1. Introduction
Since the "14th Five-Year Plan", the state has proposed to improve the quality, efficiency and competitiveness of agriculture, and thoroughly implement the action of reducing the amount of pesticides and fertilizers. General Secretary Xi Jinping emphasized at the first session of the 14th National People's Congress: "A strong agricultural country is the foundation of a strong socialist modern country, and meeting the people's needs for a better life cannot be separated from agricultural development."
Sclerotinia sclerotiorum is a devastating soil-borne plant pathogenic fungus that can cause over 60 diseases. Its host range is wide and it can infect over 600 plant species, including important economic crops such as oilseed rape, soybeans, and peanuts[1]. The plant diseases can cause huge economic losses. In the past decade, the infection area of oilseed rape sclerotinia disease in China has reached 3.1 million hectares per year, with actual yield loss exceeding 170,000 tons per year, making it the most damaging pest and disease in oilseed rape production[2]. In the United States, direct economic losses from crop diseases caused by Sclerotinia sclerotiorum exceed US $200 million annually[3]. Sclerotia has caused huge economic losses in agriculture worldwide and has become an urgent problem to be solved. At present, the pathogenic mechanism of S. sclerotiorum has not been fully elucidated, and there are few related studies on the pathogenic factors of S. sclerotiorum. Therefore, this paper reviews the pathogenic factors of S. sclerotiorum, in order to provide theoretical basis for the prevention and treatment of S. sclerotiorum.
2. Biological characteristics of Sclerotinia sclerotiorum
Sclerotinia sclerotiorum (Lib.) de Bary belongs to the phylum Ascomycota, the order Daunomycetes, and the genus Sclerotiorum. S.sclerotiorum is a necrotrophic parasitic pathogen, which mainly damages stems, leaves and fruits. S.sclerotiorum can have different symptoms in different parts of the plant, but in general it will show highly invasive pathogenic ability after invading the host epidermis, leading to rapid wilting and death of the host plant[4]. The three stages of S.sclerotiorum life are composed of ascospores, hyphae, and sclerotia. The vegetative form is white hyphae, germinated from ascospores, with septa, branching and multinucleated, which can be divided into two types: infection hyphae and branching hyphae. The outer layer of the mycelium contains melanin, and when it matures, it forms a black granular sclerotium, through which it can persist for a long time in soil or disease remnants. Under the right conditions, the sclerotium then germinates again, divided into fruiting body germination and mycelium germination. It can germinate into long pedunculated ascus disk, which is composed of colorless rod-shaped ascus and lateral silk fruiting body, ascospores in the ascus, the latter mature from the ascus, attached to the plant surface, germinate into mycelium when conditions are suitable[5].
3. Sclerotinia sclerotiorum infection model
Under humid conditions, S.sclerotiorum can grow rapidly inside the host tissue, causing necrotic, precocious, or wilting of the host tissue. After killing the host, the fungus can continue saprophytic growth on necrotic plant tissue and form numerous sclerotia in plant residues or soil. Under suitable conditions, they rapidly develop into white dense hyphal masses, and once they stop growing, they mature with dehydration and pigmentation, and the blackened outer surface is resistant to adverse conditions. Therefore, S.sclerotiorum can survive in this form for more than 10 years and can cause diseases such as sclerotinia rot, stem rot, and crown rot.
In recent years, the "two-stage" infection model (FIG. 1) was proposed[6], reflecting the complexity of the process of S.sclerotiorum infecting plants. S.sclerotiorum uses a variety of factors and complex strategies to infect and poison host plants. In the first stage, the tips of complex appsin release toxins such as oxalate and some hydrolases to digest the epidermis and penetrate the cuticle of plant cells. In the second stage, pathogens rely on toxins (especially oxalic acid) and hydrolytic enzymes to destroy and degrade the cell wall of host cells and other plant tissues, thereby achieving rapid tissue invasion while releasing nutrients to facilitate further pathogen spread[6].
