1. Introduction
Prostate cancer (PCa), also known as the epithelial malignant tumor of the prostate, primarily affects individuals under the age of 55. PCa ranks as the fourth most prevalent cancer globally, the second most frequent cancer, and the fifth leading cause of death in males [1]. In Europe, PCa is the most commonly diagnosed cancer because a high-fat diet is positively associated with PCa incidence, especially in Western countries [2, 3]. Hence, in many European countries like Germany, Spain, Italy, and the United Kingdom, the burden of direct initial medical costs for non-skin-related male cancer PCa is considerable [3]. Several treatment strategies have demonstrated effectiveness in enhancing the survival outcomes of patients with prostate cancer in clinical settings. The strategy chosen may rely on the cancer stage when it was diagnosed, tumor features, age, available medical facilities, attending physicians, and patient preferences [3]. Patients who are not candidates for surgery or who have a longer life expectancy, but experience treatment-related problems can benefit from radiation therapy. Patients with symptomatic localized tumors and those with locally progressed and extensive illness may consider hormone therapy [3]. Chemotherapy works well for tumors that don't respond to hormones; these tumors usually have advanced symptoms [3]. Nausea and vomiting are among the most dreaded adverse effects of chemotherapy for cancer patients [4]. In addition, additional gastrointestinal adverse effects of cancer treatment are commonly experienced by patients. For example, localized ulcers and pain can result from both oral and gastrointestinal mucositis, which can then induce anorexia, malabsorption, weight loss, anemia, lethargy, and an increased risk of sepsis [4]. Neutropenia is a prevalent symptom in individuals receiving chemotherapy for advanced prostate cancer, it frequently results in negative consequences [5]. In recent years, small-molecule drugs have gradually become an important means to treat PCa as a new therapeutic method. Advances in therapeutic approaches, from cytotoxic clinical chemotherapy to small-molecule drugs, can successfully extend the life span of patients. This has been shown in many patients. Particularly for drugs targeting Androgen receptor signaling pathways [6]. Abiraterone is an oral inhibitor of the cytochrome P450 c17 enzyme complex, essential for androgen synthesis. In a clinical trial conducted between April 28, 2009, and June 23, 2010, a total of 1,088 patients were randomized into two groups: one receiving a placebo and the other receiving abiraterone with prednisone. Results from the trial showed a significant therapeutic benefit in the abiraterone group, although some patients discontinued treatment due to side effects or disease progression. This review aims to systematically explore the small-molecule drugs that target the androgen receptor (AR) signaling pathway for PCa treatment, with a focus on the mechanism of action, clinical application, and several specific types of drugs.
2. Androgen receptor (AR) signaling pathway in Prostatic Cancer
AR is a multifaceted protein consisting of various discrete domains that govern gene transcription. The core structure has four principal domains, including the N-terminal domain (NTD), the hinge domain, the DNA-binding domain (DBD), and the ligand-binding domain (LBD) [7]. When androgens, such as testosterone and dihydrotestosterone, bind to the ligand-binding domain (LBD), AR activity is activated. This activation regulates the expression of genes responsive to androgens, including prostate-specific antigens [7]. Each domain engages with diverse chemical components and executes particular roles, allowing AR to facilitate a broad spectrum of physiological processes. One of the key elements in PCa progression is the AR signaling pathway. Cancer-associated fibroblasts (CAFs) affect cancer start, progression, and metastasis through many methods. AR signaling in CAFs has been demonstrated to diminish the expression of several cytokines, such as CCL2 and CXCL8, which are linked to enhanced migration of PCa cells [8]. The findings indicate that androgen receptor activation in cancer-associated fibroblasts directly influences PCa cell migration via paracrine pathways [8].
