1. Introduction
Diabetes is a chronic metabolic disorder caused by endocrine system dysfunction, leading to abnormal glucose levels. Its symptoms were first documented as early as 1500 BCE in ancient Egypt. However, it was not until 1812 that the medical community gained preliminary scientific understanding of the disease, when the first related paper was published in the New England Journal of Medicine and Surgery. Today, diabetes has become the third most life-threatening non-communicable disease globally, following only cancer and cardiovascular diseases [1]. Optimal glycemic control in patients with diabetes mellitus necessitates rigorous blood glucose monitoring. Contemporary clinical practice employs multiple modalities for glucose assessment, including capillary blood glucose monitoring via self-monitoring of blood glucose (SMBG), continuous glucose monitoring (CGM), as well as biochemical markers such as glycated hemoglobin (HbA1c) and glycated albumin (GA) (Figure 1). Notably, the Chinese Guidelines for the Prevention and Treatment of Diabetes (2024 Edition) formally recognizes SMBG as an essential component of comprehensive diabetes management and patient education, establishing it as the gold-standard method for routine glycemic surveillance in diabetic populations [2]. However, constrained by its “point-in-time” measurement paradigm, SMBG fails to capture glycemic excursions occurring nocturnally, postprandially, or during physical activity, thereby presenting critical limitations in glycemic visibility and patient compliance. In contrast, continuous glucose monitoring (CGM) systems serve as either a valuable adjunct or potential replacement for SMBG, offering dynamic glycemic profiling rather than isolated measurements while simultaneously mitigating the discomfort associated with capillary blood sampling.
CGM systems operate by quantifying glucose concentrations in interstitial fluid (ISF), which are subsequently converted to equivalent blood glucose values, thereby enabling automated monitoring of diurnal glycemic variations. This technology provides comprehensive glycemic data that elucidates patterns of glucose fluctuation, while offering distinct advantages including enhanced patient comfort, detection of previously unmonitored glycemic excursions, and compatibility with insulin pumps for closed-loop artificial pancreas systems. These superior capabilities have established CGM as a rapidly evolving next-generation monitoring technology in diabetes management [3].
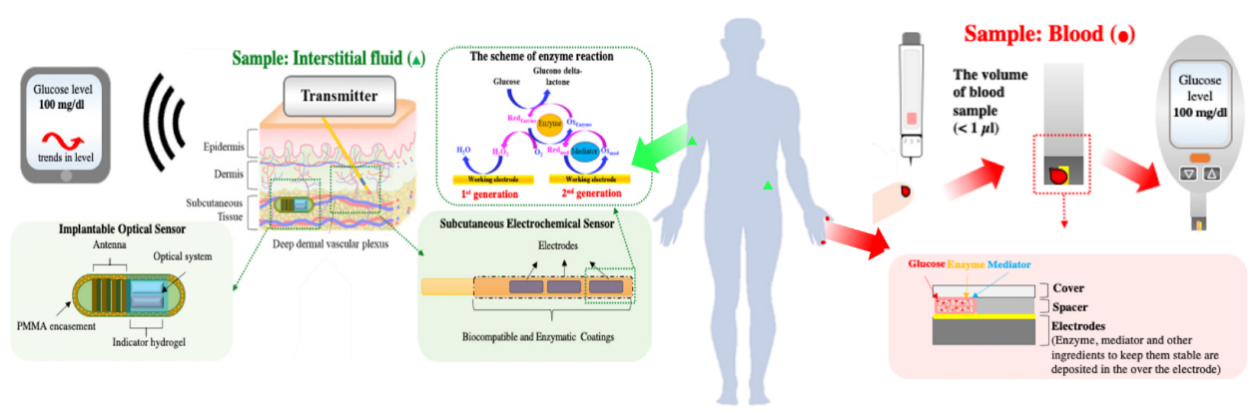
2. Technical principles and developmental trajectory of CGM systems
2.1. Technical principles of CGM systems
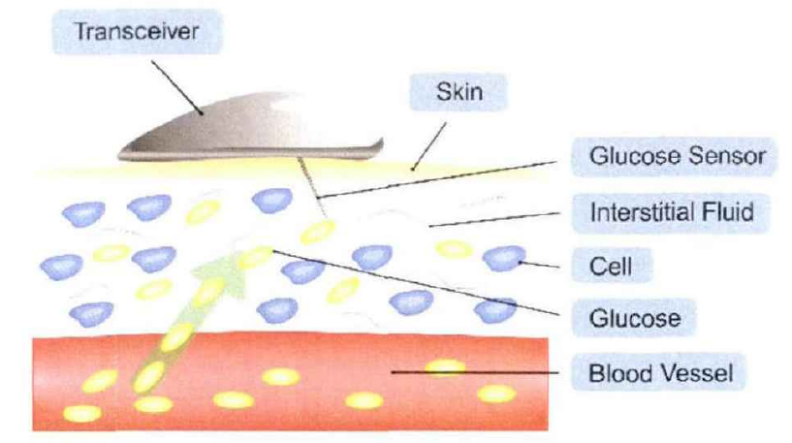
The Continuous Glucose Monitoring (CGM) system primarily consists of three components: a disposable subcutaneous microsensor, a portable external data receiver, and application software [4]. As illustrated in Figures 2 and 3, the system operates by utilizing a sensor implanted in the subcutaneous tissue to measure the glucose concentration in interstitial fluid, which is subsequently converted into blood glucose values. These data are transmitted via Bluetooth, wireless local area network (WLAN), or other connectivity protocols to the receiver. With technological advancements, the data can also be directly transmitted to application software on smartphones, computers, or cloud-based platforms.