In recent years, Ka Zhang[7]et al. also found that S.sclerotiorum, as a dead vegetative parasitic fungus, has a very short vegetative parasitic stage in the early stage of the establishment and colonization of infected plants, which may actually be a semi-living vegetative pathogen. During this period (before the first stage of the "two-stage" infection model), mycelial expansion does not cause host cell necrosis, and transient biotrophic behavior is maintained at the periphery of the colonization site. S.sclerotiorum can suppress the host immune response by secreting some non-host-selective toxins and effector proteins to achieve rapid proliferation and prepare for the transition to the necrotrophic parasitic stage. After the successful colonization of the pathogen, the host produces a large amount of reactive oxygen species, and the tissue gradually becomes necrotic, and the pathogen enters the vegetative stage of dead body.
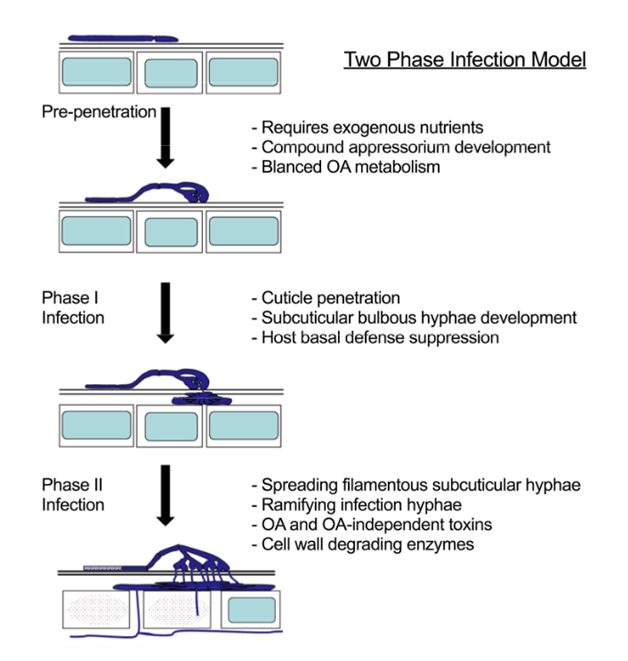
Figure 1. "Two-stage" infection model
4. Pathogenic factors and their pathogenesis
4.1. Oxalic acid
Among all the pathogenic/virulence factors of S.sclerotiorum, Oxalic acid (OA) has received the most attention and is considered as a determinant of pathogenicity[8]. OA can play different roles in multiple stages of infection. In the early stage of infection, OA can inhibit oxidative burst and callose deposition, inhibit the burst of reactive oxygen species in host plants, and contribute to the establishment of the vegetative phase of organisms[9]. Subsequently, OA induces the production of reactive oxygen species in the host, which in turn leads to host cell death.
The acidic environment induced by OA accumulation is a key step in the programmed necrosis stage of S.sclerotiorum. Oxidoreductase (OAH) catalyzes the oxidation of malonic acid to oxalic acid. The acidic environment generated by OA enhances the activity of hydrolytic enzymes, which weaken the host cell wall structure by chelating calcium ions, reduce the toxicity of calcium ion exposure, trigger cell apoptosis and programmed cell death, and allow the formation of necrotrophic fungal colonies. Additionally, it has been found that OA can manipulate the death fate of host cells, ranging from autophagy related to resistance to apoptosis related to susceptibility.
4.2. Effect proteins
Effect proteins are proteins that have the ability to regulate cell growth, differentiation, and transformation. It can transmit signals through a variety of pathways and activate different intracellular signaling pathways, thereby affecting the physiological and pathological processes of cells. S.sclerotiorum can secrete a large number of functionally diverse effector proteins. Some can suppress the immune system of the plant in the early stage of infection, and some can activate the immune response of the host plant and lead to the rapid occurrence of plant tissue necrosis. The symptom is the degradation of the cell wall of the highly active plant, which leads to rapid tissue necrosis, indicating that the initial osmotic and biotrophic periods are over, and the pathogen then expresses a different set of genes to trigger the programmed cell death of the host cell. For example, effector proteins such as the integrin SsITL and chorismate mutase SsCM1 expressed by S.sclerotiorum can help suppress plant defense responses during vegetative growth.
