necessary in these cases to ensure the medication is safe for intake [30].
Fluoroquinolones, including medications like ciprofloxacin and levofloxacin, are broad-spectrum antibiotics used to treat various bacterial infections. These medications are well-tolerated, but like many antibiotics, they can have side effects. Common side effects of fluoroquinolones include gastrointestinal disturbances and, less commonly, tendonitis and tendon rupture. There have also been reports of peripheral neuropathy and aortic aneurysm associated with fluoroquinolone use. As with any medication, it is important to minimize the risks and maximize the benefits of fluoroquinolone therapy with a healthcare professional.
Metronidazole is an antibiotic commonly used to treat anaerobic bacterial infections and certain parasitic infections [31]. It does not harm the body in most cases, with common side effects including gastrointestinal disturbances and a metallic taste. Some individuals may experience central nervous system effects such as dizziness or headache. While rare, prolonged use of high doses of metronidazole can lead to peripheral neuropathy or other serious adverse effects.
It is important for healthcare providers to monitor patients on metronidazole therapy and adjust the dosage of prescription as needed. Please note that the information provided is a summary, and it is important to consult healthcare professionals and refer to specific drug information for comprehensive dosage and administration guidelines.
5. Toxicity, Adverse Event Profile and Case Fatality Rate
All antibiotics demonstrate toxicity to a certain extent, some brings more severe symptoms. Toxicity traits can be shared amongst a group of antibiotics with the same inhibition pathway; for example, as two inhibitors of protein synthesis, both aminoglycoside and macrolide are ototoxic [32]. Nephrotoxicity and hepatotoxicity are also seen in antibiotics inhibiting Anti-30S ribosomal subunit. Furthermore, tetracycline may result in impaired growth in children, therefore, should be avoided for younger patients with an age of below 12 [8].
Out of all the Anti-50S ribosomal subunit inhibitors, chloramphenicol toxicity can cause aplastic anaemia, where the body function may get disrupted due to a stop in producing new blood cells [33]. If untreated, the chance of fatality is 70-percent within a year [34]. Amongst the inhibitors of DNA synthesis, all generations of fluoroquinolones include toxicity such as phototoxicity, Achilles’ tendon rupture, and impaired fracture healing. However, it is important to note that toxicity of metronidazole may have a more serious impact on patients; possible consequences are seizures and cerebellar dysfunction. On rare occasions of overdosage, metronidazole can result in a ventricular fibrillation [35]. This places this specific group of DNA synthesis inhibiting drugs as less preferable as a safe medicine.
6. Conclusion
In conclusion, protein synthesis antibiotics work by preventing the production of essential proteins in bacteria, while DNA synthesis antibiotics disrupt bacterial DNA replication. Protein synthesis antibiotics have a broader spectrum of activity, while DNA synthesis antibiotics may be more targeted. Both types of antibiotics can cause common side effects like gastrointestinal issues and allergic reactions, but DNA synthesis antibiotics may have additional side effects associated with certain agents. It is essential to consult a healthcare professional for personalized advice and to consider the benefits and risks of specific antibiotics based on individual circumstances.
References
[1]. MedlinePlus. (2022, January 14). Antibiotics. MedlinePlus; National Library of Medicine. https://medlineplus.gov/antibiotics.html
[2]. Rollins, D. M. (2000, August). BSCI 424 Pathogenic Microbiology -- Mechanisms of Antibiotic Action and Resistance. Science.umd.edu. https://science.umd.edu/classroom/bsci424/ Chemotherapy/AntibioticMechanisms.htm#:~:text=Five%20Basic%20Mechanisms%20of%20Antibiotic
[3]. Miller, C. (2020). 5.7 Protein Synthesis. Humanbiology.pressbooks.tru.ca, 5.7(5.7). https://humanbiology.pressbooks.tru.ca/chapter/5-6-protein-synthesis/#:~:text=Protein%20synthesis%20is%20the%20process
[4]. Blanco, A., & Blanco, G. (2017, January 1). Chapter 21 - The Genetic Information (I) (A. Blanco & G. Blanco, Eds.).