The primary ligand of the androgen receptor (AR) is testosterone, which, upon binding, induces various physiological effects. Dihydrotestosterone (DHT), a more potent form of testosterone produced in the body, binds to the AR’s LBD with higher activity. When DHT attachs to AR, it leads to the discomposition of heat shock proteins (HSPs) from the receptor, leading to the formation of an AR dimer. This dimer then translocates into the nucleus, where the DBD of AR identifies and adheres to AREs on DNA. Depending on the context, AR recruits co-regulatory proteins that either activate or suppress the transcription of target genes, such as prostate-specific antigen (PSA) (Figure 1). The AR signaling pathway is regulated by a negative feedback mechanism, which can be triggered by overactivation. In prostate cancer treatment, androgen deprivation therapy (ADT) works through lower androgen levels or inhibiting AR activity. For instance, abiraterone blocks androgen synthesis, while enzalutamide prevents androgens from binding to AR. In castration-resistant prostate cancer (CRPC), tumors continue to rely on AR signaling, even under reduced androgen levels. Approximately 30% of CRPC cases exhibit AR gene amplification, which is thought to contribute to resistance against AR signaling inhibitors (ARSI). PCa may regain sensitivity to androgen therapy with prolonged treatment. Testosterone modulates cell division, tumor development, and possibly cancer spread. These may be advantageous in androgen deprivation (AD) therapies when extensive surgery is unfeasible [9]. Androgens are essential in promoting prostate cancer during its initial phases. CRPC denotes a phase in which the disease advances or recurs despite diminished testosterone levels resulting after gonadectomy [9]. CRPC progression is associated with the amplification, mutation, and activation of alternate proliferative signaling pathways in AR genes, enabling cancer cells to persist in growth despite diminished androgen levels. These methods underscore the intricacy of androgen signaling in CRPC. Moreover, alterations in gonadotropin-releasing hormone receptor (GnRH-R) expression in PCa cells correlate with androgen sensitivity, especially in castration-resistant individuals [9]. Research indicates that elevated Gleason scores correspond with diminished GnRH-R expression levels, which is associated with resistance to GnRH analog therapies in CRPC patients exhibiting high Gleason scores. This discovery corresponds with the function of androgen signaling in the advancement of PCa. A recently identified AR-specific coregulator, melanoma antigen gene protein A11 (MAGE-11), is expressed solely in humans and non-human primates [10]. MAGE-11 enhances AR transcriptional activity by interacting directly with AR and coactivators, with its expression rising as prostate cancer progresses to castration-resistant growth. The MAGE-11 gene, part of the cancer/testicular antigen X-linked MAGE gene family, is located on the Xq28 region of the human X chromosome [10]. Under low androgen levels, MAGE-11 stabilizes AR and is crucial for forming transcriptional complexes that facilitate AR-mediated gene activation.
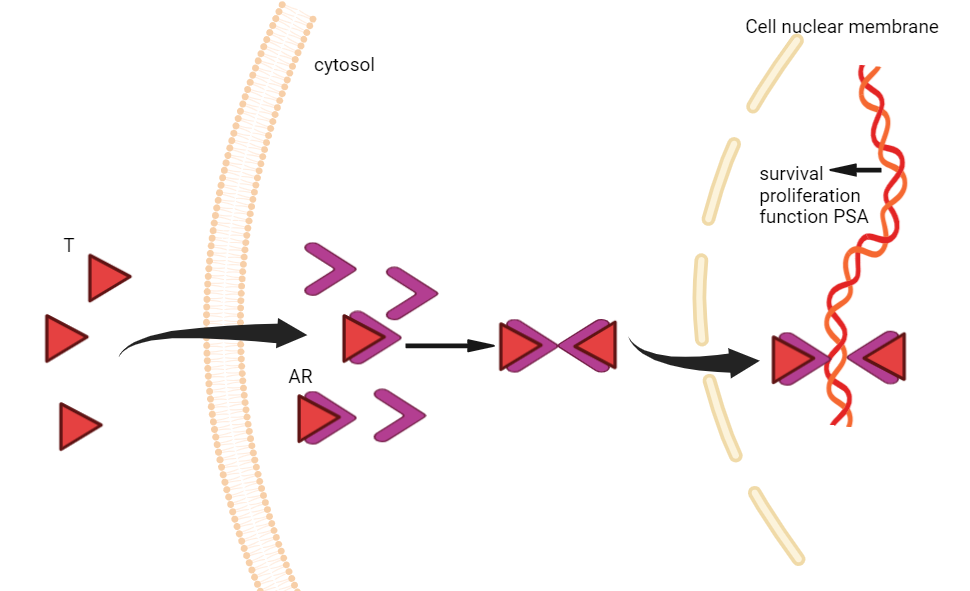
Figure 1. Graphical representation of the AR signaling pathway. T denotes testosterone, PSA stands for prostate-specific antigen, and AR stands for androgen receptor.
