1. Introduction
Esophageal squamous cell carcinoma (ESCC) is a prevalent form of malignant tumor found in the gastrointestinal tract, originating from the epithelial cells in the mucous membranes of the esophagus. ESCC tends to occur in the lower part of the oesophagus, which mainly includes the abdominal esophagus and the gastro-esophageal junction, with invasion of surrounding tissues and organs occurring during progression. Early symptoms of ESCC are not obvious and are similar to those of acute upper respiratory tract infections caused by viruses or bacteria, and are therefore not easily noticed by patients, resulting in the vast majority of patients being in the middle to late stages of the infection when the disease is diagnosed. Hundreds of thousands of cases of ESCC are diagnosed worldwide every year, and the disease develops so rapidly that it is often detected only when the condition is already quite serious, which makes squamous cell carcinoma of the esophagus not easy to be cured. The early identification, diagnosis, and treatment of ESCC play a crucial role in minimizing morbidity and mortality rates while enhancing the quality of life for patients. The dangers of ESCC are mainly manifested in the following aspects. ESCC is characterized by a high incidence rate and a rapidly progressing nature. In the initial stages, the condition often presents without any noticeable symptoms. However, some patients may experience a sensation of a foreign body in the esophagus, difficulty swallowing hard or coarse food, and a feeling of obstruction or stagnation in the throat. They may report discomfort behind the sternum, a burning sensation, and hoarseness. These subtle symptoms can significantly impact the quality of life, yet they might be easily overlooked, leading to delayed diagnosis and treatment. Once the condition worsens, it will jeopardize the health of the patients to a great extent. The clinical manifestations of ESCC are complex and varied, and symptoms such as chest pain, hemoptysis and dyspnea may occur, which seriously affect the quality of life for patients. Due to the rapid deterioration of ESCC, it often leads to distant metastasis and local infiltration, which creates uncertainty and certain difficulties in the treatment of patients. The current pathogenesis of ESCC is cumbersome, the methods of prevention and treatment are limited, and the survival rate of patients is low, these place a significant burden on the patient’s family and society.
The causative factors of ESCC are extremely diverse and are mainly associated with long-term smoking, alcohol consumption, poor dietary habits, genetic factors, chronic inflammation of the esophagus, and heterogeneous proliferation of the esophageal epithelium. At present, research in ESCC is mainly focused on clinical treatment and basic science. In terms of clinical treatment, surgical resection is the main treatment method, which can achieve better curative effect for patients with early ESCC. For advanced patients, due to the rapid progression of the disease, it may not be possible to completely remove the tumour by surgical means such as local excision or total esophagectomy to remove only part of the lesion. Therefore, it is often necessary to assist in the control of the disease by irradiation of the diseased area with high-energy radiation to kill the cancer cells, reduce the size of the tumour and the symptoms, and at the same time to protect the surrounding normal tissues with radiotherapy or chemotherapy that uses chemicals to kill the tumour cells. Immunotherapy plays a crucial role in the treatment of ESCC by harnessing the body’s immune system to combat cancer. This approach works by stimulating T-cells and other effector cells, particularly in cases where tumor gene mutations occur and the immune monitoring system fails to recognize cancerous cells. Additionally, targeted therapy and radiotherapy are vital treatment modalities for ESCC, as they focus on specific molecular targets associated with the cancer. These therapies are designed to eradicate cancer cells with enhanced precision, thereby minimizing damage to surrounding healthy tissues. Together, these innovative treatment options represent a comprehensive strategy to effectively manage ESCC.
