1. Introduction
With an estimated death of 1.82 million in 2022, this cancer has been often studied, and remains as one of the leading cases of cancer-related deaths worldwide. There are two main types of the disease, with NSCLC counting for around 85% of all cases. Despite advancements in treatments and therapeutic strategies, the treatments for lung cancer patients has remained difficult, with the relative 2-year survival rate of NSCLC increasing from 34%, for individuals diagnosed during 2009 - 2010, to 42% for diagnosed patients during 2015 - 2016. As well, improved treatment methods drove a drop in overall lung cancer mortality [1]. However, this doesn’t stop lung cancer from being the cause of most deaths relating to cancer. The persistent poor outcome, calls for a deeper understanding, and the development for more effective treatments.
The cause of the pathogenesis of lung cancer is genetic mutations that eventually drove tumorigenesis and the progression of the cancer. Amongst all of the genes, the most studied mutations which ultimately lead to lung cancer, are the mutations in epidermal growth factor receptors (EGFR), and the Kirsten rat sarcoma viral oncogene homolog (KRAS). EGFR mutations are present in 10-15% of NSCLC cases in Western populations, and up to 50% in Asian populations [2]. These mutations lead to the uncontrolled activation of the EGFR pathway and thus lead to ongoing cell proliferation, and resistance to apoptosis. Similarly, KRAS mutations were also found in around 25% of NSCLC cases, which causes continuous signaling through the RAS/MAPK pathway, also responsible for cell growth and proliferation.
These genetic alterations paved the way for the later development of targeted therapies, a treatment that targets proteins which controls how cancer cells grow, spread, and divide. The initial discovery of these treatment methods in the 1970s revolutionized the treatment landscape for cancer, for they are one of the most efficient ways to treat cancer, including lung cancer. Targeting specific mutated genes such as the EGFR gene, there are effective tyrosine kinase receptors such as gefitinib and erlotinib, which demonstrated remarkable efficacy in patients with EGFR mutations. Tyrosine kinase inhibitors are able to block specific enzymes responsible for activating many proteins through a process called phosphorylation. On the other hand, KRAS mutations, historically famous for being considered as “undruggable”, have only recently been a target with the development of KRAS G12C inhibitors, such as sotorasib and adagrasib. These inhibitors, similar to the Tyrosine Kinase inhibitors, block the signaling pathway, thus preventing cell proliferation.
Despite these advancements, resistance against these targeted therapies still stands as one of the roadblocks to the treatment of lung cancer, and many other cancers in general. In response to the challenge of therapy resistance, novel strategies are currently being explored. For example, third generation EGFR inhibitors, such as osimertinib, have been implemented to improve outcomes for the EGFR T790M resistance, and have shown promising results in clinical trials [3]. As well, combination therapies targeting multiple pathways simultaneously are also being investigated to prevent, or delay the resistance. However, the efficacy of these therapies are inevitably limited by the resistance mechanisms.
This article will aim to provide a comprehensive review of the genetic components of lung cancer, focusing on mutations in the EGFR and KRAS genes, as well as current therapeutic approaches targeting these mutations. Additionally, by synthesizing the latest research findings, the article will explore the mechanisms underlying resistance to these therapies, and discuss emerging strategies, while also offering insight into the future directions of lung cancer treatment, and contribute to the ongoing efforts to improve patient outcomes.
2. Genetic Mutations in NSCLC
Lung cancer is primarily classified into 2 types, NSCLC and SCLC. However, the cause for the two types have been characterized by distinct genetic profiles. Genetic mutations in NSCLC are pivotal in the understanding of disease mechanisms, and the development of related targeted therapies. Amongst the most common and critical mutations, are those in the EGFR and the KRAS genes. EGFR mutations are oftentimes more prevalent in non-smokers as well as East Asian populations, and causes the initiation of signaling pathways which promotes cellular survival and proliferation. These mutations often hint towards a positive response to TKIs in general as a treatment, improving treatment strategies and improving patient outcomes. Moving on, KRAS mutations, more prevalent in smokers, often results in the persistent activation of downstream signaling pathways, controlling certain parts of cell functions that often leads resistance against EGFR related therapies. To understand these mutated genes in the role of NSCLC, this section will explore the normal functions of these genes, the specific types of mutations present in these genes, and their role in the prognosis of lung cancer.