3. AR Targeting Drugs Approved for PCa Therapy
Abiraterone acetate is a famous and important medication in the treatment of metastatic CRPC, often administered alongside prednisone or methylprednisolone. Approved by the FDA, this drug is utilized for patients who do not respond to therapies aimed at lowering testosterone levels, as well as for high-risk cancer patients who are sensitive to reduced testosterone levels. The combination of abiraterone acetate and prednisone is commonly employed in managing metastatic CRPC. Abiraterone acetate selectively inhibits androgen production in prostate cancer cells, effectively delaying tumor growth by lowering androgen levels [10]. Prednisone serves as a steroid medication that can help replenish hormone levels, mitigate the adverse effects of abiraterone acetate, and improve the overall feeling and the length of life for patients, as androgen suppression may lead to adrenal insufficiency. The combination of abiraterone acetate and prednisone has been shown to significantly enhance progression-free survival and other clinical outcomes, particularly in patients with BRCA1/2 gene mutations [10]. This combination therapy may not only extend radiographic progression-free survival but also reduce the time to symptom progression and the initiation of cytotoxic chemotherapy. Treatment regimens that incorporate both medications have demonstrated good safety and efficacy in front-line patients, highlighting the importance of identifying patients with specific molecular subtypes. Bicalutamide is an FDA-approved medication that is used to treat metastatic PCa and is frequently used in combination with agonists that release luteinizing hormone (LHRH). While the specific indications for bicalutamide are well-established, ongoing studies and clinical trials continue to explore its efficacy and applications. It is crucial to emphasize that drug information is intended for educational purposes, and patients should consult a healthcare professional for advice regarding their individual conditions. As a non-steroidal anti-androgen, bicalutamide effectively blocks AR activity and tumor development in androgen-responsive PCa. It is frequently employed as a standard treatment for locally advanced, non-metastatic PCa [11]. But some factors, such as AR mutations and the presence of coactivators, like TIF2, can contribute to drug resistance. Additionally, the decrease of AR binding and adverse reactions to decrease the level of endogenous androgens are related to decreased efficacy. Notably, patients with advanced prostate cancer who have AR variants created by selective mRNA splicing or chromosomal rearrangement, in particular, AR-V7 are more resistant to enzalutamide and abiraterone [11]. Recent studies illustrate niclosamide, which targets AR-V7, can resensitize bicalutamide-resistant cells. Furthermore, nicodamid and bicalutamide together show promise in preventing the development of enzalutamide-resistant tumors, indicating a viable approach to treating prostate cancer resistance.
4. Summary
The role of small-molecule drugs in prostate cancer treatment is becoming increasingly significant, particularly in targeting androgen receptor (AR) signaling pathways. Agents such as bicalutamide, enzalutamide, and abiraterone have proven a remarkable usage in treating both hormone-sensitive and CRPC. These drugs effectively inhibit AR activity, block androgen synthesis, and alter AR signaling, thereby slowing tumor growth and enhancing patient outcomes. Current research is heavily focused on the interactions among these various drugs, with future goals aimed at reactivating silenced pathways. This involves exploring novel therapeutic combinations, researching gene therapy targeting specific molecular subtypes, and developing innovative strategies. Future studies should concentrate on creating new targeted approaches to overcome resistance to existing therapies and further improve patient outcomes. FDA-approved small-molecule drugs offer more precise and effective options for managing prostate cancer. Although treatment strategies continue to evolve and improve, prostate cancer remains a serious health concern, necessitating ongoing research to enhance prognosis and treatment outcomes.