4.3. Hydrolases
Hydrolases are a general term for a class of enzymes that catalyze hydrolysis reactions. Current studies have found that S.sclerotiorum infection can induce the expression of a large number of enzyme genes that encode hydrolytic activity, mainly encoding carbohydrate-active enzymes (carbohydrate enzymes). Most of these genes encoding Carbohydrate enzymes are from the Glycoside hydrolases (GH) and Carbohydrate esterases (CE) families, and their expression allows pathogens to infect a wide range of plant hosts. For example, the hydrolytic subtilis proteinases, which degrade plant cell wall glycoproteins, are thought to play a role in complex apposition penetration and hyphal colonization[10].
4.4. Membrane transport proteins
Plant pathogens must contend with multiple host biochemical defense mechanisms during infection. This can be achieved by avoidance (intracellular growth of pathogens to avoid extracellular phytoalexin), resistance (mutations that alter sensitivity to antimicrobial compounds), or detoxification (modification or degradation of host phytoalexin). Energy-dependent efflux of toxic phytochemicals by membrane-associated transporters is a universal detoxification mechanism that is prevalent in pathogens with a wide host range.
A total of 33 genes encoding ATP-binding cassette (ABC) transporters and 218 Major facilitator superfamily (MFS) transporters were identified in the genome of S.sclerotiorum[11]. Extensive studies have proved that ABC transporters are important for the antitoxin ability of pathogenic bacteria to tolerate plants. The Sclerotium homolog protein encoding ABC transporter BcAtr B(SS1G_13659) is up-regulated at the early stage of infection, which reduces the secretion of mycotoxins or promotes the efflux of host phytoprotectin, thereby improving the resistance of plants to Sclerotium.
4.5. Reactive Oxygen Species
Reactive Oxygen Species (ROS) produced by plants are involved in cell proliferation and differentiation, signal transduction and ion transport[12], and are part of the defense response against pathogen attack[13]. S.sclerotiorum pathogens induce host production of large amounts of ROS, which can lead to oxidative cell damage of DNA, RNA, proteins, and lipids and trigger programmed cell death. This eventually leads to host necrosis, allowing the pathogen to absorb nutrients from the necrotic tissue. It has been shown that SsNOX1, a gene encoding NADPH oxidase in S.sclerotiorum, is expressed during infection, which promotes ROS formation and sclerotium development and is also associated with the production of oxalate, a key pathogenic factor.
5. Measures for the prevention and treatment of sclerotinia caused by Sclerotinia sclerotiorum
Sclerotinia rot caused by Sclerotinia sclerotiorum is distributed worldwide and occurs from seedling to flowering stage of crops. At present, the control approaches of S.sclerotiorum mainly include rational agricultural cultivation and management, pharmaceutical control, breeding disease-resistant varieties, and biological control based on beneficial microorganisms and fungal viruses[14].
5.1. Agricultural control
In the actual control process, agricultural cultivation measures are commonly used by people as the least harmful control method to the environment. Agricultural control measures aim to reduce the number of S.sclerotiorum in the soil or to create conditions unfavorable for the development of the disease.
The most common and popular method to control the number of soil sclerotia in agricultural cultivation measures is rotation. Rotating crops susceptible to Sclerotia and non-host crops (not susceptible to Sclerotia) can disrupt the life cycle of Sclerotia, thereby reducing the number of sclerotia entering the soil every year[15]. Kharbanda[16] et al. showed that tillage measures could reduce the ability of sclerotia to produce ascus disk by burying sclerotia deeply in the soil. Another way to reduce the viability of sclerotia in soil is to control soil water content. Studies have shown that higher soil moisture negatively affects sclerotia survival.
5.2. Chemical control
The application of chemical fungicides plays an important role in the prevention and treatment of sclerotia. Studies have shown that a variety of different types of fungicides can effectively control S.sclerotiorum, including aniline pyrimidines, benzimidazoles, bisamides, demethylating inhibitors, quinones exoinhibitors (also known as methoxyacrylate fungicides) and succinate dehydrogenase inhibitors[14].