2.1. EGFR Mutation
The EGFR is a gene which codes for a receptor tyrosine kinase, and plays a key role in the regulation of cellular processes. It is a transmembrane protein with extracellular domain which ligands are able to bind to, a transmembrane domain and an intracellular tyrosine kinase domain, to conduct cellular functions. Under normal conditions, EGFR is activated upon binding to its specific ligands, then leads to receptor dimerization, which is a step required for its activation and subsequent signaling. It is a process that involves the pairing of 2 identical or similar molecules to form a complex called a dimer. The EGFR molecules undergo a change that allows them to form this dimer upon binding to a ligand. The dimerization brings the intracellular tyrosine kinase domains of the 2 receptors up close, and allows them to phosphorylate each other through a process called autophosphorylation. The phosphorylated tyrosine residues on the intracellular domains of the EGFR dimers then serve as sites for various signaling proteins to bind to. After binding, they initiate downward signaling cascades, which triggers the cell proliferation, and general functions linked with survival. By controlling these processes, EGFR maintains tissue homeostasis and normal cellular functions [4].
The EGFR group of growth factors acts as key molecules that promote and spread lung cancer. Although EGFR receptors aren’t able to penetrate through cell membranes directly, their growth factors are able to carry cellular information from EGFR via signal transduction pathways to the inside of lung cells. These signaling cascades not only contribute to lung cell growth and development, but they also have a chance of promoting lung metastasis, in which tumor cells from other parts of the body enter the lung. Mutations in the EGFR gene play an important role in the pathogenesis of NSCLC. These mutations often occur within the receptor’s tyrosine kinase domain, which leads to its constant activation without necessity for ligand binding. As shown in Figure 1, two of the mutations which take place in the EGFR gene the most, also the most prevalent in NSCLC are the exon 19 deletions and L858R point mutations in exon 21. These two mutations alone account for 88% of all EGFR mutations in NSCLC, with Exon 19 deletions accounting for 47% and L858R accounting for 41% [5]. Though they are different types of mutations, they often result in the same result, which is the continuous activation of the receptor’s tyrosine kinase domain, even in the absence of ligands. This leads to consistent autophosphorylation and aberrant initiation of signaling pathways, such as RAS/MAPK, and PI3K/AKT, that promotes uncontrolled cellular proliferation and survival.
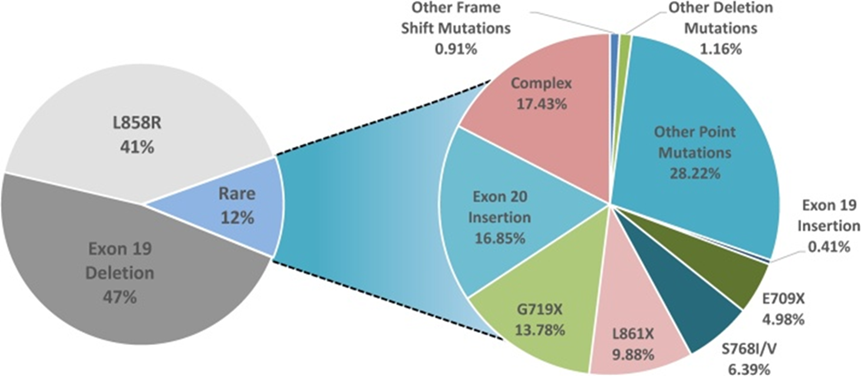
Figure 1. Mutations in NSCLC [5].