In the basic sciences, current research focuses on the following areas. Although the mechanism is still unclear, researchers hope to reveal the pathogenic mechanism of ESCC by studying the genetic variations of cancer cells and the abnormal activation of signaling pathways. For instance, the temperature-sensitive gene TRPV2 is significantly overexpressed in ESCC, and patients with high TRPV2 levels tend to have a poor prognosis. Research involving experiments conducted both in vitro and in vivo has shown that TRPV2 activates the HSP70/27 and PI3K/Akt/mTOR signaling pathways, facilitating the development and metastasis of cancer cells characterized by squamous heterogeneous hyperplasia in the esophageal epithelium [1]. Regarding prevention and screening for ESCC, early diagnosis is crucial for the treatment of ESCC. Therefore, finding high-risk groups and exploring effective screening methods have become the focus of current research. In recent years, screening using a combination of blood biomarkers and imaging can improve early detection rates. In addition, based on the molecular characteristics of ESCC cells, researchers are exploring strategies for targeted therapies. By targeting inhibitors of EGFR, VEGFR, HER2 and other signaling pathways, the growth and spread of cancer cells can be effectively blocked [2]. Immunotherapy, as one of the current hot spots in cancer treatment, has also been incorporated into research on the treatment of ESCC in recent years. Immunotherapy aims to kill cancer cells by stimulating the patient's immune system. To date, immunotherapy has achieved notable findings in a specific group of patients. Overall, some progress has been made in the study of ESCC, but many challenges remain. Some patients are resistant to existing treatments and their prognosis remains poor. Therefore, the research direction will focus on the development of individualized therapeutic strategies, targeted drug research and clinical trials to further explore the pathogenesis of ESCC and to find more effective preventive and therapeutic methods to alleviate the suffering of patients and the burden of the disease on the society. The aim of this research is to investigate the pathogenesis of ESCC caused by heat stress and the targeted, immunotherapeutic strategies based on the signaling pathways and key proteins associated with ESCC.
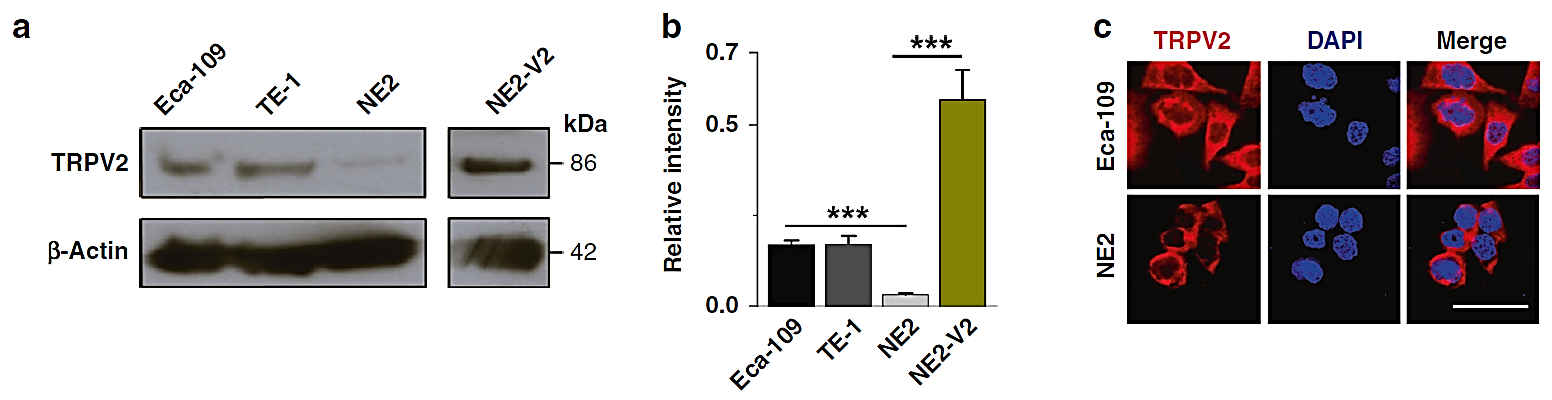
Figure 1. TRPV2 is overexpressed in ESCC cells, and its hyperactivation enhances cellular proliferation [1]. (a) Western blotting analysis. (b) Densitometric quantification analysis. (c) ICF staining analysis.
2. Pathogenesis analysis based on heat stress
Heat stress refers to the overall non-specific reactions of humans or animals in response to excessively high temperatures that surpass their thermoregulatory capabilities. Thermoregulated animals have isothermal zones, and when the surrounding temperature falls within this range, they can maintain their normal body temperature through their natural thermoregulatory mechanisms. However, when the ambient temperature exceeds the upper limit of an animal’s isothermal zone, it experiences heat stress. As an important factor leading to ESCC, environmental factors, including long-term use of overheated food, drinking overheated beverages, and repeated thermal stimulation and damage to the esophageal epithelial mucous, may greatly lead to the esophageal epithelial cell anisotropic hyperplasia, which will increase the risk of cancerous transformation to ESCC [3]. The temperature sensitivity-related gene TRPV2 is a non-selective cation channel on the cell surface, and the TRPV2 gene is activated after exposure to injurious high temperatures, i.e., physicochemical stimuli such as temperatures greater than 52 °C, and exhibits an important place in the body’s reaction to environmental pressure. In recent years, the TRPV2 gene has been shown to have oncogenic activity in a type of cancers, including breast cancer [4]. As shown in Figure 1, TRPV2 gene expression is up-regulated at both transcriptional and translational levels in cancerous cell lines compared to normal esophageal squamous epithelial cells by studying TRPV2 gene expression therein in both cell lines and cancerous tissues of ESCC. In cancerous tissue, the expression of the gene remained higher than in normal esophageal tissue.