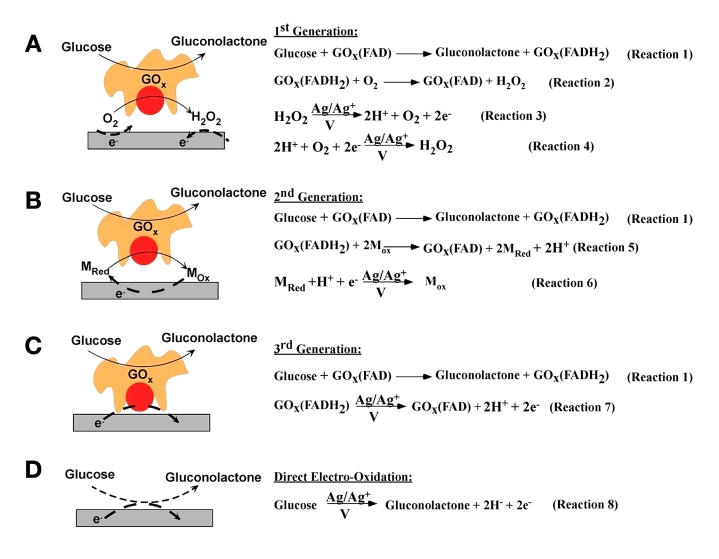
The continuous glucose monitoring (CGM) system represents a significant technological advancement in diabetes management, with its core innovation residing in the electrochemical glucose sensor architecture. As depicted in Figure 4, these sensors can be systematically classified into four distinct generations based on their electron transfer mechanisms during the glucose oxidation process [5]. Initially, the principle proposed by Clark and Lyons (1962) of utilizing glucose oxidase (GOx) and oxygen as the electron acceptor for the glucose oxidation reaction was recognized as the foundational concept of first-generation glucose sensors [6]. This principle also served as the fundamental basis for the first-generation handheld glucose sensing systems that enabled self-monitoring of blood glucose (SMBG) [7] Second-generation sensors addressed these limitations through the introduction of artificial redox mediators, creating an oxygen-independent electron transfer pathway. This advancement significantly improved operational stability and enabled system miniaturization through reduced sample volume requirements and intermittent sampling protocols [8]. At present, most commercial continuous glucose monitoring (CGM) systems are equipped with electrochemical glucose sensors that use the first or second generation sensing principles [9].Theoretical advancements have led to the conceptualization of third-generation sensors employing direct electron transfer (DET) mechanisms between enzyme and electrode [10]. While promising superior sensitivity and eliminating mediator-related complications, practical implementation faces significant challenges in maintaining consistent enzyme orientation and signal stability. Similarly, fourth-generation enzyme-free sensors, though theoretically capable of extended operational lifetimes, encounter substantial barriers in manufacturing precision and in vivo performance validation [3]. Notably, while academic research continues to explore third- and fourth-generation sensor technologies, commercial CGM systems remain constrained to first- and second-generation implementations due to their proven reliability and manufacturability.
2.2. Development of CGM systems
Commercial CGM systems can be primarily categorized into three types based on their technical characteristics: retrospective CGM (Retrospective CGM), real-time CGM (rtCGM), and intermittently scanned CGM (isCGM). These systems differ significantly in terms of data acquisition methods, functional features, and target populations (Table 1) [12].