The EGFR gene is capable of harboring a variety of mutations, which contributes to the rapid development and progression of NSCLC These mutations occur within the tyrosine kinase domain and lead to the uncontrolled activation of the receptor, leading to tumor-causing signaling. Although these other mutations might be rarer than the two previously mentioned, such as the T790M mutation in exon 20, which oftentimes arises as a secondary mutation in response to treatments, or the exon 20 insertions in general, which also leads to a tendency of drug resistance while presenting a challenge for targeted therapies [6]. EGFR mutations significantly impact patient survival, often indicating a poor prognosis. Together accounting for around 85-90% of all EGFR mutations, exon 19 deletion mutations and L858R point mutations in exon 21 are generally prone to TKIs. The presence of EGFR mutations in general, hints towards a worst prognosis for a patient with lung cancer since EGFR mutations often lead to aberrant signaling for cell proliferation, and tumorigenesis. However, in patients with late stage NSCLC, the presence of common EGFR mutations tends to be more favorable, since the OS rates are higher compared with patients with wild type mutations (Figure 2) [7].
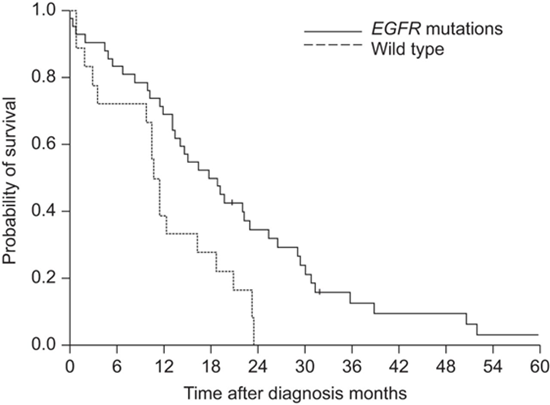
Figure 2. OS rate of patients with NSCLC mutations [8].
2.2. KRAS Mutation
The KRAS gene is a crucial component of the RAS family. These proteins play a role in transmitting signals from the receptors on the surface of the cell to the nucleus, also regulating cell growth, differentiation, and survival. KRAS functions similarly as a switch that changes between an active GDP-bound state, in which it is powered, and an inactive GDP-bound state, in which it isn’t. Under a condition in which an Epidermal growth factor binds to its respective receptor on the cell surface, KRAS is then activated by a signal transduction pathway. Active KRAS interacts with various downstream signaling pathways, such as the RAF/MEK/ERK pathway, which regulates gene expression, and the PI3K/AKT pathway, which promotes cell proliferation, survival, and differentiation. Once the KRAS gene is activated, GTP-powered KRAS starts several downstream signaling pathways, with the most notable ones still being the RAF/MEK/ERK pathway and the PI3K/AKT pathway, with the prior being responsible for controlling cell proliferation, cell cycle progressions, and the latter controlling cell survival, protein synthesis, and inhibition of apoptotic pathways. The regulation of KRAS activity is crucial in maintaining normal cell functions, and the cell responds to the corresponding growth signals, with its cycling going on and off, it prevents uncontrolled cell growth [9].
Similar to the EGFR, the KRAS gene is often involved in the pathogenesis of NSCLC. Being one of the most frequently mutated oncogenes, with a frequency of around 30% in NSCLC, while encoding a GTPase that switches on and off between an active and inactive form, the KRAS mutation factors are responsible, in the active form, of an inhibition to GTPase activity. These mutations in exons 2, 3, and 4 in KRAS lead to the constitutive activation of the gene, independently, and cause abnormal growth in cells with an ability to resist cellular apoptosis. As shown in Figure 3, 90% of KRAS mutations are located in codon 12, and the others are in codon 13, and 61. All of these mutations could lead to a similar result. KRAS G12C is the most common type of KRAS mutation in NSCLC, and that is also common in colorectal cancer. Some of the types of KRAS mutations that could participate in the development of NSCLC include G12A, G12S, G12C, G12D, G12V, G12R, G13D, Q61H, etc, located mostly on codons 12, 13, and 61. Moving on, patients with the mutated KRAS gene hints that this mutated gene is a bad prognosis factor. However, as observed in EGFR mutations too, patients with a more common mutation, such as the KRAS G12C, tend to have a higher OS rate than patients with other types of KRAS mutations. This might result from the lack of development of therapies or the lack of understanding for the specific mutations [10].