These fungicides can exhibit different modes of action: aniline pyrimidines can inhibit the reproduction of Sclerotinia by interfering with ribosomal RNA synthesis and inhibiting fungal protein biosynthesis. Dimethylamides are thought to inhibit fungal growth by activating the two-component histidine kinase signaling pathway and inhibiting osmotic signal transduction. Benzimidazoles bind tubulin, disrupt microtubule formation and highly inhibit hyphal growth.
But long-term and frequent use of chemical agents can make plants resistant to them. In addition, long-term selective pressure may also lead to resistance in other pathogens. In order to reduce the development of drug resistance and improve the efficacy, different fungicides are usually applied alternately or in combination. Although chemical fungicides can effectively control the mycelial growth of S.sclerotiorum, they have little effect on the survival of sclerotia in the soil, and long-term use has caused great pressure on the ecological environment [14].
5.3. Biological control
5.3.1. Biocontrol microorganism
The types of biological control agents mainly include fungi, bacteria, mycoviruses, plant extracts and organic amendments, etc. The main strategies for biological control of sclerotia are to inhibit mycelium growth and sclerotium germination, reduce sclerotium formation, reduce the virality of Sclerotium and induce the resistance of host plants[14].
For the biological control of S.sclerotiorum, the most commonly used fungus is Cosp. scutellaria, which can both colonize the hyphae of S.sclerotiorum and destroy its sclerotium. When the fermentation filtrate of ZS-1TN1812 mutant was sprayed on rapeseed leaves, the infection of S.sclerotiorum was significantly inhibited, indicating that the antifungal substances (AFS) produced by ZS-1TN1812 strain could be used as potential biopesticides for the control of S.sclerotiorum in rape. Trichoderma can be heavily parasitized on the hyphae and sclerotium of S.sclerotiorum, and it can secrete cell wall degrading enzymes and destroy cell wall structure [17]. Both fungi have the ability to degrade OA, a pathogenic factor of S.sclerotiorum. Pseudomonas[18] and Bacillus are the potential biocontrol bacteria of scleroderma, which mainly inhibit the germination of Scleroderma ascospores by producing antibiotic substances or directly parasitizing them.
5.3.2. Biological control agents
Biological control agents refer to the active ingredients with bactericidal and antibacterial activities extracted from some parts of plant tissues, or biological agents that are processed to control plant diseases after separation and purification of secondary metabolites[23]. It has the advantages of low toxicity, low residue, easy degradation, high biological activity, safety against non-target biosafety and not easy to cause pathogen resistance. The active substances (Balansenate I and II) extracted from the seeds of the leguminous plant Amaranth and cuminic acid extracted from the seeds of cumin showed a good inhibitory effect on S.sclerotiorum.
Although biological control agents have many advantages, they are not enough to control the spread of S.sclerotiorum to a certain extent. Only when the number of pathogens is controlled within a certain range can they effectively inhibit the development of the disease. And in the actual promotion, its cost is high, its stability is poor, the cost benefit time is slow and other problems have not been fundamentally solved.
5.4. Breeding for genetic disease resistance
Breeding disease-resistant varieties is the most economical way to control sclerotiosis, which can reduce pesticide use and field management costs, thereby improving the economic returns of crops[14]. With the development of molecular biology, transgenic technology has become an effective way to breed disease-resistant varieties. Through genetic engineering technology, the genes related to S.sclerotiorum resistance can be found and transferred into target crops, which can improve the resistance level of crops to S.sclerotiorum. When resistance genes such as oxalate-degrading enzymes (OxDC), fungal cell wall degrading enzymes (chitinase), and antimicrobial peptides (LTP) were introduced into crops, or genes that prevent host cell apoptosis were overexpressed, the resulting transgenic lines showed significantly increased resistance to S.sclerotiorum.
However, Sclerotinia sclerotiorum has strong pathogenicity, and crop resistance to S.sclerotiorum is mainly controlled by quantitative trait genes with additive effect[24]. At present, resistant varieties are mainly obtained through the study of single gene. There is still a lack of germplasm resources with high resistance to S.sclerotiorum, because there is no germplasm resources with complete resistance to S.sclerotiorum. Therefore, breeding resistant varieties is still an important way to control this fungus.
