1. Introduction
Photoredox chemistry relies on the ability of metal complexes or organic dyes to convert light energy into chemical energy by engaging in single-electron transfer. During this redox process, the catalyst enters the excited state and becomes the solid oxidizing agent that generates high-energy intermediates.1 Photoredox catalyst can increase the reaction rate while not being consumed. They are reduced and then re-oxidized in photoredox reactions and, therefore, are frequently applied as oxidizing agent in organic synthesis. Photoredox catalysts are known for their different capabilities of developing new reaction mechanisms and enabling the construction of new bonds or intermediates that were previously challenging to make. For example, the generation of alkyl radicals in the hydroxymethylation of heteroaromatic bases is made possible under room temperature by photoredox catalysis2. The Nicewicz lab utilizes photoredox chemistry to catalyze the biosynthesis of cyclobutane lignans, which are lead compounds for developing antifungal, antiviral, and anticancer drugs.3 The development and analysis of photoredox catalysts is essential because it opens doors to new possibilities for synthesizing novel and complex compounds while minimizing wastes of energy and resources. Organic photoredox dyes are vital as they are cheaper, more environmentally friendly, more readily available, and non-toxic than other metal catalysts.
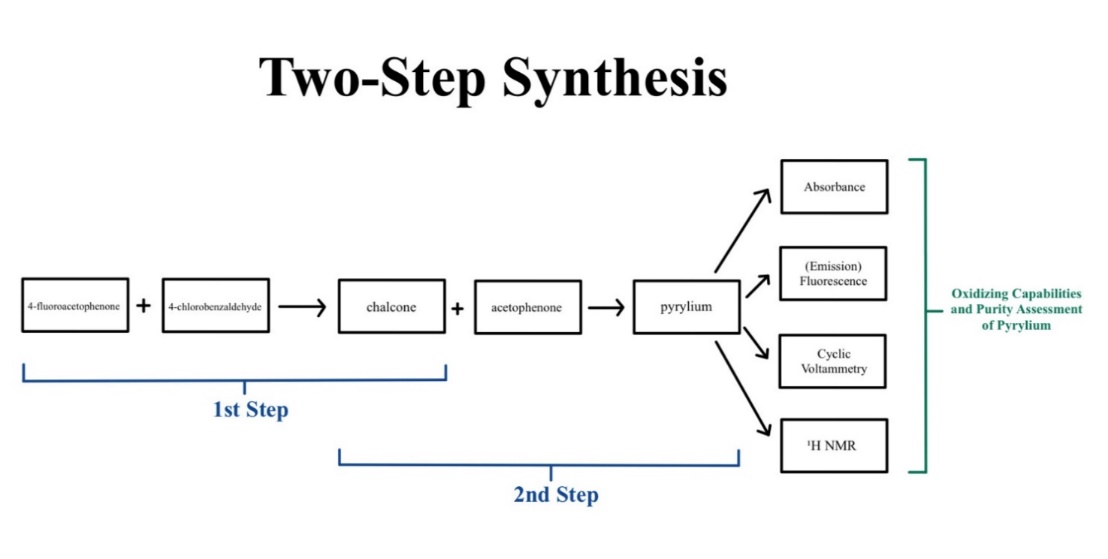
Figure 1. Overall two-step synthesis and assessments of Pyrydium.
In this experiment, pyrylium salt, a type of photoredox catalyst, is generated using a two-step synthesis to allow for different substituents in the salt. The absorbance and emission profiles and its excited state properties (with cyclic voltammetry) will be characterized to assess its capabilities as an oxidizing agent, and its reactivity in an alcohol oxidation reaction will be measured.
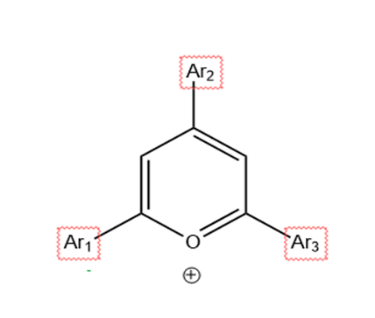
Figure 2. Generic Pyrylium structure.
2. Methods
A 1:1 (20 mmol) ratio of 4-fluoroacetophenone (2.763 g) and 4-chlorobenzaldehyde (2.800 g) were mixed with 21 mL NaOH to produce 4-chloro-4’-fluorochalcone by adding 6.4 mL of 4M NaOH dropwise, placing in an ice bath, and filtering the solution using suction filtration with 10 mL distilled water and cold EtOH. Then, 5.6 mmol of this chalcone (1.460 g) and 5.6 mmol of acetophenone (0.649 g) were mixed with 0.5 mL H2SO4 under heat for 45 minutes to produce 4-(4-chlorophenyl)-2-(4-fluorophenyl)-6-phenylpyrylium. The solution was then purified using suction filtration with 2 rinses of 10 mL cold EtOAc and crystal recrystallization with methanol.
An 1H NMR spectrum was gotten for the chalcone and the products of the alcohol oxidation reaction. In addition, a UV-Vis spectrum, emission spectrum, and cyclic voltammetry trace were also obtained for the pyrylium.
To test the oxidation ability of the pyrylium, 0.0125 mmol (0.00452 g) of pyrylium and 0.25 mmol (17 mg) of LiNO3¬¬ were mixed with 0.25 mmol (30 μL) of 1-phenylethanol and 1 mL deuterated acetonitrile. The solution was then placed in a light box for 90 minutes, and 0.47 mmol (10 μL) HMDSO internal standard was added. 1H NMR was then taken of the product.