To investigate the effect of TRPV2’s expression on the occurrence and migration of cancerous cell lines or tissues, the expression of TRPV2 in the prognostic stage of ESCC was studied using the level of TRPV2 gene expression as a classification criterion, and the obtained curves showed that compared with the group of low TRPV2 expression, the overall survival and progression-free survival period of the patients who showed the phenomenon of high TRPV2 expression were significantly shorter. As shown in Figure 2, patients with high TRPV2 gene expression exhibited significantly shorter overall survival and progression-free survival when compared to those with low TRPV2 expression. The high expression of TRPV2 gene is a typical factor that allows patients with ESCC to show poor prognosis phenomenon.
To address the specific place of TRPV2 in the development of ESCC, it was found that the condition that could significantly promote the proliferation of cancerous cells was the activation of TRPV2 gene expression by thermal stimulation at 54 °C, and the ability of cancerous cells to migrate was found to be significantly enhanced by the activation of the TRPV2 gene by the cell scratch assay. Using antagonists or knocking out the TRPV2 gene will inhibit the proliferation of cancerous cell and the migration of cancerous cell lines and tissues to some extent. The TRPV2 gene is an oncogenic factor in ESCC, and that activated TRPV2 results in the promotion of cellular heterogeneous proliferation, migration and invasion of cancerous cell lines and cancerous tissues in ESCC. High temperature (≥ 54 ℃) stimulation of the temperature-sensitive gene TRPV2 leads to the development and metastasis of ESCC. That is, heat stress causes heterogeneous proliferation of esophageal epithelial cells and promotes their development and metastasis, which is an important pathogenic mechanism leading to ESCC.
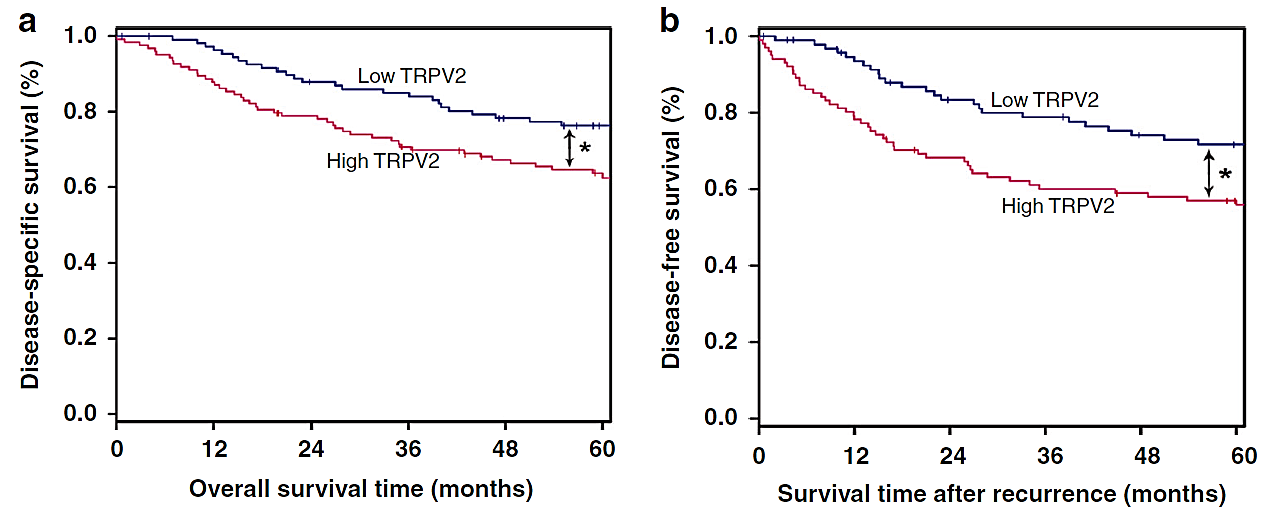
Figure 2. Elevated levels of TRPV2 expression were linked to poorer survival outcomes in ESCC [1]. (a) Survival curves. (b) Disease-free survival analysis.
