1. Introduction
Thyroid cancer is the ninth most frequent cancer globally, according to the GLOBOCAN 2020 cancer incidence and mortality database kept by the International Agency for Research on Cancer of the World Health Organization [1]. It is a malignant tumor from the thyroid gland, which is a crucial endocrine organ that produces hormones that regulate growth, development, and metabolism. The most common types of thyroid cancer are divided into three histological types: differentiated thyroid cancer, including papillary carcinoma and follicular carcinoma; medullary thyroid carcinoma; as well as Anaplastic thyroid cancer (ATC) [1]. ATC is known for being highly aggressive and lethal, and most patients survive less than one year. Notably, approximately 45% of ATC patients carry the BRAF V600E mutation, a genetic variant that has a significant impact on the development of different types of cancer, especially thyroid cancer [1].
The presence of BRAF V600E mutation can result in the activation of BRAF kinase within the mitogen-activated protein kinase (MAPK) cell signaling pathway, leading to enhanced cell proliferation and survival, ultimately causing uncontrolled cell growth and contributing to the development of cancer in various tumors [2]. However, this mutation has become a therapeutic target for many cancers, and targeting BRAF through selective inhibitors is one of the successful strategies in treating tumors. BRAF inhibitors like trametinib, vemurafenib, and dabrafenib have demonstrated effectiveness in treating BRAF V600E mutant tumors [2]. Dabrafenib has been widely studied and used in clinical trials, showing significant therapeutic potential.
Dabrafenib, a selective inhibitor of BRAF V600E, was selected due to it has shown great potential in the treatment of various cancers [3]. Its use in thyroid cancer, especially in patients with BRAF V600E mutations, has achieved good results. Clinical trials have explored the use of dabrafenib as a monotherapy and in conjunction with MEK inhibitors such as trametinib to improve its therapeutic effect and overcome resistance mechanisms [3]. Clinical trials have shown that dabrafenib can effectively inhibit tumor growth and induce apoptosis [3].
This review summarizes dabrafenib's current importance in treating thyroid cancer, focusing on its mechanism of action, clinical efficacy, and safety. The research will focus on dabrafenib potential future applications and its use in clinical practice, as well as any possible advantages of combining it with other treatments. This article emphasizes the significance of dabrafenib for patients with BRAF V600E mutations, emphasizing its potential as a promising treatment in the treatment of thyroid cancer.