2.2.1. Retrospective CGM
Retrospective CGM monitors glucose concentrations in interstitial fluid via a subcutaneously implanted glucose sensor but does not display the wearer’s glucose levels in real time. Data analysis is only possible after downloading the recorded information post-monitoring, enabling the evaluation of glycemic trends and patterns. However, this system lacks real-time hypoglycemia or hyperglycemia alert functionality and exhibits inherent data latency.The first CGM system approved by the FDA in 1999—the Medtronic MiniMed—operated in retrospective mode with a limited lifespan of only three days [10].Due to its delayed data feedback, it was primarily used in hospital settings, allowing physicians to assess long-term glycemic variability, detect asymptomatic hyperglycemia/hypoglycemia (e.g., nocturnal hypoglycemia), and optimize treatment regimens. This makes it suitable for patients who do not require immediate insulin dosage adjustments.
|
Category |
Principle |
Characteristics |
Applicable population |
Representative products |
|
Retrospective CGM |
After the device collects data, it is stored. Users need to download the data for analysis after the monitoring period ends. |
Real-time blood glucose data cannot be provided. It is only used for doctor's diagnosis or retrospective analysis of patients. |
Patients who do not need to adjust the insulin dose immediately. |
Medtronic MiniMed |
|
Real-time CGM (rtCGM) |
The sensor continuously monitors blood sugar and transmits data in real time to the receiving device (such as a mobile phone or insulin pump), which can provide alerts for high and low blood sugar. |
It provides real-time blood glucose data, is suitable for all-day blood glucose management, and can be combined with insulin pumps to form a closed-loop system. |
Patients with type 1 diabetes or type 2 diabetes who require intensive insulin therapy |
Dexcom G7、Medtronic Guardian Connect |
|
intermittently scanned CGM (isCGM) |
The sensor continuously monitors blood sugar but does not automatically push data. Users need to manually scan and read it. |
There is no need for frequent fingertip blood sampling. Some versions support high and low blood sugar alerts. |
Patients with type 2 diabetes and limited budgets. |
Abbott FreeStyle Libre 2/3 |
|
Other CGM |
Glucose indicator hydrogels based on fluorescence and diboric acid and a micro optical detection system are used to measure blood glucose. The data is transmitted to a mobile application via an intelligent transmitter and requires regular calibration. |
Based on optical principles, surgical implantable devices require an external emitter and have an extremely long service life and high accuracy. |
- |
Senseonics Eversense |
2.2.2. Real-time CGM (rtcgm)
Real-time CGM (rtCGM) also monitors interstitial glucose levels via a subcutaneous sensor but differs from retrospective CGM by wirelessly transmitting glucose readings, trend directions, and rate of change to a receiver or smartphone in real time. Additionally, it features proactive alerts to warn users of impending hypoglycemia or hyperglycemia.By eliminating the data delay inherent in retrospective CGM, rtCGM enables at-home glucose monitoring and self-management. Most commercial CGM systems, such as the Dexcom G7 and Medtronic Guardian Connect, utilize real-time monitoring, offering extended wear durations (up to 7 days) and glucose updates every 1-5 minutes. These systems also include customizable high/low glucose alarms, reducing the risk of acute complications. As such, rtCGM is particularly suitable for patients with type 1 diabetes or insulin-dependent type 2 diabetes.
2.2.3. Intermittently scanned CGM (iscgm)
Intermittently scanned CGM (isCGM), pioneered by Abbott’s FreeStyle Libre, employs an on-demand scanning mechanism. Unlike rtCGM, it does not require fingerstick calibration and continuously measures glucose levels, but data are only accessible when the user actively scans the sensor with a reader or smartphone. However, its effectiveness depends on scan frequency and patient compliance.The latest FreeStyle Libre 3 (2020) features a 70% smaller form factor, extended wear time (14-30 days), improved accuracy, and optional glucose alarms. Due to its affordability, isCGM is widely adopted by patients with type 2 diabetes and those with budget constraints.
2.2.4. Other CGM systems
While most CGM systems fall into the three categories above,Senseonics Eversense [13], approved by the FDA in 2018, represents a distinct fully implantable CGM system.Unlike conventional CGMs, it utilizes a fluorescent, boronic acid-based hydrogel sensor paired with a miniature optical detection system to measure glucose levels. Data are transmitted via an external smart transmitter to a mobile app, though periodic calibration is required.The Senseonics Eversense series has gained recognition for its exceptional longevity and high accuracy. The latest Eversense 365 (2024) boasts an unprecedented 365-day wear time.However, its adoption is limited by the need for surgical implantation, an external transmitter, and a complex usage protocol, raising concerns among some users.
3. Core reporting metrics and evaluation framework for CGM systems
3.1. Core reporting metrics of CGM systems
The core reporting metrics of CGM systems can be categorized into three primary groups (Table 2):Glycemic Control Metrics- These classical indicators reflect the average level of glycemic control, including:Time in Range (TIR),Time Above Range (TAR),Time Below Range (TBR),Glucose Management Indicator (GMI),Mean Glucose,Glycemic Variability (GV) Metrics,Ambulatory Glucose Profile (AGP) Parameters.The GV metrics and AGP parameters specifically characterize glucose fluctuations, representing a unique advantage of CGM systems in assessing dynamic glycemic patterns [2].
