1. Introduction
Inflammation is an important protective mechanism of the body against the invasion of foreign pathogens. Nonresolving inflammation is a major driver of disease. Perpetuation of inflammation is an inherent risk because inflammation can damage tissue and necrosis can provoke inflammation[1]. Current approaches to overcome the inflammation include the use of immune selective anti-inflammatory derivatives, selective glucocorticoid receptor agonist, resolvins and protectins and TNF inhibitors[2]. Tumor necrosis factor alpha (TNF-α) is a cytokine that has pleiotropic effects on various cell types. It has been identified as a major regulator of inflammatory responses and is known to be involved in the pathogenesis of some inflammatory and autoimmune diseases. TNF-α exists in a soluble and transmembrane form. One of the first forms of TNF-α that is made is called transmembrane TNF-α. This needs to be changed by TACE, a membrane-bound disintegrin metalloproteinase, so that the soluble form of TNF-α can be released. In general, sTNF-α binds to its receptors, mainly TNFR1 and TNFR2, and then transmits molecular signals for biological functions such as inflammation and cell death[3].ADAM17, also known as TNF-α converting enzyme or TACE, is now known to process over 80 different substrates. Many of these substrates are mediators of inflammation.ADAM17 plays a crucial role in the body's inflammatory responses and immune system by cleaving adhesion molecules expressed on vascular endothelial cells, leukocytes, and platelets, thereby affecting the adhesion, aggregation, and extravasation processes of leukocytes, as well as the formation of thrombi. It also regulates the initiation, progression, resolution, and chronicity of inflammatory responses by affecting cytokines, chemokines, and their receptors on leukocytes. Therefore, ADAM17 has established its position as a therapeutic target for inflammatory diseases[4].
2. The introduction of ADAM17
2.1. The structure of ADAM17
The protease ADAM17, also known as tumor necrosis factor a (TNF- a)-converting enzyme (TACE), is a type I transmembrane cell-surface metalloprotease that is widely expressed in various tissues and cell types[5-7]. The ADAM17 protein consists of a prodomain, a metalloproteinase domaina, catalytic domain, a disintegrin domain, a cysteine-rich membrane proximal domain, a single transmembrane domain and an intracellular cytoplasmic domain (Fig. 1). The prodomain consists of amino acid residues at positions 18 to 214, and its primary function is to inhibit the metalloproteinase activity of ADAM17 and prevent premature activation of ADAM17 in cells[8]. The metalloproteinase domain uses a Zn2+ ion for the catalysis that is coordinated to three histidines of the conserved binding motif HEXXHXXGXXH[9].The deintegrin domain facilitates interactions with other proteins.Proximal to the stalk region are a transmembrane domain, majorly involved in ADAM17 interaction with its essential regulators iRhom1 and 2, and an intracellular cytoplasmic domain whose physiological function is still unclear[10].
.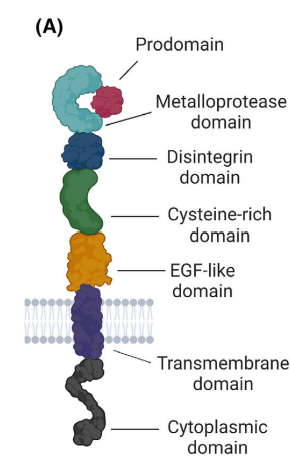
Figure 1: The structure of ADAM17 [11].
2.2. The function of ADAM17
The protease A Disintegrin And Metalloproteinase 17 (ADAM17) plays a central role in the pathophysiology of several diseases. ADAM17 is involved in the cleavage and shedding of at least 80 known membranetethered proteins, which subsequently modulate several intracellular signaling pathways, and therefore alter cell behavior. Dysregulated expression and/or activation of ADAM17 has been linked to a wide range of autoimmune and inflammatory diseases, cancer, and cardiovascular disease[11]. ADAM17 plays a decisive role in inflammation, as it can cleave and thereby activate cytokines and cytokine receptors. The most prominent examples are the cytokine Tumor Necrosis Factor α (TNFα) and the TNF receptors 1 and 2, which are all ADAM17 substrates, and the cytokine Interleukin-6 (IL-6), whose IL-6 receptor is also a substrate for ADAM17[12].The majority of the pro-inflammatory activities of TNF-α are attributed to its soluble form, for which it has to be proteolytically cleaved from the cell. This process is facilitated by the protease ADAM17, which was therefore initially named TACE (TNF-α converting enzyme) . High serum levels of TNF-α are found in severe inflammatory conditions like sepsis.