Host-induced gene silencing (HIGS) can also be used to construct resistant cultivars. HIGS is a technology based on RNA interference, which can express artificial RNAi vectors targeting important genes of pathogens (e.g., OAH gene of S.sclerotiorum in the host. In the process of host-pathogen interaction, small interfering RNA or double-stranded RNA produced by the host is taken up by the pathogen to silence the expression of corresponding genes of the pathogen. Thus, the pathogenic ability of the pathogen is reduced, and the host can acquire resistance to the pathogen. At present, HIGS technology has been successfully used in the control of pathogens, viruses and nematodes. Recently, Xu[25] et al. have shown that RAS signaling genes can be used as host-induced gene silencing targets to control fungal diseases caused by Scleroderma.
6. Summary and Prospect
Sclerotinia sclerotiorum has a wide range of host plants. It deploys a variety of factors and complex strategies to establish and infect host plants, which can reduce the yield and quality of a variety of commercial crops. In the past decade, many genes involved in pathogen development and pathogenesis have been identified. In the two-stage infection model, pathogens may inhibit plant defense responses by producing OA, ROS, CWDEs and other pathogenic factors in complex appressorium penetration or primary invasion hyphae, resulting in host cell death and thus achieve the goal of infection. At present, the research on the pathogenic genes of S.sclerotiorum is still incomplete, and it is difficult to establish the relationship between the signaling pathways of different special biological processes. At present, the control of S.sclerotiorum mainly relies on agricultural cultivation measures and chemical fungicides, but both of these two methods have certain disadvantages. In particular, the long-term abuse of chemical fungicides has made S.sclerotiorum resistant to some commonly used fungicides, resulting in increased agricultural production costs, environmental pollution, affecting the health of humans and animals, and killing beneficial microorganisms.
More powerful methods, such as genome-wide CRISPR, should be developed in addition to traditional reverse genetics research analyses during future studies of S.sclerotiorum to enhance research on this economically important fungus. Through the application of quantitative resistance breeding and stage-specific defense phenotypic screening or genome editing technology, it is expected to obtain varieties with certain resistance and safely apply to the prevention and control of sclerotia.
References
[1]. Yan X , Kevin A , Lei T , et al.A forward genetic screen in Sclerotinia sclerotiorum revealed the transcriptional regulation of its sclerotial melanisation pathway.[J].Molecular plant-microbe interactions : MPMI, 2021, 35(3):
[2]. YANG Qingbo, LIU Wancai, HUANG Chong. Statistics and analysis of oilseed rape losses caused by main diseases and insect pests in recent 10 years[J/OL]. Plant Protection, 2018, 44(3): 24-30. DOI:10.16688/j.zwbh.2017340.
[3]. Liu Ling. The function of GATA transcription factor in the growth development and pathogenic process of Sclerotinia sclerotiorum (Lib.) de Bary[D/OL]. Jilin University, 2019[2024-05-23]. https://kns.cnki.net/kcms2/article/abstract?v=7qHjwMDcsG0e8Gd7Pyu76_YlPhRjCjK7PtB0CXr0Eaes9YnQQN6w9ZFOZOBgST9hjDGvCSjfLb2vYmvm1RkdtXaJttJUMQdksbKIKem8fHSqbnueWX9L80BU1K_exYJ6yP16oCb58lsn3CBQil3TfQ==&uniplatform=NZKPT&language=CHS.
[4]. Xia S , Xu Y , Hoy R , et al.The Notorious Soilborne Pathogenic Fungus Sclerotinia sclerotiorum: An Update on Genes Studied with Mutant Analysis[J].Pathogens, 2019, 9(1):27-27.
[5]. Zhang Xiaojuan, Zhang Yu, Hu Shengwu. Progress on Resistance Mechanism of Sclerotinia sclerotiorum and Genetic Breeding Program on Disease Resistant Rapeseed[J/OL]. Molecular Plant Breeding, 2016, 14(3): 704-711. DOI:10.13271/j.mpb.014.000704.