3. Development of targeted therapy and immunotherapy in ESCC
Targeted immunotherapy encompasses both targeted therapy and immunotherapy. Targeted therapy is a treatment that operates at the cellular and molecular level, focusing on specific cancer-causing sites, such as protein molecules or gene fragments within tumor cells. By creating a corresponding therapeutic drug, it can be introduced into the body to selectively bind to and act on these cancer-causing sites, leading to the death of tumor cells while sparing the surrounding normal tissue cells. Based on the different targeting sites, tumor-targeted therapy can be categorized into two primary types: tumor cell-targeted therapy and tumor vascular-targeted therapy. Tumor cell-targeted therapy targets specific antigens or receptors found on the surface of tumor cells, whereas tumor vascular-targeted therapy focuses on specific antigens or receptors present on the surface of endothelial cells in tumor-associated new capillaries. While the targeted nature of monoclonal antibodies against tumor cells enhances their concentration in local tumor tissue, the process tends to be slower as these large molecules must first pass through the vascular endothelial barrier to reach their tumor cell targets. In contrast, vascular-targeted drugs have the significant advantage of rapidly accumulating in high concentrations at the target site following administration.
Immunotherapy is a therapeutic approach designed to artificially modulate the immune system, either by enhancing or suppressing its functions, depending on whether the immune status is low or high. The ultimate goal of this treatment is to effectively manage or cure various diseases. There are numerous immunotherapy techniques available, each suited for treating a wide range of health conditions. Specifically, immunotherapy for tumors focuses on invigorating the body’s immune response, enabling it to utilize its own immune capabilities to attack and destroy cancer cells and tumor tissues. Unlike traditional treatments such as surgery, chemotherapy, radiotherapy, or targeted therapy that primarily focus on directly targeting tumor cells, immunotherapy shifts the focus to the body’s immune system itself as the primary target.
Because the incidence rate and mortality rate of ESCC are extremely high, its causative factors are extremely complex, diversified and variable, and because there are no obvious symptoms in the early stage, most of the patients are diagnosed only in the middle and late stages of the disease, resulting in a poor prognosis, and the role of the conventional treatments, such as surgical resection of the tumour, radiotherapy and chemotherapy, is already very minimal. Therefore, molecular targeted therapy and immunotherapy have successfully broken through the bottleneck period in the development of therapeutic strategies for ESCC [5].
For molecular targeted therapy, the target can distinguish cancerous cells from healthy cells, so an ideal target is the key to the success of targeted therapy. In recent years, epidermal growth factor (EGFR), vascular endothelial growth factor (VEGF), and its receptor (VEGFR), human epidermal growth factor receptor 2 (HER-2), the cellular tight junction protein CLAN18, mammalian target of rapamycin (mTOR), the complex kinase receptor (MET), and apoptosis inhibitory proteins (XIAP). If targeting EGFR, which is a tyrosine kinase receptor, it can cause downstream tyrosine kinase activation. Numerous researches have shown that tumour development is closely related to the abnormal expression of EGFR [6]. In ESCC, however, the frequency of high expression of the EGFR gene is slightly higher than in other cases, reaching approximately 60-70%, and has been shown to be an independent risk factor leading to a very poor prognostic situation in patients [7].
For immunotherapy, in order to prevent tumours from achieving immune escape by expressing immune checkpoint protein molecules that inhibit T-cell immunity, immunotherapy for ESCC has been rapidly developed in recent years to allow the immune response to proceed normally by re-establishing the recognition and killing ability of immune cells. To date, the immunotherapy for ESCC has primarily focused on programmed death receptor 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). For instance, in the case of PD-1/PD-L1 interactions, it has been demonstrated that prolonged expression of PD-1 on T cells can result in T lymphocyte exhaustion [8]. PD-L1 is a ligand for PD-1 and is found in various human cancer cells. It helps tumors evade the immune system by interacting with PD-1 receptors on T cells. Consequently, by blocking the interaction between PD-1 on T cells and PD-L1 on tumor cells, we enable the immune system to activate effectively and mount an anti-tumor response [9].