|
Core indicators for blood sugar control |
|||||||
|
Index |
Definition and calculation |
Clinical significance |
Target values |
||||
|
TIR |
The proportion of time when the glucose value is within the target range (typically 3.9-10.0 mmol/L) |
It is strongly correlated with the risk of diabetic complications. For every 10% increase in TIR, the microvascular risk decreases by 40% |
General population: >70% Elderly/High-risk: >50% |
||||
|
TAR |
The proportion of time when the glucose value was >10.0 mmol/L (which can be divided into two levels: TAR1:10.0-13.9 mmol/L; TAR2:>13.9 |
It reflects postprandial hyperglycemia or insufficient insulin. Long-term elevation increases the risk of macrovascular disease |
TAR1 <25% TAR2 <5% |
||||
|
TBR |
The proportion of time with glucose values <3.9 mmol/L (which can be divided into two levels: TBR1:3.0-3.9 mmol/L;) TBR2:<3.0 |
Hypoglycemic events are associated with cognitive impairment and cardiovascular events, with particular attention to TBR2 (severe hypoglycemia). |
TBR1 <4% TBR2 <1% |
||||
|
GMI |
Equivalent value of HbA1c estimated based on average glucose value (formula: GMI=3.31+0.02392× average blood glucose) |
For predicting the long-term blood glucose control level, a deviation of less than 0.5% from the laboratory HbA1c is considered reliable |
Individualized setting (generally <7.0%) |
||||
|
Average glucose value |
The arithmetic mean of all glucose values during the monitoring period |
It directly reflects the overall blood glucose level, but the volatility needs to be evaluated in combination with TIR/TBR |
Individualized target (generally <8.3 mmol/L) |
||||
|
Index |
Definition and calculation |
Clinical significance |
||||||
|
Standard Deviation (SD) |
The standard deviation of blood glucose levels reflects the degree of dispersion |
When SD <1/3, the average blood glucose value is stable. SD>1/2 indicates high-risk fluctuations |
||||||
|
MAGE (Mean Amplitude of Blood Glucose Fluctuation) |
Calculate the average value of the peak-valley difference of intraday blood glucose fluctuations (only count fluctuations greater than 1SD) |
MAGE>3.9 mmol/L significantly increases the risk of oxidative stress and vascular endothelial injury |
||||||
|
CONGA (Continuous Overlapping Net blood glucose Effect) |
Calculate the continuous fluctuations of blood sugar changes at fixed time intervals (such as 1 hour) |
Evaluate postprandial blood glucose drift and the timeliness of drug effects |
3.2. Evaluation framework of CGM systems
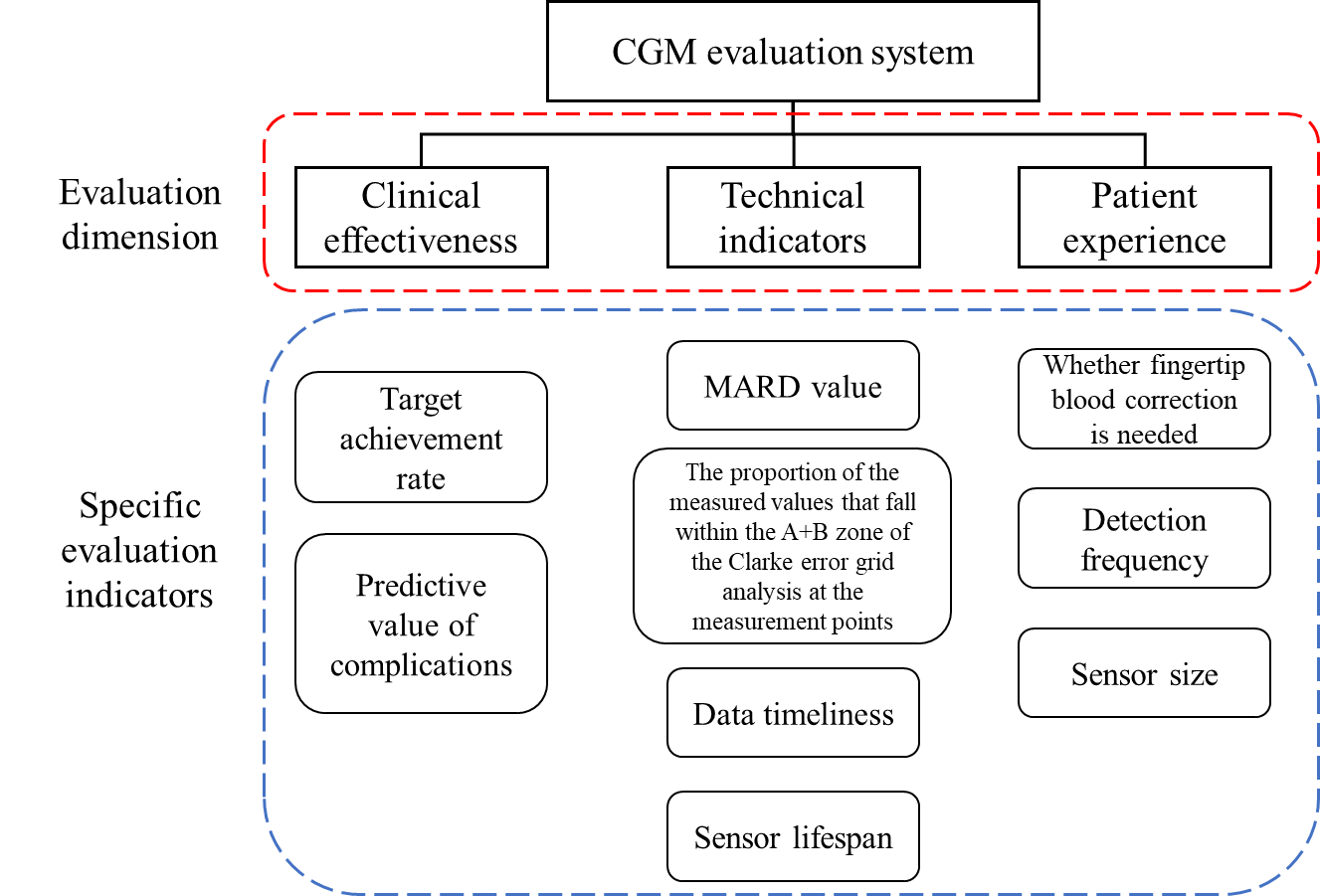
The evaluation framework of CGM systems primarily assesses three key dimensions: clinical efficacy, technical performance, and patient experience (Figure 5).First,clinical efficacy serves as the critical criterion for determining the practical utility of a CGM system, evaluated through core target achievement rate and predictive value for complications.Second,technical performance constitutes the core parameters for system development and optimization, with key challenges reflected in the following metrics:MARD (Mean Absolute Relative Difference),Proportion of measurements falling within Zones A+B in Clarke Error Grid Analysis,Data timeliness,Sensor lifespan.Lastly,patient experiencefocuses on comfort and usability, influenced by factors such as:Need for fingerstick calibration,Receiver design,Sensor size,Monitoring frequency,Measurable glucose range,Biocompatibility.This structured evaluation ensures a comprehensive assessment of CGM systems from both clinical and user-centric perspectives [3].