[6]. Xiaofei L , A J R .Mechanisms of Broad Host Range Necrotrophic Pathogenesis in Sclerotinia sclerotiorum.[J].Phytopathology, 2018, 108(10):PHYTO06180197RVW.
[7]. ZHANG Ka, LI Hao-jie, ZHANG Jin-fang, et al. Research progress on the resistance of Brassica napus to Sclerotinia sclerotiorum [J/OL]. Chinese Journal of Oil Crop Sciences, 2023, 45(6): 1095-1102. DOI:10.19802/j.issn.1007-9084.2023212.
[8]. Liangsheng X , Meichun X , David W , et al.pH dependency of sclerotial development and pathogenicity revealed by using genetically defined oxalate-minus mutants of Sclerotinia sclerotiorum.[J].Environmental microbiology, 2015, 17(8):2896-909.
[9]. Maginnis S M .Virus–Receptor Interactions: The Key to Cellular Invasion[J].Journal of Molecular Biology, 2018, 430(17):2590-2611.
[10]. Olivieri F , Zanetti E M , Oliva R C , et al.Characterization of an Extracellular Serine Protease of Emphasis Type="Italic"Fusarium eumartii/Emphasis and its Action on Pathogenesis Related Proteins[J].European Journal of Plant Pathology, 2002, 108(1):63-72.
[11]. Keisuke H , Henk-Jan S , A M W D .Bcmfs1, a novel major facilitator superfamily transporter from Botrytis cinerea, provides tolerance towards the natural toxic compounds camptothecin and cercosporin and towards fungicides.[J].Applied and environmental microbiology, 2002, 68(10):4996-5004.
[12]. Julia F , Vadim D , F H J B , et al.Reactive oxygen species produced by NADPH oxidase regulate plant cell growth.[J].Nature, 2003, 422(6930):442-6.
[13]. BAKER C, ORLANDI E. Active Oxygen in Plant Pathogenesis[J/OL]. ANNUAL REVIEW OF PHYTOPATHOLOGY, 1995, 33: 299-321. DOI:10.1146/annurev.py.33.090195.001503.
[14]. LI Enchen, XI Zheng, ZHU Na, et al. Research Advances on Biocontrol of Sclerotinia Rot caused by Sclerotinia sclerotiorum[J]. Journal of Yunnan Agricultural University, 2023, 38(4): 714-724.
[15]. Derbyshire C M , Denton‐Giles M .The control of sclerotinia stem rot on oilseed rape ( Brassica napus ): current practices and future opportunities[J].Plant Pathology, 2016, 65(6):859-877.
[16]. Kharbanda P , Tewari J .Integrated management of canola diseases using cultural methods[J].Canadian Journal of Plant Pathology, 2009, 18(2):168-175.
[17]. VINALE F, SIVASITHAMPARAM K, GHISALBERTI E L, et al. Trichoderma-plant-pathogen interactions[J/OL]. SOIL BIOLOGY & BIOCHEMISTRY, 2008, 40(1): 1-10. DOI:10.1016/j.soilbio.2007.07.002.
[18]. Aeron A , Dubey C R , Maheshwari K D , et al.Multifarious activity of bioformulated Pseudomonas fluorescens PS1 and biocontrol of Sclerotinia sclerotiorum in Indian rapeseed (Brassica campestris L.)[J].European Journal of Plant Pathology, 2011, 131(1):81-93.
[19]. Xiaojia H , P D R , E J M , et al.Formulations of the endophytic bacterium Bacillus subtilis Tu-100 suppress Sclerotinia sclerotiorum on oilseed rape and improve plant vigor in field trials conducted at separate locations.[J].Canadian journal of microbiology, 2011, 57(7):539-46.
[20]. Hu X , Roberts P D , Xie L , et al.Formulations of Bacillus subtilis BY-2 suppress Sclerotinia sclerotiorum on oilseed rape in the field[J].Biological Control, 2014, 7054-64.
[21]. Chen Z , Gao T , Liang S , et al.Molecular mechanism of resistance of Fusarium fujikuroi to benzimidazole fungicides[J].FEMS Microbiology Letters, 2014, 357(1):77-84.