2. BRAF in thyroid cancer
2.1. BRAF gene
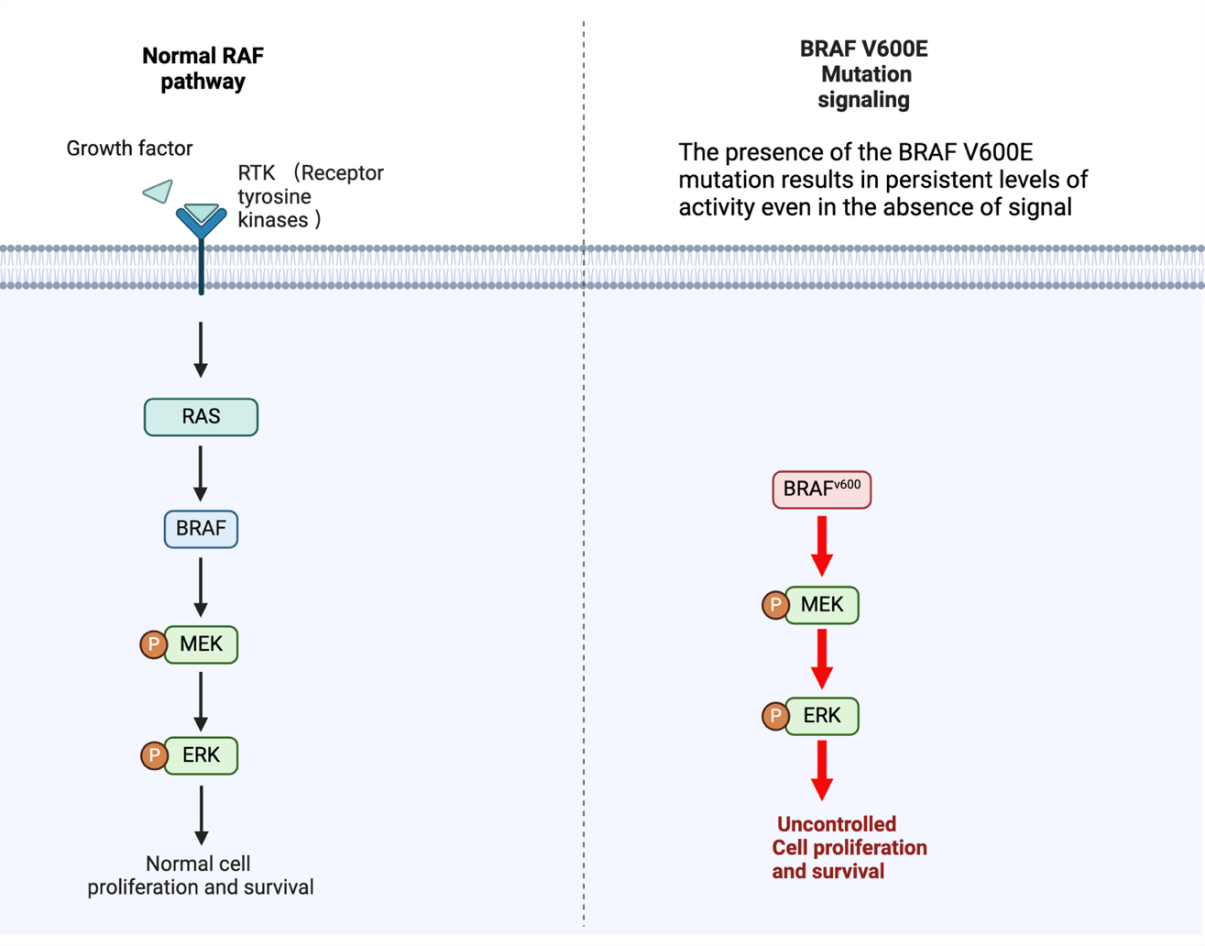
The RAS/MAPK signaling pathway depends on the serine/threonine protein kinase B-Raf, which is encoded by the BRAF gene (Figure 1). RAS/MAPK signaling transmits signals from cell surface receptors to the cell nucleus and then passes through multiple phosphorylation and dephosphorylation steps to promote cell proliferation and survival. It is essential for regulating cell growth, differentiation and survival [4]. The most common mutation within the BRAF gene is called BRAF V600E, and it results from glutamic acid being substituted by valine. It causes the B-Raf protein to have persistent activity. This mutant protein continuously activates the RAS/MAPK pathway, regardless of external signals, to form RAF dimers, resulting in uncontrolled cell proliferation and survival [4]. This abnormal signaling is a key driver of the pathogenesis of multiple cancers, including melanoma, colorectal cancer and thyroid cancer. As recent studies have elucidated, the continuous activation of this pathway due to BRAF mutation promotes tumor growth and resistance to apoptosis, making it a key target for cancer treatment. By selectively preventing this abnormal signaling by reducing RAF dimers, targeted treatment with BRAF inhibitors has demonstrated a substantial therapeutic effect [4], thereby reducing tumor growth and improving patient outcomes.
2.2. BRAF V600E mutation in thyroid cancer
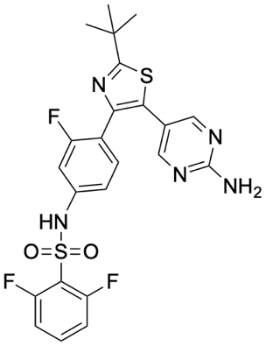
The BRAF V600E mutation plays an important role in thyroid cancer, especially in ATC. The presence of the BRAF V600E mutation results in sustained levels of activity even in the absence of a signal. This mutation causes unrestricted activation of the MAPK signaling pathway, which causes uncontrolled cell proliferation, survival, and, ultimately, tumor formation [2]. Once the MAPK pathway is activated, it enhances cancer-promoting activities, such as increased cell growth and enhanced ability to spread to other areas. BRAF inhibitors (such as dabrafenib, etc.) specifically target this mutation and can effectively block abnormal signaling pathways [2]. Figure 2 shows the structure of dabrafenib. By inhibiting BRAF, tumor growth and metastasis can be significantly reduced, making it an effective drug for the treatment of thyroid cancer. In various studies, the ability of dabrafenib and other BRAF inhibitors to be used in combination with other drugs has been explored to significantly enhance the therapeutic effect on thyroid cancer.