In addition, ADAM17 plays a role in tumor growth, angiogenesis, invasion, and metastasis, and is an important target for tumor research[13]. In addition, ADAM17 plays a role in tumor growth, angiogenesis, invasion, and metastasis, and is an important target for tumor research.
3. The application of ADAM17 in the process of inflammation
3.1. ADAM17 Substrates That Mediate Inflammatory Processes
Besides TNF-α and substrates involved in tumor immuno-surveillance, there are over 20 substrates for ADAM17 that are regulators of inflammation[4]. The paper will only discuss the several recently reported ADAM17 substrates here.
IL-1R2, or Interleukin-1 Receptor Type 2, is a protein that is part of the interleukin-1 (IL-1) family of cytokines. This is a receptor that binds to IL-1, specifically IL-1β. Unlike IL-1R1 (Interleukin-1 Receptor Type 1), it does not send signals and is known as a decoy receptor. This means that IL-1R2 can bind IL-1β and prevent it from interacting with the signaling receptor IL-1R1, thereby acting as a regulator to control the inflammatory response mediated by IL-1[14].Soluble IL-1R2, produced by ADAM17 processing, can therefore bind to IL-1 and possibly dampen the immune response.It indicatess that an ADAM17 inhibitor would be anti-inflammatory due to increasing receptor levels of IL1-R2 on the cell surface.
Neogenin is a regulator of inflammatory processes. Acute inflammation is dampened when neogenin is endogenously repressed. Studies using functional inhibition of neogenin resulted in a significant attenuation of inflammatory peritonitis [15]. Neogenin also promotes pulmonary inflammation during lung injury, and its inhibition reduces hepatic ischemia-reperfusion injury.
ADAM17 is capable of cleaving IL-6R, which is a primary mediator of both the classic and trans-signaling pathways of IL-6. In the classic signaling pathway of IL-6, IL-6 binds to the membrane-bound IL-6R and promotes the resolution of inflammation by activating STAT1 and STAT3. By cutting the extracellular domain of IL-6R, ADAM17 turns on the trans-signaling pathway of IL-6. This is linked to the development of many inflammatory diseases, such as rheumatoid arthritis[16].
3.2. The role of ADAM17 in inflammatory disorder
3.2.1. Role of ADAM17 in Emphysema and COPD
Also known as TACE (TNF-α converting enzyme), ADAM17 (a disintegrin and metalloprotease-17) is a membrane-anchored proteinase that is ubiquitously expressed in human lung tissue and its expression is upregulated in lung diseases including asthma, COPD, and endotoxin-induced acute lung injury. Aberrant activation of ADAM17 influences several features of emphysema pathology, including pulmonary inflammation, cell proliferation, and epithelial barrier function. ADAM17 plays a significant role in the activated shedding of EGFR ligands (TGF‐α, amphiregulin, epiregulin, HB‐EGF) and cleaves membrane‐bound TNF-α, IL6R, TNF‐R, NOTCH receptors, L‐selectin, ICAM‐1, and E‐cadherin . This is important in emphysema as elevated levels of IL6R are observed in peripheral blood leukocytes of patients with COPD , and its genetic variants are linked with COPD severity . In addition, ADAM17 is reported to further activate membrane responses depending on its phosphorylation status[17-18].
3.2.2. Role of ADAM17 in Lung neutrophilic inflammation
ADAM17 plays an important role in lung neutrophilic inflammation, involving several aspects such as macrophage proliferation, regulation of the inflammatory response, neutrophil phenotype conversion, L-selectin shedding, and prevention of emphysema.Macrophages are prominent cells in acute and chronic inflammatory diseases. Using an acute peritonitis model, Tang et, al. identify a significant defect in macrophage proliferation in mice lacking the leukocyte transmembrane protease ADAM17. The defect is associated with decreased levels of macrophage colony-stimulating factor 1 (CSF-1) in the peritoneum and is rescued by intraperitoneal injection of CSF-1. Cell surface CSF-1 (csCSF-1) is one of the substrates of ADAM17. Their results demonstrate a novel mechanism whereby ADAM17 promotes macrophage proliferation in states of acute and chronic inflammation[19]. Dreymueller et al. Found ADAM17 promotes endothelial and epithelial permeability of smooth muscle and epithelial cells, transendothelial leukocyte migration, and production of inflammatory mediators[19] .