[5]. ScienceDirect; Academic Press. https://www.sciencedirect.com/science/article/abs/pii/ B9780128035504000215
[6]. What are the similarities between protein synthesis and DNA replication? | AAT Bioquest. (2022, June 1). Www.aatbio.com. https://www.aatbio.com/resources/faq-frequently-asked-questions/what-are-the-similarities-between-protein-synthesis-and-dna-replication
[7]. Climent, E., Benaiges, D., & Pedro-Botet, J. (2021). Hydrophilic or Lipophilic Statins? Frontiers in Cardiovascular Medicine, 8. https://doi.org/10.3389/fcvm.2021.687585
[8]. Moore, D. W., MD. (n.d.). Antibiotic Classification & Mechanism - Basic Science - Orthobullets. https://www.orthobullets.com/basic-science/9059/antibiotic-classification-and-mechanism
[9]. Gentamicin sulfate | CAS 1405-41-0. (n.d.). SCBT - Santa Cruz Biotechnology. https://www.scbt.com/p/gentamicin-sulfate-1405-41-0
[10]. Neomycin sulfate | CAS 1405-10-3. (n.d.). SCBT - Santa Cruz Biotechnology. https://www.scbt.com/p/neomycin-sulfate-1405-10-3
[11]. Hilmer, S. N., Tran, K., Rubie, P., Wright, J. D., Gnjidic, D., Mitchell, S. J., Matthews, S., & Carroll, P. R. (2011). Gentamicin pharmacokinetics in old age and frailty. British Journal of Clinical Pharmacology, 71(2), 224–231. https://doi.org/10.1111/j.1365-2125.2010.03825.x
[12]. Fohner, A. E., Sparreboom, A., Altman, R. B., & Klein, T. E. (2017). PharmGKB summary. Pharmacogenetics and Genomics, 27(4), 164–167. https://doi.org/10.1097/ fpc.0000000000000270
[13]. Kirkness, C. M., Seal, D. V., & Hay, J. (n.d.). Topical chloramphenicol: Use or abuse? nature.com. https://www.nature.com/articles/eye199597.pdf
[14]. Zhitnitskiy, P. (n.d.). Chapter 7 – Lincosamides. Pressbooks. https://open.lib.umn.edu/ swinedrugs/chapter/lincosamides/
[15]. Villa, G., Maggio, P., De Gaudio, A. R., Novelli, A., Antoniotti, R., Fiaccadori, E., & Adembri, C. (2016). Effects of continuous renal replacement therapy on linezolid pharmacokinetic/pharmacodynamics: a systematic review. Critical Care, 20(1). https://doi.org/10.1186/s13054-016-1551-7
[16]. Lipophilicity of antibacterial fluoroquinolones | DrugBank Online. (n.d.). DrugBank. https://go.drugbank.com/articles/A178843
[17]. Muñoz, C., Palacio, D. A., & Rivas, B. L. (2020). EFFECT OF SOLVENT BEHAVIOR OF NALIDIXIC ACID BY ULTRAVIOLET SPECTROSCOPY. Journal of the Chilean Chemical Society, 65(3), 4885–4887. https://doi.org/10.4067/s0717-97072020000204885
[18]. Ciprofloxacin hydrochloride - ALX-380-288 - Enzo Life Sciences. (2022, May 18). https://www.enzolifesciences.com/ALX-380-288/ciprofloxacin-.-hydrochloride/
[19]. Moxifloxacin Hydrochloride | CAS 186826-86-8. (n.d.). SCBT - Santa Cruz Biotechnology. https://www.scbt.com/p/moxifloxacin-hydrochloride-186826-86-8
[20]. Lecturio. (n.d.). https://app.lecturio.com/#/article/3133
[21]. Redgrave, L. S., Sutton, S. B., Webber, M. A., & Piddock, L. J. V. (2014, August). Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. cell.com. https://www.cell.com/trends/microbiology/pdf/S0966-842X%2814%2900089-4.pdf
[22]. Fluoroquinolones. (n.d.). Lecturio. https://app.lecturio.com/#/article/3030
[23]. Bush, N. G., Diez-Santos, I., Abbott, L., & Maxwell, A. (2020). Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules, 25(23), 5662. https://doi.org/10.3390/molecules25235662
[24]. Metronidazole: Uses, interactions, mechanism of action | DrugBank Online. (n.d.). DrugBank. https://go.drugbank.com/drugs/DB00916
[25]. Osmosis - Antibiotics - Metronidazole: Nursing Pharmacology - Osmosis Video Library. (n.d.). Osmosis. https://www.osmosis.org/learn/Antibiotics_-_Metronidazole:_Nursing_Pharmacology
[26]. Weir, C. B. (2023, June 26). Metronidazole. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK539728/#:~:text=Go%20to%3A-,Mechanism%20of%20Action,cell%20death%20in%20susceptible%20organisms.