References
[1]. Wang, L., Lu, B., He, M., Wang, Y., Wang, Z., & Du, L. (2022). Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Frontiers in Public Health, 10, 811044–811044.
[2]. Hanash, K. A., Al-othaimeen, A., Kattan, S., Lindstedt, E., Al-zahrani, H., Merdad, T., Peracha, A., Kardar, A. H., Aslam, M., & Al-akkad, A. (2000). Prostatic Carcinoma: A Nutritional Disease? Conflicting Data From the Kingdom of Saudi Arabia. The Journal of Urology, 164(5), 1570–1572.
[3]. Fourcade, R. O., Benedict, Á., Black, L. K., Stokes, M. E., Alcaraz, A., & Castro, R. (2010). Treatment costs of prostate cancer in the first year after diagnosis: a short‐term cost of illness study for France, Germany, Italy, Spain and the UK. BJU International, 105(1), 49–56.
[4]. Urata, S., Izumi, K., Hiratsuka, K., Maolake, A., Natsagdorj, A., Shigehara, K., Iwamoto, H., Kadomoto, S., Makino, T., Naito, R., Kadono, Y., Lin, W., Wufuer, G., Narimoto, K., & Mizokami, A. (2018). C‐C motif ligand 5 promotes migration of prostate cancer cells in the prostate cancer bone metastasis microenvironment. Cancer Science, 109(3), 724–731.
[5]. Tahir Manzoor Lone, Nadeem, M., Khan, A., Umair, M., Hamid, M., & Masaba Masood. (2024). Neutropenia: Frequency and Management Outcomes in Women with Breast Cancer Receiving Anthracycline Based Chemotherapy. Pakistan Armed Forces Medical Journal, 74(4), 901-.
[6]. Ryan, C. J., Smith, M. R., Fizazi, K., Saad, F., Mulders, P. F. A., Sternberg, C. N., Miller, K., Logothetis, C. J., Shore, N. D., Small, E. J., Carles, J., Flaig, T. W., Taplin, M.-E., Higano, C. S., de Souza, P., de Bono, J. S., Griffin, T. W., De Porre, P., Yu, M. K., … Rathkopf, D. E. (2015). Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology, 16(2), 152–160.
[7]. Contreras, H. R., Lopez-Moncada, F., & Castellon, E. A. (2020). Cancer stem cell and mesenchymal cell cooperative actions in metastasis progression and hormone resistance in prostate cancer: Potential role of androgen and gonadotropin releasing hormone receptors (Review). International Journal of Oncology, 56(5), 1075–1082.
[8]. Saleem, M., Siddique, H. R., Ganju, R., Mishra, S. K., & Aburatani, H. (2012). Abstract 3917: Regulatory role of ROBO-1, a novel tumor suppressor on Androgen receptor and Wnt signaling during castration-resistant prostate cancer development: A novel molecular target for gene therapy. Cancer Research (Chicago, Ill.), 72(8_Supplement), 3917–3917.
[9]. Obinata, D., Takayama, K., Inoue, S., & Takahashi, S. (2024). Exploring androgen receptor sign19aling pathway in prostate cancer: A path to new discoveries. International Journal of Urology, 31(6), 590–597.
[10]. Wilson, E. M. (2010). Androgen receptor molecular biology and potential targets in prostate cancer. Therapeutic Advances in Urology, 2(3), 105–117.
[11]. Liu, C., Armstrong, C. M., Lou, W., Lombard, A. P., Cucchiara, V., Gu, X., Yang, J. C., Nadiminty, N., Pan, C.-X., Evans, C. P., & Gao, A. C. (2017). Niclosamide and Bicalutamide Combination Treatment Overcomes Enzalutamide- and Bicalutamide-Resistant Prostate Cancer. Molecular Cancer Therapeutics, 16(8), 1521–1530.