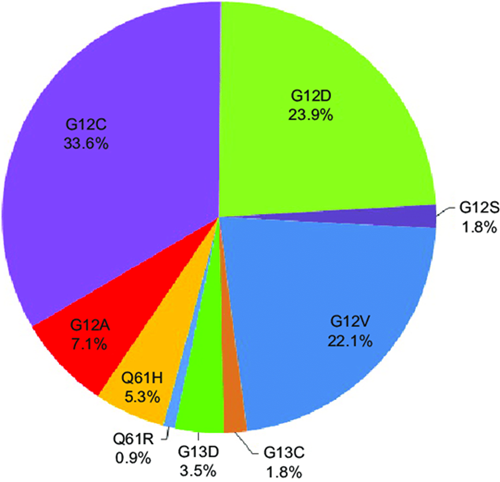
Figure 3. Different types of KRAS mutations in patients [11]
3. Advancements in Targeted therapies
Lung cancer, particularly NSCLC, has seen multiple significant advancements in targeted therapies over recent years. These therapies had focused on specific genetic mutations that drive cancer growth and progression. This section will briefly go over several therapies targeting the mutated EGFR and KRAS gene in NSCLC.
3.1. EGFR Targeted Therapies
Targeted therapies for EGFR mutations have improved outcomes for NSCLC patients significantly. First generation EGFR inhibitors, such as erlotinib, and gefitinib, work by binding to the ATP-binding site of the EGFR tyrosine kinase, thus inhibiting its activity. These drugs are overall effective in patients with specific EGFR mutations, such as exon 19 deletions, and the commonly seen L858R mutation in exon 21. They prevent EGFR from activating downstream signaling pathways which promotes tumor growth, and thus blocks cell proliferation, leading to cell death. Second-generation EGFR inhibitors are inhibitors, used when there is resistance to the first generation inhibitors but not specifically due to one type of mutation, such as afatinib, binds closely to EGFR and other members of the ErbB family, offering a more general field of activities and a possibility of overcoming the various mutations as resistance to first-generation inhibitors. The irreversible binding leads to a more sustained inhibition of the EGFR pathway, however it couldn’t target more random and specific mutations. If the patient continues to develop strong resistances to first generation and second generation inhibitors, third generation EGFR inhibitors are then utilized. Osimertinib is a third generation EGFR inhibitor that specifically targets the T790M resistance mutation, which frequently happens. Similar to the second generation inhibitors, osimertinib inhibits EGFR by binding to both sites of the T790M resistance mutation. The T790M mutation alters the shape of the ATP binding sites, which makes it harder for the first and second generation inhibitors. By specifically targeting this mutation, osimertinib is able to inhibit EGFR signaling even in the presence of this mutation. However, most mutations in response to treatments are unique, and a generalized treatment for these specific mutations is hard to develop [12].
3.2. KRAS Targeted Therapies
Similar to EGFR mutations, KRAS mutations are also prevalent in NSCLC. These mutations, particularly adenocarcinomas, have been historically known to be challenging to target. Recent developments have led to KRAS inhibitors such as sotorasib and adagrasib specifically targeting the KRAS G12C mutation. They often lock KRAS in its inactive GDP-bound state, thus inhibiting downstream signaling pathways essential for tumor growth. These first generation inhibitors have been showing promising results in preclinical and clinical studies, providing a cure to patients with the G12C variant. Olomorasib is a recently evaluated, second generation inhibitor of the KRAS G12C that just announced its presence on June 1, 2024. However, due to the fact that KRAS resistance mutations aren’t as traceable as EGFR mutations, olomorasib isn’t able to accommodate all patients. Being a selective second-generation inhibitor of the KRAS G12C protein, there will still be a significant portion of limitations for patients with KRAS G12C mutated cancer types. As for third generation inhibitors, they are still under development currently, and there hasn’t been any clear example that has stood out yet. These inhibitors have been aiming to improve the limitations of earlier generations, and that they are designed to target a broader range of KRAS mutations while overcoming the resistance mutations to second generation inhibitors. However, there have been notable cases of combination therapies that are viable in the treatment of the mutated KRAS gene, such as combining KRAS inhibitors with Immune Checkpoint Inhibitors, or MEK inhibitors to provide a more comprehensive blockage of a certain pathway, or to improve the immune response against tumor cells [13].