| |
Figure 1. BRAF Gene Mechanism and Mutation Pathway. In response to growth factors, RTKs are activated, which subsequently activate RAS. RAS then activates BRAF, which in turn phosphorylates MEK. Activated MEK phosphorylates ERK, leading to normal cell proliferation and survival. However, the constitutive activation of BRAF caused by the BRAF V600E mutation phosphorylates MEK and ERK continuously even in the absence of external growth signals. External factors in unregulated signaling allow cells to proliferate and survive uncontrolled. RAS, Rat Sarcoma; RAF, Rapidly Accelerated Fibrosarcoma; MEK, Mitogen-Activated Protein Kinase Kinase; and ERK, Extracellular Signal-Regulated Kinase. Figure credit: original. Figure made from Biorender.com. | |
| |
Figure 2. Structure of Dabrafenib. | |
3. Application of dabrafenib on thyroid cancer
3.1. Therapeutic effects of dabrafenib
Studies have shown that dabrafenib needs to be used in combination with other drugs to have a better effect in the treatment of ATC. Kimura et al. used a mouse model to study the effect of combined inhibition of BRAF and MEK on the activity of cancer stem cells (CSCs) in ATC. The study demonstrated that inhibition of the MEK pathway combined with dabrafenib can significantly reduce the number of cancer stem cells in ATC, which are often associated with tumorigenicity, cancer metastasis and drug resistance [5]. The team implanted human tumor cells into immunocompromised mice. Tumor volume was measured over time, and the CSC population was quantified using flow cytometry to determine the results [5]. Dabrafenib alone has little effect on inhibiting the activity of CSCs, but combined treatment ultimately leads to a significant reduction in tumor volume, indicating that simultaneous targeting of the BRAF and MEK pathways may improve the therapeutic effect, but CSCs cannot be eliminated, which is also the reason for the recurrence of ATC [5]. This study emphasized that drug combinations have a significant effect on cancer stem cells and emphasizes the importance of preventing recurrence and improving the treatment effect of ATC patients.
3.2. Combination therapy of dabrafenib and other treatments
Studies have demonstrated the synergistic effect of dabrafenib and other drugs on the AKT/hTERT pathway (Figure 3). The AKT/hTERT pathway is essential for the development of thyroid cancer, especially for telomerase activation, which maintains cell immortality [6]. However, thyroid cancer cells have developed resistance to dabrafenib and reduced its therapeutic effect. So, another study by Liao et al. emphasized the role of dabrafenib combined with melatonin in ATC cells and found that the combination significantly inhibited the AKT/hTERT signaling pathway and expression [6]. The method involved treating ATC cell lines with different concentrations of melatonin and dabrafenib in combination. The effects on cell viability, apoptosis, and AKT/hTERT signaling were assessed using Western blotting, flow cytometry, and quantitative PCR [6]. The results of this study indicate that melatonin can increase dabrafenib inhibitory effect on cell migration, invasion, and proliferation as well as support dabrafenib-induced apoptosis and cell cycle arrest in ATC cells [6]. Inhibition of AKT signaling leads to decreased expression of the telomerase catalytic subunit hTERT, which in turn leads to decreased telomerase activity, limiting the ability of cancer cells to evade apoptosis, thereby promoting cell death and reducing proliferation. The results of this study suggest that melatonin can enhance the efficacy of dabrafenib, providing a potential strategy for overcoming drug resistance and improving therapeutic efficacy.