4. Clinical applications of CGM systems
4.1. Application of CGM systems in type 1 diabetes (T1DM)
Type 1 diabetes mellitus (T1DM) is a metabolic disorder characterized by hyperglycemia resulting from autoimmune destruction of pancreatic β-cells, leading to insulin deficiency. It predominantly affects children, adolescents, and perimenopausal individuals, with rapid onset, high glycemic variability, and an increased risk of diabetic ketoacidosis (DKA). Lifelong insulin therapy is required, but it often leads to significant glucose fluctuations and hypoglycemia [14]. According to the 2025 Standards of Medical Care in Diabetes by the American Diabetes Association (ADA) and the 25th National Academic Conference of the Chinese Diabetes Society (CDS 2023) [15], CGM systems are strongly recommended for T1DM patients, particularly those with frequent hypoglycemia. Beyond real-time glucose monitoring, integrating CGM with insulin pumps optimizes insulin dosing, improves HbA1c, and significantly increases Time in Range (TIR). More importantly, CGM helps prevent hypoglycemic episodes, especially nocturnal hypoglycemia, reducing acute complications [16].
4.2. Application of CGM systems in type 2 diabetes (T2DM)
Type 2 diabetes mellitus (T2DM) is a chronic condition primarily driven by insulin resistance and progressive β-cell dysfunction. It commonly occurs in adults due to genetic and environmental factors (e.g., sedentary lifestyle, overnutrition). Early symptoms are often subtle, leading to delayed diagnosis until complications arise. However, T2DM can be managed through lifestyle and pharmacological interventions [17]. Both ADA 2025and CDS 2023recommend CGM for T2DM patients on multiple daily insulin injections [15]. For those on oral medications,intermittent CGMcan assess the immediate impact of diet and exercise, serving as a behavioral and therapeutic adjustment tool to enhance self-management and adherence [18].
4.3. Application of CGM systems in other diabetes subtypes
Gestational Diabetes Mellitus (GDM) is a transient condition triggered by pregnancy-related hormones (e.g., placental lactogen), inducing insulin resistance and hyperglycemia during mid-to-late gestation. GDM increases maternal and fetal complications, though glucose levels typically normalize postpartum. However, affected women face a higher future risk of T2DM [2]. Guidelines from the UK National Institute for Health and Care Excellence (NICE) and the Chinese Perinatal Medicine/Diabetes Society Consensus endorse CGM for GDM screening and management, enabling early intervention to improve maternal-fetal safety and reduce adverse pregnancy outcomes.