4. Challenges and Limitations
While developing treatments for (NSCLC), there have been two critical challenges : The Resistance and Off-Target effects, as well as Patient-Specific Responses. These two issues not only complicate the effectiveness of modern therapies, but also highlight the limitations which must be addressed to improve patient outcomes.
4.1. Resistance and Off-Target Effects
Targeted therapies and Immunotherapies for NSCLC have been steadily showing significant promise. However, challenges still exist. One of the most pressing issues for these methods of treatment is the development of resistance to these treatments. Drug resistance has been a major cause for therapeutic failures in NSCLC. It can occur through various mechanisms, which include changes in cell mechanisms, and activation of pro-survival and anti-apoptosis pathways. As mentioned before, tumor cells are capable of developing secondary mutations in response to a first generation drug, such as the T790M mutation, which works to diminish the drug's efficacy. Similarly, ALK inhibitors may also lose effectiveness due to secondary mutations such as L1196M, which results in further disease progression. This mutation has been caused by the tumor’s number of heterogeneous cells with different characteristics. This diversity has been caused by the amount of tumor cells, called Cancer stem cells, which have abilities to initiate tumors, and are highly self-renewing [14]. Overall, the presence of these factors contributes towards disease progression and the worsened prognosis of the patient.
Another challenge which is related to the responses towards the treatments, are off-target effects. These effects arise when treatments not only affect the targeted cells, but also some of the nearby tissues, which could lead to unwanted effects. For example, when monoclonal antibodies, a specific treatment in which are designed to target specific antigens, are used on patients, they might also bind to similar antigens in nearby healthy cells, leading to the mistakened attack on those cells.
4.2. Patient-Specific Responses
Another challenge that still resides, is the patient specific responses. The effectiveness of NSCLC treatments can vary widely amongst patients, and this is influenced by both the genetic and epigenetic factors. These factors pose a variability and raise a challenge in the overall management in the development of a cure for a certain disease. Knowing a therapy that might work for one patient, but could be ineffective for another, could lead to the necessity of developing different therapies targeted for different populations with different characteristics in response to treatments targeting the same disease, and therefore hindering the development of an all-encompassing treatment of certain diseases. For example, patients with EGFR mutations can vary in responses to tyrosine kinase inhibitors. Others might respond well while others might have worse responses due to additional secondary mutations, or differences in the metabolism of the drug. In response to these limitations, personalized medicines have been implemented, to specifically fit a person’s response to diseases and drugs. However, the cost required in the development of these treatments is high, and not a lot of individuals have the privilege to these types of treatments, which causes a disparity in access to life-saving therapies, ultimately leading to unequal treatment outcomes and vastly different OS rate for patients.
4.3. Future Treatments
Despite significant challenges and limitations in current treatments to NSCLC, including issues with resistance, off-target effects, and patient-specific responses, emerging therapies to NSCLC still offer promising solutions. As mentioned earlier, personalized medicine is one of the developments that is at the forefront, which offers promising future solutions. A method which could understand a person’s genetic information of an individual person, as well as the way their genes interact with each other and the environment, is genomic profiling. Treatments can be tailored based on an individual’s unique genetic makeup. This approach allows the overcoming of the variability amongst patient responses by precisely targeting each mutation that arises to treatment methods in each patient. However, oftentimes these personalized medicines require a lot of effort, therefore the cost and the availability of these drugs won’t be widespread. A possibility to improve this may be drug repurposing, to lower the cost of a drug by finding cheaper replacement. As well, there have also been innovations in immunotherapy, such as enhanced Immune Checkpoint inhibitors, and specific antibodies, which are being developed to address the limitations of pre-existing therapies by better modulating the immune system. Additionally, new drug delivery methods, such as using nanoparticles and monoclonal antibodies to carry cytotoxic agents are currently being explored to improve the precision and efficacy of various treatments, while working towards reducing off-target effects and enhancing drug delivery directly to oncogenes, and not the healthy tissues around. To encapsulate, while challenges still remain, these emerging strategies and developments have provided a peak into the future of cancer treatment potential therapies.