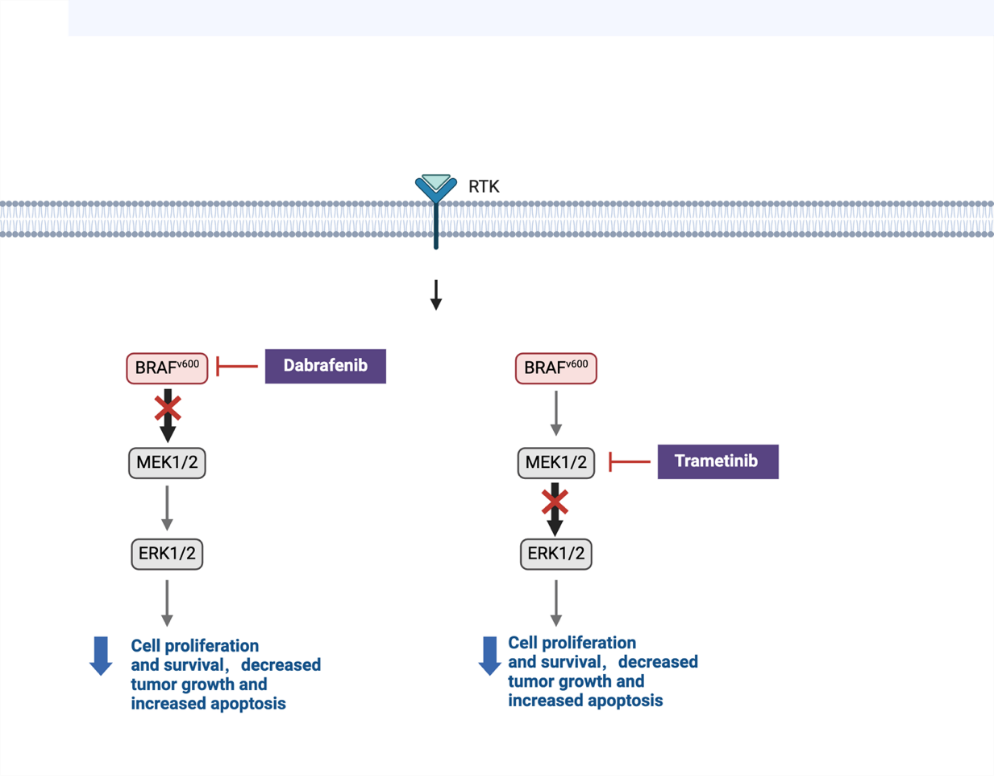
Figure 3. Inhibition of the BRAF V600E mutation pathway by dabrafenib and Trametinib. Dabrafenib inhibits the BRAF V600E mutation, resulting in reduced MEK1/2 and ERK1/2 activity, which in turn reduces cell proliferation and survival, ultimately leading to reduced tumor growth and increased apoptosis. Trametinib inhibits MEK1/2 downstream of the BRAF V600E mutation, resulting in reduced ERK1/2 activity, reduced cell proliferation and survival, reduced tumor growth, and increased apoptosis. Both inhibitors can effectively disrupt the MAPK signaling pathway, thus serving as drugs to improve tumors. RAS, Rat Sarcoma; RAF, Rapidly Accelerated Fibrosarcoma; MEK, Mitogen-Activated Protein Kinase Kinase; and ERK, Extracellular Signal-Regulated Kinase. Figure credit: original. Figure made from Biorender.com.
The effects of the MEK inhibitor trametinib and the BRAF inhibitor dabrafenib on the growth arrest and viability of four ATC cell lines (ACT-1, OCUT-2, OCUT-4, and OCUT-6) were examined in recent research. The researchers treated the cell lines with different concentrations of dabrafenib and trametinib [7]. Cell viability was assessed using the MTT assay, and cell cycle analysis was performed by flow cytometry to determine the effects on the G0/G1 phase. The phosphorylation levels of MEK and ERK were also assessed using Western blotting [7]. The study found that dabrafenib was most effective in OCUT-2 and OCUT-4 cell lines carrying the BRAF V600E mutation. It can significantly inhibit the viability of BRAF mutant cells by downregulating MEK/ERK phosphorylation, leading to G0/G1 cell cycle arrest [7]. Trametinib reduced cell viability in all cell lines by downregulating ERK phosphorylation, but its response to OCUT-4 in the ATC cell line was very weak, and drug resistance may exist [7]. Overall, thyroid cancer can be effectively treated with dabrafenib and trametinib combined (Table 1).