3.2.3. ADAM17 in Arthritis
TNF-α is a pro-inflammatory cytokine that is critical to the pathogenesis of Arthritis.ADAM17 can cleave the membrane-bound TNF-α precursor, releasing soluble TNF-α, which is one of its key roles in RA (Rheumatoid Arthritis)[21].IL-6 is another pro-inflammatory cytokine associated with RA. ADAM17 is also involved in the cleavage of the IL-6 receptor (IL-6R), which is a primary mediator of the IL-6 signaling pathway.ADAM17 modifies cell-cell and cell-matrix interactions, such as adhesion molecules L-selectin, ICAM, VCAM, etc., to adjust the speed at which immune cells enter the site of inflammatory response. At the same time, it controls the progress of the inflammatory response by regulating the secretion levels of soluble inflammatory factors like TNFα and IFN-γ. ADAM17 might be able to help treat RA because it impacts many processes, including the release of cytokines that cause inflammation, the control of inflammatory responses at different levels, and the management of immune cell functions.
4. Challenges and future trends
This review provides a detailed introduction to the structural features of ADAM17 and its interaction with various ligands, including epidermal growth factor receptor ligands, pro-inflammatory cytokines, adhesion molecules, and pro-amyloid precursor proteins. Additionally, this article summarizes the role of ADAM17 in the initiation and progression of various inflammatory diseases. Therefore, ADAM17 is an important target for the treatment of inflammation and many other inflammation-induced or inflammation-related diseases. Therefore, the development of selective ADAM17 inhibitors is very necessary. Currently, existing small molecule inhibitors of ADAM17 have shown poor selectivity, skeletal muscle toxicity, hepatotoxicity, lack of efficacy, and no antibodies have entered clinical trials. A contributing factor to the lack of progress of ADAM17 inhibitors in the clinic has been the adverse side-effects and toxicities that accompanied the use of early generation small molecular weight chemical inhibitors of ADAM17, which lacked specificity by targeting other proteases (e.g., ADAM10) . However, these earlier studies have since triggered the advent of a new generation of highly specific antiADAM17 antibodies and the ADAM17 prodomain inhibitor that show potent efficacy in preclinical disease models, with the promise to further develop and refine for future clinical implementation .
5. Conclusion
This review provides a detailed introduction to the structural features of ADAM17 and its interaction with various ligands, including epidermal growth factor receptor ligands, pro-inflammatory cytokines, adhesion molecules, and pro-amyloid precursor proteins. Additionally, this article summarizes the role of ADAM17 in the initiation and progression of various inflammatory diseases. Therefore, ADAM17 is an important target for the treatment of inflammation and many other inflammation-induced or inflammation-related diseases. This review mainly introduces the relationship between ADAM17 and inflammation, but ADAM17 also plays an important role in the occurrence and development of many malignant tumors. A substantial number of preclinical studies have indicated that ADAM17 inhibitors can suppress the growth of some malignant tumors, but they have been halted at Phase 2 clinical trials due to reasons such as hepatotoxicity. The specific mechanisms by which ADAM17 treats diseases such as inflammation and cancer still need to be further explored and refined. Even though many possible ADAM17 substrates have been found, it is still hard to make therapeutic ADAM17 inhibitors because there are still a lot of mechanisms to learn and figure out. The basic mechanisms of ADAM17 in tissue homeostasis or developmental processes are somewhat understood, but its role in pathological mechanisms appears to be increasingly complex. The challenge lies in identifying the molecular bridges that can connect these mechanisms to gain a comprehensive perspective. More research should be done in the future on the substrates of ADAM17 and how they work in cancer and inflammation, especially those substrates that have to do with tumor immune surveillance, drug and radiation resistance, heart hypertrophy, and inflammatory bowel disease. Further development of more selective ADAM17 inhibitors is needed to reduce side effects and toxicity, especially hepatotoxicity.
References
[1]. Nathan C, Ding A. Nonresolving inflammation. Cell. 2010 Mar 19;140(6):871-82. doi: 10.1016/j.cell.2010.02.029.