5. Conclusion
This paper has provided a comprehensive analysis of the genetic mutations associated with NSCLC, with a focus on EGFR and KRAS mutations. The research has highlighted the advancements in targeted therapies designed to address those mutations, while detailing their effectiveness in improving patient survival rates. However, challenges such as the development of drug resistance, off-target effects, and patient specific responses have been major blockades in the current treatment development. The value of these findings are their potential to inform future research and clinical practices. By understanding, holistically, the mechanisms of resistance and the limitations of existing treatments, researchers are able to better design strategies that are more personalized and effective to diseases. This study shows the importance of the comprehensive view at treating certain diseases, that even though personalized medicine could work for these varied responses, there are still factors that should be considered, such as the cost, and applicability, while allowing the audience to look at more ways to improve this solution, such as drug repurposing. These insights can aid future research efforts towards ultimately overcoming the limitations of current treatments, and improving the overall prognosis result for NSCLC patients. However, this paper also acknowledges limitations in its scope. The analysis focused primarily on EGFR and KRAS mutations, in one type of lung cancer, NSCLC leaving a handful of other significant mutations, such as the ALK mutation, and TP53 mutations. Aswell, many other emerging treatment methods might remain unknown, as they are still in early stages of development. Additionally, limitations of treatments also remain vague, particularly in understanding the variability in patient responses functions. Looking into the future, this research should aim to address these problems in expanding the view to a broader range of genetic mutations, while exploring a more diverse range of combination therapies which could relieve resistance. There’s also a need for more clinical trials to evaluate the long-term effectiveness of emerging therapies. The goal of this research is to engage the audience in looking more into the field of treatments for lung cancer in particular, in developing more adaptable and viable treatment strategies which could meet the more diverse needs of NSCLC patients, ultimately improving their chances of long-term survival.
References
[1]. Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians, 2021, 71(1): 7–33.
[2]. Hsu, W.-H., Yang, J. C.-H., Mok, T. S., & Loong, H. H. Overview of current systemic management of EGFR-mutant NSCLC. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 2018, 29(suppl_1): i3–i9.
[3]. Eun Ki Chung, Seung Hyun Yong, Eun Hye Lee, Eun Young Kim, Yoon Soo Chang, & Sang Hoon Lee. New Targeted Therapy for Non-Small Cell Lung Cancer. Tuberculosis & Respiratory Diseases, 2023, 86(1): 1–13.
[4]. Endang Purba, Saita, E., & Maruyama, I. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The “Rotation Model”. Cells, 2017, 6(2): 13–13.
[5]. Harrison, P. T., Vyse, S., & Huang, P. H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Seminars in Cancer Biology, 2020, 61: 167–179.
[6]. Nguyen, K.-S. H., Kobayashi, S., & Costa, D. B. Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non–Small-Cell Lung Cancers Dependent on the Epidermal Growth Factor Receptor Pathway. Clinical Lung Cancer, 2009, 10(4): 281–289.
[7]. Rolof G.P. Gijtenbeek, Ronald A.M. Damhuis, van, et al. Overall survival in advanced epidermal growth factor receptor mutated non-small cell lung cancer using different tyrosine kinase inhibitors in The Netherlands: a retrospective, nationwide registry study. The Lancet Regional Health - Europe, 2023, 27: 100592–100592.
[8]. Wu, S.-G., Hu, F.-C., Chang, Y.-L., et al. Frequent EGFR mutations in nonsmall cell lung cancer presenting with miliary intrapulmonary carcinomatosis. European Respiratory Journal, 2012, 41(2): 417–424.
[9]. Sylwia Jančík, Jiří Drábek, Radzioch, D., & Marián Hajdúch. Clinical Relevance of KRAS in Human Cancers. Journal of Biomedicine and Biotechnology, 2010, 2010: 1–13.
[10]. Reita, D., Pabst, L., Erwan Pencreach, Guérin, E., et al. Direct Targeting KRAS Mutation in Non-Small Cell Lung Cancer: Focus on Resistance. Cancers, 2022, 14(5): 1321–1321.
[11]. Zheng D, Wang R, Zhang Y, et al. The prevalence and prognostic significance of KRAS mutation subtypes in lung adenocarcinomas from Chinese populations. OncoTargets and therapy, 2016: 833-843.