[27]. Searle, G. D. (n.d.). FLAGYL®: (metronidazole) capsules. accessdata.fda.gov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
[28]. Frontiers in Cellular Neuroscience. (2021). Neurotoxicity of Aminoglycoside Antibiotics and Detection Strategies. https://www.frontiersin.org/articles/10.3389/fncel.2021.692762
[29]. UpToDate. (n.d.). Aminoglycosides. https://www.uptodate.com/contents/aminoglycosides
[30]. Ecological and Evolutionary Research, Society for General Microbiology. (2018). Neomycin Sulfate. https://journals.asm.org/doi/10.1128/ecosalplus.esp-0002-2018
[31]. Medscape. (n.d.). Metronidazole. https://emedicine.medscape.com/article/857679-overview
[32]. Joo, Y., Cruickshanks, K. J., Klein, B. E., Klein, R., Hong, O., & Wallhagen, M. (2018). Prevalence of ototoxic medication use among older adults in Beaver Dam, Wisconsin. Journal of the American Association of Nurse Practitioners, 30(1), 27–34. https://doi.org/10.1097/jxx.0000000000000011
[33]. Aplastic anemia - Symptoms & causes - Mayo Clinic. (2022, February 11). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/aplastic-anemia/symptoms-causes/syc-20355015
[34]. Aplastic Anemia. (n.d.). St. Jude Children’s Research Hospital. https://www.stjude.org/ disease/aplastic-anemia.html
[35]. Elgassim, M. a. M., Saied, A. S. S., Mustafa, M. A., Abdelrahman, A. M., AlJaufi, I., & Salem, W. (2022). A Rare Case of Metronidazole Overdose Causing Ventricular Fibrillation. Cureus. https://doi.org/10.7759/cureus.24728
Cite this article
Wang,X.;Liu,D.;Li,Y.;Zhang,D. (2024). Comparative analysis of antibiotics: Inhibition of protein synthesis versus DNA synthesis. Theoretical and Natural Science,44,57-63.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. MedlinePlus. (2022, January 14). Antibiotics. MedlinePlus; National Library of Medicine. https://medlineplus.gov/antibiotics.html
[2]. Rollins, D. M. (2000, August). BSCI 424 Pathogenic Microbiology -- Mechanisms of Antibiotic Action and Resistance. Science.umd.edu. https://science.umd.edu/classroom/bsci424/ Chemotherapy/AntibioticMechanisms.htm#:~:text=Five%20Basic%20Mechanisms%20of%20Antibiotic
[3]. Miller, C. (2020). 5.7 Protein Synthesis. Humanbiology.pressbooks.tru.ca, 5.7(5.7). https://humanbiology.pressbooks.tru.ca/chapter/5-6-protein-synthesis/#:~:text=Protein%20synthesis%20is%20the%20process
[4]. Blanco, A., & Blanco, G. (2017, January 1). Chapter 21 - The Genetic Information (I) (A. Blanco & G. Blanco, Eds.).
[5]. ScienceDirect; Academic Press. https://www.sciencedirect.com/science/article/abs/pii/ B9780128035504000215
[6]. What are the similarities between protein synthesis and DNA replication? | AAT Bioquest. (2022, June 1). Www.aatbio.com. https://www.aatbio.com/resources/faq-frequently-asked-questions/what-are-the-similarities-between-protein-synthesis-and-dna-replication
[7]. Climent, E., Benaiges, D., & Pedro-Botet, J. (2021). Hydrophilic or Lipophilic Statins? Frontiers in Cardiovascular Medicine, 8. https://doi.org/10.3389/fcvm.2021.687585
[8]. Moore, D. W., MD. (n.d.). Antibiotic Classification & Mechanism - Basic Science - Orthobullets. https://www.orthobullets.com/basic-science/9059/antibiotic-classification-and-mechanism
[9]. Gentamicin sulfate | CAS 1405-41-0. (n.d.). SCBT - Santa Cruz Biotechnology. https://www.scbt.com/p/gentamicin-sulfate-1405-41-0
[10]. Neomycin sulfate | CAS 1405-10-3. (n.d.). SCBT - Santa Cruz Biotechnology. https://www.scbt.com/p/neomycin-sulfate-1405-10-3
[11]. Hilmer, S. N., Tran, K., Rubie, P., Wright, J. D., Gnjidic, D., Mitchell, S. J., Matthews, S., & Carroll, P. R. (2011). Gentamicin pharmacokinetics in old age and frailty. British Journal of Clinical Pharmacology, 71(2), 224–231. https://doi.org/10.1111/j.1365-2125.2010.03825.x
[12]. Fohner, A. E., Sparreboom, A., Altman, R. B., & Klein, T. E. (2017). PharmGKB summary. Pharmacogenetics and Genomics, 27(4), 164–167. https://doi.org/10.1097/ fpc.0000000000000270