Cite this article
Wang,Z. (2024). Small-Molecule Drugs target Androgen Receptor (AR) signaling pathway for Prostatic Cancer Treatment. Theoretical and Natural Science,65,73-77.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2024 Workshop: Computational Proteomics in Drug Discovery and Development from Medicinal Plants
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Wang, L., Lu, B., He, M., Wang, Y., Wang, Z., & Du, L. (2022). Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. Frontiers in Public Health, 10, 811044–811044.
[2]. Hanash, K. A., Al-othaimeen, A., Kattan, S., Lindstedt, E., Al-zahrani, H., Merdad, T., Peracha, A., Kardar, A. H., Aslam, M., & Al-akkad, A. (2000). Prostatic Carcinoma: A Nutritional Disease? Conflicting Data From the Kingdom of Saudi Arabia. The Journal of Urology, 164(5), 1570–1572.
[3]. Fourcade, R. O., Benedict, Á., Black, L. K., Stokes, M. E., Alcaraz, A., & Castro, R. (2010). Treatment costs of prostate cancer in the first year after diagnosis: a short‐term cost of illness study for France, Germany, Italy, Spain and the UK. BJU International, 105(1), 49–56.
[4]. Urata, S., Izumi, K., Hiratsuka, K., Maolake, A., Natsagdorj, A., Shigehara, K., Iwamoto, H., Kadomoto, S., Makino, T., Naito, R., Kadono, Y., Lin, W., Wufuer, G., Narimoto, K., & Mizokami, A. (2018). C‐C motif ligand 5 promotes migration of prostate cancer cells in the prostate cancer bone metastasis microenvironment. Cancer Science, 109(3), 724–731.
[5]. Tahir Manzoor Lone, Nadeem, M., Khan, A., Umair, M., Hamid, M., & Masaba Masood. (2024). Neutropenia: Frequency and Management Outcomes in Women with Breast Cancer Receiving Anthracycline Based Chemotherapy. Pakistan Armed Forces Medical Journal, 74(4), 901-.
[6]. Ryan, C. J., Smith, M. R., Fizazi, K., Saad, F., Mulders, P. F. A., Sternberg, C. N., Miller, K., Logothetis, C. J., Shore, N. D., Small, E. J., Carles, J., Flaig, T. W., Taplin, M.-E., Higano, C. S., de Souza, P., de Bono, J. S., Griffin, T. W., De Porre, P., Yu, M. K., … Rathkopf, D. E. (2015). Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology, 16(2), 152–160.
[7]. Contreras, H. R., Lopez-Moncada, F., & Castellon, E. A. (2020). Cancer stem cell and mesenchymal cell cooperative actions in metastasis progression and hormone resistance in prostate cancer: Potential role of androgen and gonadotropin releasing hormone receptors (Review). International Journal of Oncology, 56(5), 1075–1082.
[8]. Saleem, M., Siddique, H. R., Ganju, R., Mishra, S. K., & Aburatani, H. (2012). Abstract 3917: Regulatory role of ROBO-1, a novel tumor suppressor on Androgen receptor and Wnt signaling during castration-resistant prostate cancer development: A novel molecular target for gene therapy. Cancer Research (Chicago, Ill.), 72(8_Supplement), 3917–3917.
[9]. Obinata, D., Takayama, K., Inoue, S., & Takahashi, S. (2024). Exploring androgen receptor sign19aling pathway in prostate cancer: A path to new discoveries. International Journal of Urology, 31(6), 590–597.
[10]. Wilson, E. M. (2010). Androgen receptor molecular biology and potential targets in prostate cancer. Therapeutic Advances in Urology, 2(3), 105–117.
[11]. Liu, C., Armstrong, C. M., Lou, W., Lombard, A. P., Cucchiara, V., Gu, X., Yang, J. C., Nadiminty, N., Pan, C.-X., Evans, C. P., & Gao, A. C. (2017). Niclosamide and Bicalutamide Combination Treatment Overcomes Enzalutamide- and Bicalutamide-Resistant Prostate Cancer. Molecular Cancer Therapeutics, 16(8), 1521–1530.