Molecular targeted therapies and immunotherapies offer innovative approaches for the treatment of ESCC. These therapeutic modalities not only enhance our awareness of cancer biology but also pave the way for the development of more effective treatment strategies. As research in this field continues to evolve, it is critical to explore these avenues further, as they hold significant promise for improving patient outcomes in ESCC. Future investigations should focus on optimizing these therapies and understanding their mechanisms to develop integrated treatment plans that can ultimately lead to better survival rates and patients’ quality of life.
4. Conclusion
The analysis concluded that high temperature (≥ 54 ℃) stimulation of the temperature-sensitive gene TRPV2 leads to the development and metastasis of ESCC, which promotes the heterogeneous proliferation of esophageal epithelial cells and thus achieves the pathogenic result. For targeted therapy, the target distinguishes cancerous cells from healthy cells, for example, by targeting the EGFR gene, it was found that in ESCC, the frequency of high expression of the EGFR gene was slightly higher than in other cases, reaching about 60-70%, which was proved to be the cause of poor prognosis of the treatment. For immunotherapy, the normal operation of immune function is achieved by rebuilding the recognition and killing ability of immune cells. For example, PD-L1 evades the attack of the body’s immune system by combining with PD-1 on the surface of T-cells, so it can activate the body’s immune system by blocking the combination between PD-1 on T-cells and PD-L1 on tumour cells, thus exerting the anti-tumour effect. In summary, molecular targeted therapy and immunotherapy can provide a direction for future research on therapeutic strategies for ESCC.
References
[1]. Huang R, Li S, Tian C, et al. 2022 British Journal of Cancer 127(8) 1424-1439
[2]. Wang B. Study on the correlation between EGFR gene and PI3K/Akt/mTOR signalling pathway in non-small cell lung cancer tissues. Chinese People's Liberation Army Medical College, 2014.
[3]. Tai W P, Nie G J, Chen M J, et al. 2017 Medicine 96(50) e9325
[4]. Elbaz M, Ahirwar D, Xiaoli Z, et al. 2018 Oncotarget 9(71) 33459
[5]. Ma Z, Xu W, Jin Y, et al. 2024 Pharmacy Practice and Service 42(6) 231-237
[6]. Yamaoka T, Ohba M, Ohmori T. 2017 International journal of molecular sciences 18(11) 2420
[7]. Hanawa M, Suzuki S, Dobashi Y, et al. 2006 International journal of cancer 118(5) 1173-1180
[8]. Iwai Y, Ishida M, Tanaka Y, et al. 2002 Proceedings of the National Academy of Sciences 99(19) 12293-12297
[9]. Yang W, Xing X, Yeung S C J, et al. 2022 Journal for immunotherapy of cancer 10(1) e003497
Cite this article
He,Z. (2024). Analysis of the pathogenesis of esophageal squamous cell carcinoma. Theoretical and Natural Science,62,40-44.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Huang R, Li S, Tian C, et al. 2022 British Journal of Cancer 127(8) 1424-1439
[2]. Wang B. Study on the correlation between EGFR gene and PI3K/Akt/mTOR signalling pathway in non-small cell lung cancer tissues. Chinese People's Liberation Army Medical College, 2014.
[3]. Tai W P, Nie G J, Chen M J, et al. 2017 Medicine 96(50) e9325
[4]. Elbaz M, Ahirwar D, Xiaoli Z, et al. 2018 Oncotarget 9(71) 33459
[5]. Ma Z, Xu W, Jin Y, et al. 2024 Pharmacy Practice and Service 42(6) 231-237
[6]. Yamaoka T, Ohba M, Ohmori T. 2017 International journal of molecular sciences 18(11) 2420
[7]. Hanawa M, Suzuki S, Dobashi Y, et al. 2006 International journal of cancer 118(5) 1173-1180
[8]. Iwai Y, Ishida M, Tanaka Y, et al. 2002 Proceedings of the National Academy of Sciences 99(19) 12293-12297
[9]. Yang W, Xing X, Yeung S C J, et al. 2022 Journal for immunotherapy of cancer 10(1) e003497