Table 1. Dabrafenib target in thyroid cancer signaling
Reported Target | Function/ Pathway of target | Effect | Ref |
BRAF V600E | This results in the MAPK pathway being continuously activated, which in turn causes uncontrolled proliferation of cells and survival, which in turn leads to the development of tumors. | Inhibitor | [1-4] |
MEK | A member of the MAPK signaling cascade and a downstream kinase of BRAF | Inhibitor | [3,5,6] |
ERK | Extracellular signal-regulated kinases are involved in the MAPK pathway and promote cell proliferation and survival | Inhibitor | [6,7] |
Cancer Stem Cells (CSCs) | Cells with strong self-renewal ability led to tumor formation | Reduction | [5] |
AKT/hTERT Pathway | Pathway involve maintain chromosome stability, and lead to cell immortalization and tumor formation. | Inhibitor | [6] |
ACT-1, OCUT-2, OCUT-4, and OCUT-6 | BRAF V600E mutant ATC cell line | Reduce cell viability and induce cell apoptosis | [7] |
These in vitro and in vivo investigations obvious demonstrate the benefits of dabrafenib and its efficacy in treating thyroid cancer, especially ATC. It can improve and treat ATC by inhibiting signal pathways, reducing cell viability and the ability to avoid death, and targeting cancer stem cells in thyroid cancer. However, the effect of single dabrafenib is not very prominent. Thus, application of dabrafenib in combination with other drugs may achieve better results.
4. Clinical trials of dabrafenib
Clinical trials have investigated the efficacy of dabrafenib alone and in combination with other drugs in the treatment of BRAF V600E mutant thyroid cancer (Table 2). It plays an important role in evaluating the effectiveness of new treatments for thyroid cancer. Rothenberg et al. conducted a phase II clinical trial to investigate whether dabrafenib can stimulate BRAF V600E mutant metastatic papillary thyroid cancer (PTC). 10 patients with iodine-resistant, BRAF V600E mutant metastatic PTC were enrolled in the research [8]. Patients received 150 mg of dabrafenib orally twice a day for a duration of 25 days. Results showed that 6 of the 10 patients experienced significant responses after receiving dabrafenib treatment. Dabrafenib effectively induced tumor redifferentiation, allowing previously resistant tumors to be treated with radioactive iodine [8]. These findings suggest that dabrafenib can restore iodine sensitivity in some patients and provide a prospective therapeutic approach for PTC therapy.
Another important trial was analyzed from the Phase II ROAR (Rare Tumor Agnostic Research) (NCT02034110) basket research, a multicenter, open-label trial. In this study, patients with BRAF V600E mutant anaplastic thyroid carcinoma (ATC) were examined for the safety and effectiveness of dabrafenib and trametinib combination treatment. [3]. 36 patients with BRAF V600E mutant ATC were involved in the research and received treatment with a combination of trametinib (2 mg once day of the week) and dabrafenib (150 mg twice daily). The primary outcome was that the combination therapy showed a significant overall response rate (ORR) of 56%, meaning that a significant portion of patients achieved a partial or complete response [3]. At the same time, the duration of response (DOR) rate was 50%, and there was also an improvement in progression-free survival (PFS) and overall survival (OS) [3]. This indicates that BRAF V600E mutant ATC could be successfully treated with dabrafenib and trametinib, providing a promising novel therapy option for this type of cancer.
Table 2. Studies and clinical trials involving dabrafenib in thyroid cancer
Authors | Year | Design | Subjects | Treatment | Results | |
Kimura et al. | 2024 | Mouth model | Mouse model with human tumor cells | Dabrafenib + MEK inhibitor | reduction in tumour volume, and when combine two drugs can reduce CSC | [5] |
Liao et al. | 2020 | In vitro study | ATC cell lines | Dabrafenib + melatonin | inhibited the AKT/hTERT pathway, increased apoptosis, reduced cell proliferation, promoting cell death | [6] |
Kurata K et al. | 2016 | In vitro study | four ATC cell lines (ACT-1, OCUT-2, OCUT-4, and OCUT-6) | Dabrafenib + trametinib | Dabrafenib is effective in OCUT-2 and OCUT-4, downregulating MEK/ERK phosphorylation, inhibiting BRAF mutant cell viability, and G0/G1 cell cycle arrest | [7] |
Rothenberg SM et al. | 2015 | Phase II, open label | 10 people with BRAF V600E mutant metastatic PTC that is iodine-resistant | 150 mg twice day for 25 days of dabrafenib | 6 of 10 patients showed tumor response and restored iodine sensitivity | [8] |
Subbiah V et al. | 2022 | Phase II, ROAR basket study | 36 patients with BRAF V600E mutant anaplastic thyroid cancer (ATC) | Trametinib 2 mg once daily + dabrafenib 150 mg twice a day | The overall response rate (ORR) was 56%, the duration of response (DOR) rate was 50%, and there was significant improvement in progression-free survival and overall survival. | [3] |
In general, both clinical trials have given positive comments on dabrafenib. In these two clinical trials, dabrafenib has shown strong potential and significant improvement in thyroid cancer, both as a single therapy or in combination with other drugs. Further clinical trials are required to verify the effects of dabrafenib, prevent the side effects, and contribute to precise medication in thyroid cancer patients.