Additionally, CGM is beneficial for other diabetes subtypes, including: Monogenic diabetes (e.g., MODY), Secondary diabetes (e.g., pancreatic disease-induced), Drug-induced diabetes, Post-pancreatic transplant diabetes. These conditions exhibit high heterogeneity and require personalized management [2]. Both ADA and CDS advocate CGM use in these populations to facilitate precision medicine.
4.4. Application of CGM systems in hypoglycemia management
Compared to hyperglycemia, hypoglycemia poses more immediate health risks, yet it is often overlooked in clinical practice. As one of the most common acute complications of diabetes, hypoglycemia can occur in any diabetic patient, potentially leading to coma, fractures, arrhythmias, or even death [13]. CGM systems update glucose readings every 3–5 minutes, providing continuous trend data to detect hypoglycemia (<3.9 mmol/L) in real time. Advanced devices (e.g.,Abbott FreeStyle Libre, Sinocare H6 series) feature hypoglycemia alarms (auditory/vibratory alerts), enabling prompt intervention—particularly critical for patients with hypoglycemia unawareness.
5. Future directions and challenges of CGM systems
5.1. Multi-parameter integrated devices
One of the future trends in CGM system development is multi-parameter integration, which combines key metabolic indicators such as blood glucose, blood pressure, and lipids to achieve more comprehensive physiological monitoring. Leveraging multi-sensor fusion technology, such systems will provide more precise data support for the management of diabetes and related metabolic disorders. However, this field currently faces numerous technical challenges, including improving sensor accuracy, optimizing data integration, and controlling device energy consumption. Therefore, future research should focus on cutting-edge technologies such as flexible electronics, biosensors, and microfluidic chips to enhance measurement accuracy, user comfort, and long-term stability, thereby advancing the clinical application of multi-parameter monitoring systems [19].
5.2. Ai-driven personalized medicine
Artificial intelligence (AI)-driven personalized medicine represents another critical direction in CGM technology development. Its core lies in constructing precise metabolic risk prediction models through big data analytics and machine learning techniques [20]. Based on long-term glucose monitoring data, AI can provide personalized health intervention strategies, including optimized insulin dosing, dietary planning, and exercise regimens. However, challenges such as inconsistent data quality, privacy protection, and clinical feasibility remain. Thus, future research must further develop technologies such as federated learning, digital twins, and multi-modal data fusion to improve the robustness and generalizability of AI models, enabling more accurate, secure, and individualized diabetes management [21].
5.3. Artificial pancreas
Artificial pancreas technology embodies the ultimate goal of CGM systems—achieving fully automated glucose regulation through real-time CGM, intelligent algorithms, and closed-loop insulin delivery systems, thereby reducing patient burde [22]. Recent advancements in dual-hormone closed-loop systems, adaptive AI control, and remote monitoring have significantly enhanced the precision and stability of artificial pancreas systems. Nevertheless, existing systems still face technical bottlenecks in device miniaturization, battery life, and precise insulin delivery. Consequently, future research should prioritize key areas such as advanced materials, energy management, and intelligent control algorithms to improve the portability, durability, and autonomous adaptability of artificial pancreas systems, thereby driving further intelligence and automation in diabetes management [23].
6. Conclusion
With continuous advancements in glucose management and monitoring technologies,continuous glucose monitoring (CGM) systems have evolved as a critical tool in diabetes care, progressing from retrospective monitoring to real-time and flash monitoring. Their technical principles have matured, and their applications have expanded significantly.
Compared to traditional fingerstick blood glucose monitoring, CGM systems measure glucose concentrations in interstitial fluid, providing continuous, dynamic glucose profiles that enable more sensitive detection of glycemic fluctuations. By integrating core reporting metrics with a comprehensive evaluation framework, CGM systems offer more precise glycemic assessment for clinical decision-making. They are now widely used in the management of type 1 diabetes, type 2 diabetes, gestational diabetes, and patients at risk of hypoglycemia, significantly improving glycemic control and quality of life.
Looking ahead, CGM systems are advancing toward multi-functional integration and AI-driven intelligence, with the potential to achieve fully automated, closed-loop glucose control, optimize personalized treatment strategies, and support comprehensive chronic disease management. Despite current challenges—such as sensor stability, user adherence, cost, and data interpretation—CGM systems are poised to play an increasingly vital role in precision diabetes management and lifelong health interventions.
References
[1]. Polonsky, K.S.. The past 200 years in diabetes [J]. The New England Journal of Medicine , 2012 , 367(14): 1332-1340.
[2]. Diabetes Society of Chinese Medical Association Chinese Guidelines for the Prevention and Treatment of Diabetes (2024 Edition) [J]. Chinese Journal of Diabetes, 2025, 17(01): 16-139.