[12]. Lovly, C. M., Iyengar, P., & Gainor, J. F. Managing Resistance to EFGR- and ALK-Targeted Therapies. American Society of Clinical Oncology Educational Book, 2017, 37: 607–618.
[13]. Friedlaender, A., Drilon, A., Weiss, G. J., et al. KRAS as a druggable target in NSCLC: Rising like a phoenix after decades of development failures. Cancer Treatment Reviews, 2020, 85: 101978–101978.
[14]. Venus Sosa Iglesias, Giuranno, L., Dubois, L. J., Theys, J., & Vooijs, M. Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting?. Frontiers in Oncology, 2018, 8.
Cite this article
Feng,W. (2024). Advancements in Targeted Therapies Based on the EGFR, and KRAS Genetic Mutations in Lung Cancer. Theoretical and Natural Science,67,22-29.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. Cancer Statistics, 2021. CA: A Cancer Journal for Clinicians, 2021, 71(1): 7–33.
[2]. Hsu, W.-H., Yang, J. C.-H., Mok, T. S., & Loong, H. H. Overview of current systemic management of EGFR-mutant NSCLC. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 2018, 29(suppl_1): i3–i9.
[3]. Eun Ki Chung, Seung Hyun Yong, Eun Hye Lee, Eun Young Kim, Yoon Soo Chang, & Sang Hoon Lee. New Targeted Therapy for Non-Small Cell Lung Cancer. Tuberculosis & Respiratory Diseases, 2023, 86(1): 1–13.
[4]. Endang Purba, Saita, E., & Maruyama, I. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The “Rotation Model”. Cells, 2017, 6(2): 13–13.
[5]. Harrison, P. T., Vyse, S., & Huang, P. H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Seminars in Cancer Biology, 2020, 61: 167–179.
[6]. Nguyen, K.-S. H., Kobayashi, S., & Costa, D. B. Acquired Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Non–Small-Cell Lung Cancers Dependent on the Epidermal Growth Factor Receptor Pathway. Clinical Lung Cancer, 2009, 10(4): 281–289.
[7]. Rolof G.P. Gijtenbeek, Ronald A.M. Damhuis, van, et al. Overall survival in advanced epidermal growth factor receptor mutated non-small cell lung cancer using different tyrosine kinase inhibitors in The Netherlands: a retrospective, nationwide registry study. The Lancet Regional Health - Europe, 2023, 27: 100592–100592.
[8]. Wu, S.-G., Hu, F.-C., Chang, Y.-L., et al. Frequent EGFR mutations in nonsmall cell lung cancer presenting with miliary intrapulmonary carcinomatosis. European Respiratory Journal, 2012, 41(2): 417–424.
[9]. Sylwia Jančík, Jiří Drábek, Radzioch, D., & Marián Hajdúch. Clinical Relevance of KRAS in Human Cancers. Journal of Biomedicine and Biotechnology, 2010, 2010: 1–13.
[10]. Reita, D., Pabst, L., Erwan Pencreach, Guérin, E., et al. Direct Targeting KRAS Mutation in Non-Small Cell Lung Cancer: Focus on Resistance. Cancers, 2022, 14(5): 1321–1321.
[11]. Zheng D, Wang R, Zhang Y, et al. The prevalence and prognostic significance of KRAS mutation subtypes in lung adenocarcinomas from Chinese populations. OncoTargets and therapy, 2016: 833-843.
[12]. Lovly, C. M., Iyengar, P., & Gainor, J. F. Managing Resistance to EFGR- and ALK-Targeted Therapies. American Society of Clinical Oncology Educational Book, 2017, 37: 607–618.
[13]. Friedlaender, A., Drilon, A., Weiss, G. J., et al. KRAS as a druggable target in NSCLC: Rising like a phoenix after decades of development failures. Cancer Treatment Reviews, 2020, 85: 101978–101978.
[14]. Venus Sosa Iglesias, Giuranno, L., Dubois, L. J., Theys, J., & Vooijs, M. Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting?. Frontiers in Oncology, 2018, 8.