5. Conclusion
Dabrafenib has shown great potential in the treatment of BRAF V600E mutant thyroid cancer, especially ATC. BRAF V600E mutation leads to persistent activation of the MAPK pathway, which promotes the malignant growth of tumors. Dabrafenib, as a selective BRAF inhibitor, can effectively disrupt this pathway, reduce cell proliferation, and induce apoptosis. Studies have shown that dabrafenib, especially when used in combination with MEK inhibitors such as trametinib, can effectively inhibit tumor growth and target CSCs associated with tumor recurrence and resistance. Clinical trials such as the ROAR study reported high response rates and prolonged progression-free survival in patients treated with the combination of dabrafenib and trametinib, indicating its effectiveness in treating malignant thyroid cancer. The use of dabrafenib, especially in combination with other therapeutic agents, has advanced the treatment prospects of thyroid cancer. By specifically targeting the BRAF V600E mutation, dabrafenib provides a precision medicine approach that can improve patient outcomes and reduce tumor recurrence, indicating important implications for the development of targeted cancer therapies. Future studies should focus on optimizing dabrafenib combination therapies to improve its efficacy and reduce resistance. In addition, further clinical trials are needed to validate these findings in larger patient populations and explore the benefits of combining dabrafenib with new drugs from other pathways.
References
[1]. Chen DW, Lang BHH, McLeod DSA, Newbold K and Haymart MR 2023 Lancet. 401 1531-1544
[2]. Gouda MA and Subbiah V P 2023 ESMO Open. 8 100788
[3]. Subbiah V, et al. 2022 Ann Oncol. 33 406-415
[4]. Poulikakos PI, Sullivan RJ and Yaeger R. 2022 Clin. Cancer Res. 28 4618-4628.
[5]. Kimura T, Doolittle WKL, Kruhlak M, Zhao L, Hwang E, Zhu X, Tang B, Wolcott KM and Cheng SY. 2024 Thyroid. 34 484-495.
[6]. Liao Y, Gao Y, Chang A, Li Z, Wang H, Cao J, Gu W and Tang R. 2020 J Cell. Mol. Med. 24 12119-12130.
[7]. Kurata K, Onoda N, Noda S, Kashiwagi S, Asano Y, Hirakawa K and Ohira M. 2016 Int J Oncol. 49 2303-2308.
[8]. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH and Wirth LJ. 2015 Clin. Cancer Res. 21 1028-35.
Cite this article
Wen,B. (2024). Therapeutic role of dabrafenib on thyroid cancer. Theoretical and Natural Science,62,12-18.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Chen DW, Lang BHH, McLeod DSA, Newbold K and Haymart MR 2023 Lancet. 401 1531-1544
[2]. Gouda MA and Subbiah V P 2023 ESMO Open. 8 100788
[3]. Subbiah V, et al. 2022 Ann Oncol. 33 406-415
[4]. Poulikakos PI, Sullivan RJ and Yaeger R. 2022 Clin. Cancer Res. 28 4618-4628.
[5]. Kimura T, Doolittle WKL, Kruhlak M, Zhao L, Hwang E, Zhu X, Tang B, Wolcott KM and Cheng SY. 2024 Thyroid. 34 484-495.
[6]. Liao Y, Gao Y, Chang A, Li Z, Wang H, Cao J, Gu W and Tang R. 2020 J Cell. Mol. Med. 24 12119-12130.
[7]. Kurata K, Onoda N, Noda S, Kashiwagi S, Asano Y, Hirakawa K and Ohira M. 2016 Int J Oncol. 49 2303-2308.
[8]. Rothenberg SM, McFadden DG, Palmer EL, Daniels GH and Wirth LJ. 2015 Clin. Cancer Res. 21 1028-35.