[3]. Lee I, Probst D, Klonoff D, et al. Continuous glucose monitoring systems-Current status and future perspectives of the flagship technologies in biosensor research [J]. Biosensors and Bioelectronics, 2021, 181: 113054.
[4]. Singh L G, Levitt D L, SatyarenggaATYARENGGA M, et al. Continuous Glucose Monitoring in General Wards for Prevention of Hypoglycemia: Results From the Glucose Telemetry System Pilot Study [J]. Journal of Diabetes Science and Technology, 2020, 14(4): 783-790.
[5]. Chen Wei. Research on Subcutaneous Implantable Microsensor Technology for Continuous Glucose Monitoring [D]. Zhejiang University, 2018.
[6]. Updike S J, Hicks G P. The enzyme electrode [J]. Nature, 1967, 214(5092): 986-988.
[7]. JR L C C. Membrane polarographic electrode system and method with electrochemical compensation: US3539455A [P]. 1970-11-10 [2025-05-09].
[8]. Wang J. Electrochemical Glucose Biosensors [J]. Chemical Reviews, 2008, 108(2): 814-825.
[9]. Yamada S. Historical achievements of self-monitoring of blood glucose technology development in Japan [J]. Journal of Diabetes Science and Technology, 2011, 5(5): 1300-1306.
[10]. Yamashita Y, Lee I, Loew N, et al. Direct electron transfer (DET) mechanism of FAD dependent dehydrogenase complexes ∼from the elucidation of intra- and inter-molecular electron transfer pathway to the construction of engineered DET enzyme complexes∼ [J]. Current Opinion in Electrochemistry, 2018, 12: 92-100.
[11]. Vaddiraju S, Burgess D J, Tomazos I, et al. Technologies for continuous glucose monitoring: current problems and future promises [J]. Journal of Diabetes Science and Technology, 2010, 4(6): 1540-1562.
[12]. Wang Chaoping, Yuan Xiaoli, Wang Min, et al. Research progress on the clinical application of remote continuous glucose monitoring [J]. Modern Clinical Nursing, 2023, 22(11): 82-88.
[13]. HEALTH C for D and R. Eversense E3 Continuous Glucose Monitoring (CGM) System – P160048/S021 [J/OL]. FDA, 2023 [2025-05-09].
[14]. Diabetes Society of Chinese Medical Association Diagnosis and Treatment Guidelines for Type 1 Diabetes mellitus in China (2021 Edition) [J]. Chinese Journal of Diabetes, 2022, 14(11): 1143-1250.
[15]. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025 | Diabetes Care | American Diabetes Association [EB/OL]. [2025-05-09].
[16]. Lu Wudan, Liu Junxiang. Application of Continuous Glucose Monitoring System in Evaluating Islet Function of type 1 Diabetes Mellitus [J]. New World of Diabetes, 2024, 27(20): 184-187, 198.
[17]. Diabetes Society of Chinese Medical Association Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes Mellitus (2020 Edition) [J]. Chinese Journal of Diabetes, 2021, 13(04): 315-409.
[18]. Ping Yanru, Jia Zhumin, Huang Jiarong, et al. The application progress and prospect of continuous glucose monitoring in patients with type 2 diabetes mellitus [J]. Chinese Clinical Research, 2024, 37(7): 1008-1012.
[19]. Turksoy K, Hajizadeh I, Hobbs N, et al. Multivariable Artificial Pancreas for Various Exercise Types and Intensities [J]. Diabetes Technology & Therapeutics, 2018, 20(10): 662-671.
[20]. Priyadarshini R G, Narayanan S. Analysis of blood glucose monitoring – a review on recent advancements and future prospects [J]. Multimedia Tools and Applications, 2024, 83(20): 58375-58419.
[21]. Villena G W, Mobashsher A T, Abbosh A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors [J/OL]. Sensors, 2019, 19(4): 800.
[22]. Li Xiaolei, Qi Xiaoling, Xu Liwu, et al. Observation on the Therapeutic Effect of Continuous Glucose Monitoring System Combined with Insulin Pump in the Treatment of Newly diagnosed Type 2 Diabetes Patients [J]. Chinese Journal of Diabetes, 2016, 24(3): 219-222.
[23]. Bergenstal R M, Klonoff D C, Garg S K, et al. Threshold-Based Insulin-Pump Interruption for Reduction of Hypoglycemia [J/OL]. New England Journal of Medicine, 2013, 369(3): 224-232.
Cite this article
Xu,J. (2025). Technological Innovations and Clinical Implementation of Continuous Glucose Monitoring (CGM) Systems. Theoretical and Natural Science,114,16-26.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Extended Reality (XR) Applications in Medical Imaging
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Polonsky, K.S.. The past 200 years in diabetes [J]. The New England Journal of Medicine , 2012 , 367(14): 1332-1340.