[13]. Kirkness, C. M., Seal, D. V., & Hay, J. (n.d.). Topical chloramphenicol: Use or abuse? nature.com. https://www.nature.com/articles/eye199597.pdf
[14]. Zhitnitskiy, P. (n.d.). Chapter 7 – Lincosamides. Pressbooks. https://open.lib.umn.edu/ swinedrugs/chapter/lincosamides/
[15]. Villa, G., Maggio, P., De Gaudio, A. R., Novelli, A., Antoniotti, R., Fiaccadori, E., & Adembri, C. (2016). Effects of continuous renal replacement therapy on linezolid pharmacokinetic/pharmacodynamics: a systematic review. Critical Care, 20(1). https://doi.org/10.1186/s13054-016-1551-7
[16]. Lipophilicity of antibacterial fluoroquinolones | DrugBank Online. (n.d.). DrugBank. https://go.drugbank.com/articles/A178843
[17]. Muñoz, C., Palacio, D. A., & Rivas, B. L. (2020). EFFECT OF SOLVENT BEHAVIOR OF NALIDIXIC ACID BY ULTRAVIOLET SPECTROSCOPY. Journal of the Chilean Chemical Society, 65(3), 4885–4887. https://doi.org/10.4067/s0717-97072020000204885
[18]. Ciprofloxacin hydrochloride - ALX-380-288 - Enzo Life Sciences. (2022, May 18). https://www.enzolifesciences.com/ALX-380-288/ciprofloxacin-.-hydrochloride/
[19]. Moxifloxacin Hydrochloride | CAS 186826-86-8. (n.d.). SCBT - Santa Cruz Biotechnology. https://www.scbt.com/p/moxifloxacin-hydrochloride-186826-86-8
[20]. Lecturio. (n.d.). https://app.lecturio.com/#/article/3133
[21]. Redgrave, L. S., Sutton, S. B., Webber, M. A., & Piddock, L. J. V. (2014, August). Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. cell.com. https://www.cell.com/trends/microbiology/pdf/S0966-842X%2814%2900089-4.pdf
[22]. Fluoroquinolones. (n.d.). Lecturio. https://app.lecturio.com/#/article/3030
[23]. Bush, N. G., Diez-Santos, I., Abbott, L., & Maxwell, A. (2020). Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules, 25(23), 5662. https://doi.org/10.3390/molecules25235662
[24]. Metronidazole: Uses, interactions, mechanism of action | DrugBank Online. (n.d.). DrugBank. https://go.drugbank.com/drugs/DB00916
[25]. Osmosis - Antibiotics - Metronidazole: Nursing Pharmacology - Osmosis Video Library. (n.d.). Osmosis. https://www.osmosis.org/learn/Antibiotics_-_Metronidazole:_Nursing_Pharmacology
[26]. Weir, C. B. (2023, June 26). Metronidazole. StatPearls - NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK539728/#:~:text=Go%20to%3A-,Mechanism%20of%20Action,cell%20death%20in%20susceptible%20organisms.
[27]. Searle, G. D. (n.d.). FLAGYL®: (metronidazole) capsules. accessdata.fda.gov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
[28]. Frontiers in Cellular Neuroscience. (2021). Neurotoxicity of Aminoglycoside Antibiotics and Detection Strategies. https://www.frontiersin.org/articles/10.3389/fncel.2021.692762
[29]. UpToDate. (n.d.). Aminoglycosides. https://www.uptodate.com/contents/aminoglycosides
[30]. Ecological and Evolutionary Research, Society for General Microbiology. (2018). Neomycin Sulfate. https://journals.asm.org/doi/10.1128/ecosalplus.esp-0002-2018
[31]. Medscape. (n.d.). Metronidazole. https://emedicine.medscape.com/article/857679-overview
[32]. Joo, Y., Cruickshanks, K. J., Klein, B. E., Klein, R., Hong, O., & Wallhagen, M. (2018). Prevalence of ototoxic medication use among older adults in Beaver Dam, Wisconsin. Journal of the American Association of Nurse Practitioners, 30(1), 27–34. https://doi.org/10.1097/jxx.0000000000000011
[33]. Aplastic anemia - Symptoms & causes - Mayo Clinic. (2022, February 11). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/aplastic-anemia/symptoms-causes/syc-20355015
[34]. Aplastic Anemia. (n.d.). St. Jude Children’s Research Hospital. https://www.stjude.org/ disease/aplastic-anemia.html
[35]. Elgassim, M. a. M., Saied, A. S. S., Mustafa, M. A., Abdelrahman, A. M., AlJaufi, I., & Salem, W. (2022). A Rare Case of Metronidazole Overdose Causing Ventricular Fibrillation. Cureus. https://doi.org/10.7759/cureus.24728