[22]. Gao , Han , Chen , et al.Biological control of oilseed rape Sclerotinia stem rot by Bacillus subtilis strain Em7[J].Biocontrol Science and Technology, 2014, 24(1):39-52.
[23]. TAN Haijun. Review and prospect of biological pesticides in China [J/OL]. World Pesticide, 2022, 44(4): 16-27+54. DOI:10.16201/j.cnki.cn10-1660/tq.2022.04.03.
[24]. Wu J , Cai G , Tu J , et al.Identification of QTLs for resistance to sclerotinia stem rot and BnaC.IGMT5.a as a candidate gene of the major resistant QTL SRC6 in Brassica napus.[J].PLoS ONE, 2017, 8(7):e67740.
[25]. YAN XU, TAN J, LU J, et al. RAS signalling genes can be used as host‐induced gene silencing targets to control fungal diseases caused by Sclerotinia sclerotiorum and Botrytis cinerea[J/OL]. Plant Biotechnology Journal, 2024, 22(1): 262-277. DOI:10.1111/pbi.14184.
Cite this article
Wang,Y. (2024). Progress in the study of pathogenic factors of Sclerotinia sclerotiorum. Theoretical and Natural Science,59,137-144.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yan X , Kevin A , Lei T , et al.A forward genetic screen in Sclerotinia sclerotiorum revealed the transcriptional regulation of its sclerotial melanisation pathway.[J].Molecular plant-microbe interactions : MPMI, 2021, 35(3):
[2]. YANG Qingbo, LIU Wancai, HUANG Chong. Statistics and analysis of oilseed rape losses caused by main diseases and insect pests in recent 10 years[J/OL]. Plant Protection, 2018, 44(3): 24-30. DOI:10.16688/j.zwbh.2017340.
[3]. Liu Ling. The function of GATA transcription factor in the growth development and pathogenic process of Sclerotinia sclerotiorum (Lib.) de Bary[D/OL]. Jilin University, 2019[2024-05-23]. https://kns.cnki.net/kcms2/article/abstract?v=7qHjwMDcsG0e8Gd7Pyu76_YlPhRjCjK7PtB0CXr0Eaes9YnQQN6w9ZFOZOBgST9hjDGvCSjfLb2vYmvm1RkdtXaJttJUMQdksbKIKem8fHSqbnueWX9L80BU1K_exYJ6yP16oCb58lsn3CBQil3TfQ==&uniplatform=NZKPT&language=CHS.
[4]. Xia S , Xu Y , Hoy R , et al.The Notorious Soilborne Pathogenic Fungus Sclerotinia sclerotiorum: An Update on Genes Studied with Mutant Analysis[J].Pathogens, 2019, 9(1):27-27.
[5]. Zhang Xiaojuan, Zhang Yu, Hu Shengwu. Progress on Resistance Mechanism of Sclerotinia sclerotiorum and Genetic Breeding Program on Disease Resistant Rapeseed[J/OL]. Molecular Plant Breeding, 2016, 14(3): 704-711. DOI:10.13271/j.mpb.014.000704.
[6]. Xiaofei L , A J R .Mechanisms of Broad Host Range Necrotrophic Pathogenesis in Sclerotinia sclerotiorum.[J].Phytopathology, 2018, 108(10):PHYTO06180197RVW.
[7]. ZHANG Ka, LI Hao-jie, ZHANG Jin-fang, et al. Research progress on the resistance of Brassica napus to Sclerotinia sclerotiorum [J/OL]. Chinese Journal of Oil Crop Sciences, 2023, 45(6): 1095-1102. DOI:10.19802/j.issn.1007-9084.2023212.
[8]. Liangsheng X , Meichun X , David W , et al.pH dependency of sclerotial development and pathogenicity revealed by using genetically defined oxalate-minus mutants of Sclerotinia sclerotiorum.[J].Environmental microbiology, 2015, 17(8):2896-909.
[9]. Maginnis S M .Virus–Receptor Interactions: The Key to Cellular Invasion[J].Journal of Molecular Biology, 2018, 430(17):2590-2611.