[2]. Diabetes Society of Chinese Medical Association Chinese Guidelines for the Prevention and Treatment of Diabetes (2024 Edition) [J]. Chinese Journal of Diabetes, 2025, 17(01): 16-139.
[3]. Lee I, Probst D, Klonoff D, et al. Continuous glucose monitoring systems-Current status and future perspectives of the flagship technologies in biosensor research [J]. Biosensors and Bioelectronics, 2021, 181: 113054.
[4]. Singh L G, Levitt D L, SatyarenggaATYARENGGA M, et al. Continuous Glucose Monitoring in General Wards for Prevention of Hypoglycemia: Results From the Glucose Telemetry System Pilot Study [J]. Journal of Diabetes Science and Technology, 2020, 14(4): 783-790.
[5]. Chen Wei. Research on Subcutaneous Implantable Microsensor Technology for Continuous Glucose Monitoring [D]. Zhejiang University, 2018.
[6]. Updike S J, Hicks G P. The enzyme electrode [J]. Nature, 1967, 214(5092): 986-988.
[7]. JR L C C. Membrane polarographic electrode system and method with electrochemical compensation: US3539455A [P]. 1970-11-10 [2025-05-09].
[8]. Wang J. Electrochemical Glucose Biosensors [J]. Chemical Reviews, 2008, 108(2): 814-825.
[9]. Yamada S. Historical achievements of self-monitoring of blood glucose technology development in Japan [J]. Journal of Diabetes Science and Technology, 2011, 5(5): 1300-1306.
[10]. Yamashita Y, Lee I, Loew N, et al. Direct electron transfer (DET) mechanism of FAD dependent dehydrogenase complexes ∼from the elucidation of intra- and inter-molecular electron transfer pathway to the construction of engineered DET enzyme complexes∼ [J]. Current Opinion in Electrochemistry, 2018, 12: 92-100.
[11]. Vaddiraju S, Burgess D J, Tomazos I, et al. Technologies for continuous glucose monitoring: current problems and future promises [J]. Journal of Diabetes Science and Technology, 2010, 4(6): 1540-1562.
[12]. Wang Chaoping, Yuan Xiaoli, Wang Min, et al. Research progress on the clinical application of remote continuous glucose monitoring [J]. Modern Clinical Nursing, 2023, 22(11): 82-88.
[13]. HEALTH C for D and R. Eversense E3 Continuous Glucose Monitoring (CGM) System – P160048/S021 [J/OL]. FDA, 2023 [2025-05-09].
[14]. Diabetes Society of Chinese Medical Association Diagnosis and Treatment Guidelines for Type 1 Diabetes mellitus in China (2021 Edition) [J]. Chinese Journal of Diabetes, 2022, 14(11): 1143-1250.
[15]. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025 | Diabetes Care | American Diabetes Association [EB/OL]. [2025-05-09].
[16]. Lu Wudan, Liu Junxiang. Application of Continuous Glucose Monitoring System in Evaluating Islet Function of type 1 Diabetes Mellitus [J]. New World of Diabetes, 2024, 27(20): 184-187, 198.
[17]. Diabetes Society of Chinese Medical Association Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes Mellitus (2020 Edition) [J]. Chinese Journal of Diabetes, 2021, 13(04): 315-409.
[18]. Ping Yanru, Jia Zhumin, Huang Jiarong, et al. The application progress and prospect of continuous glucose monitoring in patients with type 2 diabetes mellitus [J]. Chinese Clinical Research, 2024, 37(7): 1008-1012.
[19]. Turksoy K, Hajizadeh I, Hobbs N, et al. Multivariable Artificial Pancreas for Various Exercise Types and Intensities [J]. Diabetes Technology & Therapeutics, 2018, 20(10): 662-671.
[20]. Priyadarshini R G, Narayanan S. Analysis of blood glucose monitoring – a review on recent advancements and future prospects [J]. Multimedia Tools and Applications, 2024, 83(20): 58375-58419.
[21]. Villena G W, Mobashsher A T, Abbosh A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors [J/OL]. Sensors, 2019, 19(4): 800.
[22]. Li Xiaolei, Qi Xiaoling, Xu Liwu, et al. Observation on the Therapeutic Effect of Continuous Glucose Monitoring System Combined with Insulin Pump in the Treatment of Newly diagnosed Type 2 Diabetes Patients [J]. Chinese Journal of Diabetes, 2016, 24(3): 219-222.
[23]. Bergenstal R M, Klonoff D C, Garg S K, et al. Threshold-Based Insulin-Pump Interruption for Reduction of Hypoglycemia [J/OL]. New England Journal of Medicine, 2013, 369(3): 224-232.