[2]. Dhingra AK, Chopra B, Dass R, Mittal SK. An update on Anti-inflammatory Compounds: A Review. Antiinflamm Antiallergy AgentsMedChem.2015;14(2):81-97.doi:10.2174/1871523014666150514102027.
[3]. Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021 Mar 8;22(5):2719. doi: 10.3390/ijms22052719.
[4]. Moss, M. L. and D. Minond (2017). "Recent Advances in ADAM17 Research: A Promising Target for Cancer and Inflammation." Mediators of Inflammation 2017.
[5]. Saad MI, Rose-John S & Jenkins BJ (2019) ADAM17: an emerging therapeutic target for lung cancer. Cancers 11, 1218.
[6]. Saad MI, McLeod L, Hodges C, Vlahos R, Rose-John S, Ruwanpura S & Jenkins BJ (2021) ADAM17 deficiency protects against pulmonary emphysema. Am J Respir Cell Mol Biol 64, 183–195.
[7]. Gooz M (2010) ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol 45, 146–169.
[8]. Grötzinger J, Lorenzen I, Düsterhöft S. Molecular insights into the multilayered regulation of ADAM17: The role of the extracellular region [J]. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 2017, 1864(11) : 2088-2095.
[9]. Calligaris M, Cuffaro D, Bonelli S, Spanò DP, Rossello A, Nuti E, Scilabra SD. Strategies to Target ADAM17 in Disease: From its Discovery to the iRhom Revolution. Molecules. 2021 Feb 10;26(4):944. doi: 10.3390/molecules26040944.
[10]. Zunke, F.; Rose-John, S. The shedding protease ADAM17: Physiology and pathophysiology. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1864, 2059–2070.
[11]. Saad MI, Jenkins BJ. The protease ADAM17 at the crossroads of disease: revisiting its significance in inflammation, cancer, and beyond. FEBS J. 2024 Jan;291(1):10-24. doi: 10.1111/febs.16923.
[12]. Düsterhöft S, Lokau J, Garbers C. The metalloprotease ADAM17 in inflammation and cancer. Pathol Res Pract. 2019 Jun;215(6):152410. doi: 10.1016/j.prp.2019.04.002.
[13]. G.D. Kalliolias, L.B. Ivashkiv, TNF biology, pathogenic mechanisms and emerging therapeutic strategies, Nat. Rev. Rheumatol. 12 (1) (2016) 49–62
[14]. Vanessa A. Peters, Jennifer J. et, al. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain, Behavior, and Immunity. 2013.32:1-8
[15]. Schlegel M, Körner A, Kaussen T, et al. Inhibition of neogenin fosters resolution of inflammation and tissue regeneration. J Clin Invest. 2018 . 128(10):4711-4726.
[16]. Yan I, Schwarz J, Lücke K, et, al. ADAM17 controls IL-6 signaling by cleavage of the murine IL-6Rα from the cell surface of leukocytes during inflammatory responses. J Leukoc Biol. 2016 May;99(5):749-60.
[17]. Saad MI, McLeod L, Hodges C, et, al. ADAM17 deficiency protects against pulmonary emphysema. Am J Respir Cell Mol Biol. 2021. 64:183–195.
[18]. Jundi B, Geraghty P. ADAM17: A Therapeutic Target for Patients with Emphysema? Am J Respir Cell Mol Biol. 2021. 64(2):155-157.
[19]. Tang J, Frey JM, Wilson CL, et, al. Neutrophil and Macrophage Cell Surface Colony-Stimulating Factor 1 Shed by ADAM17 Drives Mouse Macrophage Proliferation in Acute and Chronic Inflammation. Mol Cell Biol. 2018. 38(17):e00103-18.
[20]. Dreymueller D, Uhlig S, Ludwig A. ADAM-family metalloproteinases in lung inflammation: potential therapeutic targets. Am J Physiol Lung Cell Mol Physiol. 2015. 308(4):L325-43.
[21]. Wong E, Cohen T, Romi E et, al. Harnessing the natural inhibitory domain to control TNFa converting enzyme (TACE) activity in vivo. Sci Rep 2016. 6: 1–12.
Cite this article
Peng,G. (2025). Research on the Role of ADAM17 in the Development of Inflammation. Theoretical and Natural Science,82,102-107.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Nathan C, Ding A. Nonresolving inflammation. Cell. 2010 Mar 19;140(6):871-82. doi: 10.1016/j.cell.2010.02.029.