[10]. Olivieri F , Zanetti E M , Oliva R C , et al.Characterization of an Extracellular Serine Protease of Emphasis Type="Italic"Fusarium eumartii/Emphasis and its Action on Pathogenesis Related Proteins[J].European Journal of Plant Pathology, 2002, 108(1):63-72.
[11]. Keisuke H , Henk-Jan S , A M W D .Bcmfs1, a novel major facilitator superfamily transporter from Botrytis cinerea, provides tolerance towards the natural toxic compounds camptothecin and cercosporin and towards fungicides.[J].Applied and environmental microbiology, 2002, 68(10):4996-5004.
[12]. Julia F , Vadim D , F H J B , et al.Reactive oxygen species produced by NADPH oxidase regulate plant cell growth.[J].Nature, 2003, 422(6930):442-6.
[13]. BAKER C, ORLANDI E. Active Oxygen in Plant Pathogenesis[J/OL]. ANNUAL REVIEW OF PHYTOPATHOLOGY, 1995, 33: 299-321. DOI:10.1146/annurev.py.33.090195.001503.
[14]. LI Enchen, XI Zheng, ZHU Na, et al. Research Advances on Biocontrol of Sclerotinia Rot caused by Sclerotinia sclerotiorum[J]. Journal of Yunnan Agricultural University, 2023, 38(4): 714-724.
[15]. Derbyshire C M , Denton‐Giles M .The control of sclerotinia stem rot on oilseed rape ( Brassica napus ): current practices and future opportunities[J].Plant Pathology, 2016, 65(6):859-877.
[16]. Kharbanda P , Tewari J .Integrated management of canola diseases using cultural methods[J].Canadian Journal of Plant Pathology, 2009, 18(2):168-175.
[17]. VINALE F, SIVASITHAMPARAM K, GHISALBERTI E L, et al. Trichoderma-plant-pathogen interactions[J/OL]. SOIL BIOLOGY & BIOCHEMISTRY, 2008, 40(1): 1-10. DOI:10.1016/j.soilbio.2007.07.002.
[18]. Aeron A , Dubey C R , Maheshwari K D , et al.Multifarious activity of bioformulated Pseudomonas fluorescens PS1 and biocontrol of Sclerotinia sclerotiorum in Indian rapeseed (Brassica campestris L.)[J].European Journal of Plant Pathology, 2011, 131(1):81-93.
[19]. Xiaojia H , P D R , E J M , et al.Formulations of the endophytic bacterium Bacillus subtilis Tu-100 suppress Sclerotinia sclerotiorum on oilseed rape and improve plant vigor in field trials conducted at separate locations.[J].Canadian journal of microbiology, 2011, 57(7):539-46.
[20]. Hu X , Roberts P D , Xie L , et al.Formulations of Bacillus subtilis BY-2 suppress Sclerotinia sclerotiorum on oilseed rape in the field[J].Biological Control, 2014, 7054-64.
[21]. Chen Z , Gao T , Liang S , et al.Molecular mechanism of resistance of Fusarium fujikuroi to benzimidazole fungicides[J].FEMS Microbiology Letters, 2014, 357(1):77-84.
[22]. Gao , Han , Chen , et al.Biological control of oilseed rape Sclerotinia stem rot by Bacillus subtilis strain Em7[J].Biocontrol Science and Technology, 2014, 24(1):39-52.
[23]. TAN Haijun. Review and prospect of biological pesticides in China [J/OL]. World Pesticide, 2022, 44(4): 16-27+54. DOI:10.16201/j.cnki.cn10-1660/tq.2022.04.03.
[24]. Wu J , Cai G , Tu J , et al.Identification of QTLs for resistance to sclerotinia stem rot and BnaC.IGMT5.a as a candidate gene of the major resistant QTL SRC6 in Brassica napus.[J].PLoS ONE, 2017, 8(7):e67740.
[25]. YAN XU, TAN J, LU J, et al. RAS signalling genes can be used as host‐induced gene silencing targets to control fungal diseases caused by Sclerotinia sclerotiorum and Botrytis cinerea[J/OL]. Plant Biotechnology Journal, 2024, 22(1): 262-277. DOI:10.1111/pbi.14184.