[2]. Dhingra AK, Chopra B, Dass R, Mittal SK. An update on Anti-inflammatory Compounds: A Review. Antiinflamm Antiallergy AgentsMedChem.2015;14(2):81-97.doi:10.2174/1871523014666150514102027.
[3]. Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021 Mar 8;22(5):2719. doi: 10.3390/ijms22052719.
[4]. Moss, M. L. and D. Minond (2017). "Recent Advances in ADAM17 Research: A Promising Target for Cancer and Inflammation." Mediators of Inflammation 2017.
[5]. Saad MI, Rose-John S & Jenkins BJ (2019) ADAM17: an emerging therapeutic target for lung cancer. Cancers 11, 1218.
[6]. Saad MI, McLeod L, Hodges C, Vlahos R, Rose-John S, Ruwanpura S & Jenkins BJ (2021) ADAM17 deficiency protects against pulmonary emphysema. Am J Respir Cell Mol Biol 64, 183–195.
[7]. Gooz M (2010) ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol 45, 146–169.
[8]. Grötzinger J, Lorenzen I, Düsterhöft S. Molecular insights into the multilayered regulation of ADAM17: The role of the extracellular region [J]. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 2017, 1864(11) : 2088-2095.
[9]. Calligaris M, Cuffaro D, Bonelli S, Spanò DP, Rossello A, Nuti E, Scilabra SD. Strategies to Target ADAM17 in Disease: From its Discovery to the iRhom Revolution. Molecules. 2021 Feb 10;26(4):944. doi: 10.3390/molecules26040944.
[10]. Zunke, F.; Rose-John, S. The shedding protease ADAM17: Physiology and pathophysiology. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1864, 2059–2070.
[11]. Saad MI, Jenkins BJ. The protease ADAM17 at the crossroads of disease: revisiting its significance in inflammation, cancer, and beyond. FEBS J. 2024 Jan;291(1):10-24. doi: 10.1111/febs.16923.
[12]. Düsterhöft S, Lokau J, Garbers C. The metalloprotease ADAM17 in inflammation and cancer. Pathol Res Pract. 2019 Jun;215(6):152410. doi: 10.1016/j.prp.2019.04.002.
[13]. G.D. Kalliolias, L.B. Ivashkiv, TNF biology, pathogenic mechanisms and emerging therapeutic strategies, Nat. Rev. Rheumatol. 12 (1) (2016) 49–62
[14]. Vanessa A. Peters, Jennifer J. et, al. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain, Behavior, and Immunity. 2013.32:1-8
[15]. Schlegel M, Körner A, Kaussen T, et al. Inhibition of neogenin fosters resolution of inflammation and tissue regeneration. J Clin Invest. 2018 . 128(10):4711-4726.
[16]. Yan I, Schwarz J, Lücke K, et, al. ADAM17 controls IL-6 signaling by cleavage of the murine IL-6Rα from the cell surface of leukocytes during inflammatory responses. J Leukoc Biol. 2016 May;99(5):749-60.
[17]. Saad MI, McLeod L, Hodges C, et, al. ADAM17 deficiency protects against pulmonary emphysema. Am J Respir Cell Mol Biol. 2021. 64:183–195.
[18]. Jundi B, Geraghty P. ADAM17: A Therapeutic Target for Patients with Emphysema? Am J Respir Cell Mol Biol. 2021. 64(2):155-157.
[19]. Tang J, Frey JM, Wilson CL, et, al. Neutrophil and Macrophage Cell Surface Colony-Stimulating Factor 1 Shed by ADAM17 Drives Mouse Macrophage Proliferation in Acute and Chronic Inflammation. Mol Cell Biol. 2018. 38(17):e00103-18.
[20]. Dreymueller D, Uhlig S, Ludwig A. ADAM-family metalloproteinases in lung inflammation: potential therapeutic targets. Am J Physiol Lung Cell Mol Physiol. 2015. 308(4):L325-43.
[21]. Wong E, Cohen T, Romi E et, al. Harnessing the natural inhibitory domain to control TNFa converting enzyme (TACE) activity in vivo. Sci Rep 2016. 6: 1–12.