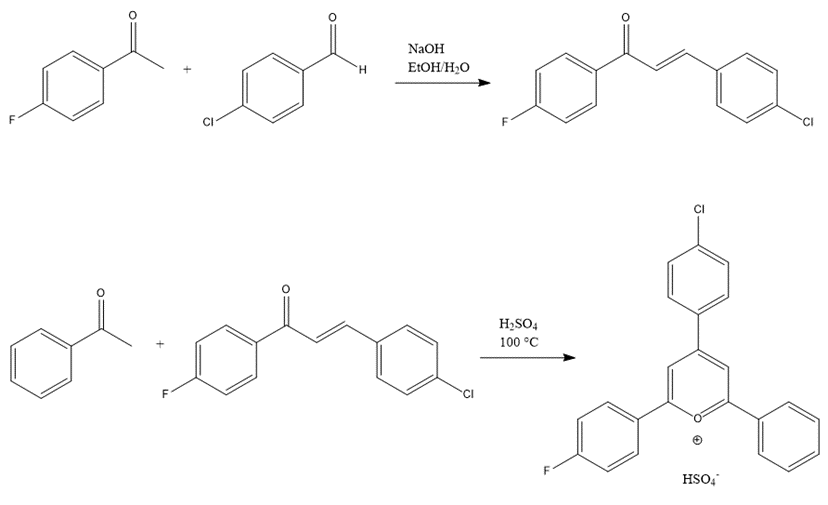
Figure 3. Two synthesis reaction steps and products.
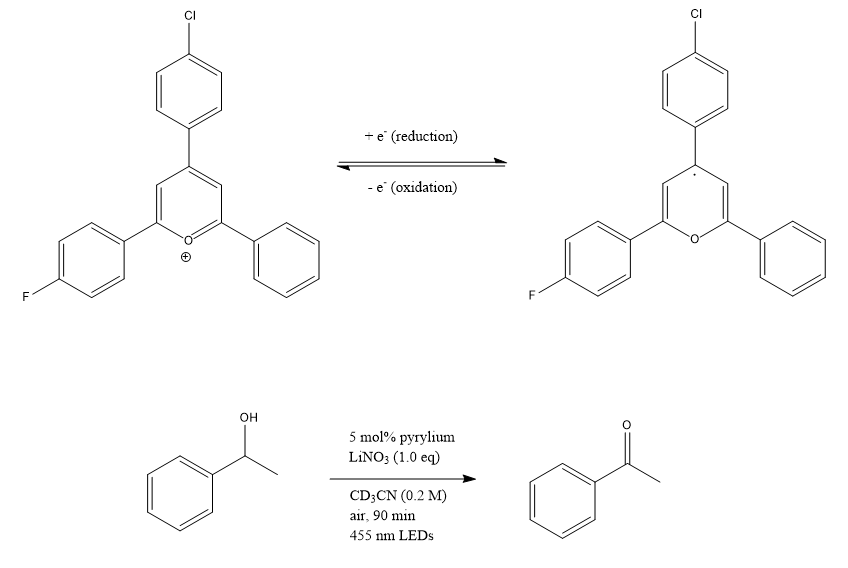
Figure 4. Oxidation reaction.
3. Results and discussion
The pyrylium salt synthesis in this experiment has two steps: the first step of chalcone synthesis includes 4-fluorocetophenone and 4-chlorobenzaldehyde as starting materials; the second step is the reaction of the chalcone product with acetophenone. For the pryrylium salt to be a potent oxidizing agent, the more electron deficient it is, the bigger its tendency to receive electrons during photoredox reactions. Therefore, the substituents selected in this pyrylium synthesis are electron withdrawing groups, such as fluoro and chloro, which have large electronegativity values. In addition to the type of substituents, their location on the benzene rings is also selected to be 1,4 instead of 1,2 to minimize the negative effects of hindrance on reactions. This experiment included an elevated amount of starting materials, leading to a higher pyrylium product for subsequent analyses. The crude pyrylium salt product weighed 0.525 g. The purified product, 2-fluorophenyl-4-chlorophenyl-6-phenylpyrylium, weighed 0.200 g (5.53 mmol, 10% yield; 38% recovery from crude)4.
Theoretical yield (g)=(0.0054 mol acetophenone) × (1/1) × 361.82 g/mol = 1.954 g mmol product = 0.200g / (361.82g/mol) = 0.000553 mol = 5.53 mmol
\( \% yield = (0.200 g) / (1.954 g) × 100\% = 10\% \)
\( \% recovery = (0.200 g) / (0.525 g) × 100\% = 38 \% \)
The UV-Vis spectrum of the synthesized pyrylium salt has a maximum absorbance peak of 0.4. at the approximate wavelength of 320 nm (λmaxabs). This wavelength value is not within the visible light region as expected for pyrylium salt, indicating the absence of pure product yielded or a low concentration of pyrylium in the measured sample. The low absorbance wavelength signifies a low conjugation and, therefore, poor oxidizing ability of the product due to impurity or low yield. Therefore, a slightly more concentrated pyrylium sample should be made for the UV-Vis measurement, and in order to improve the purity, the recrystallization step should have been repeated.
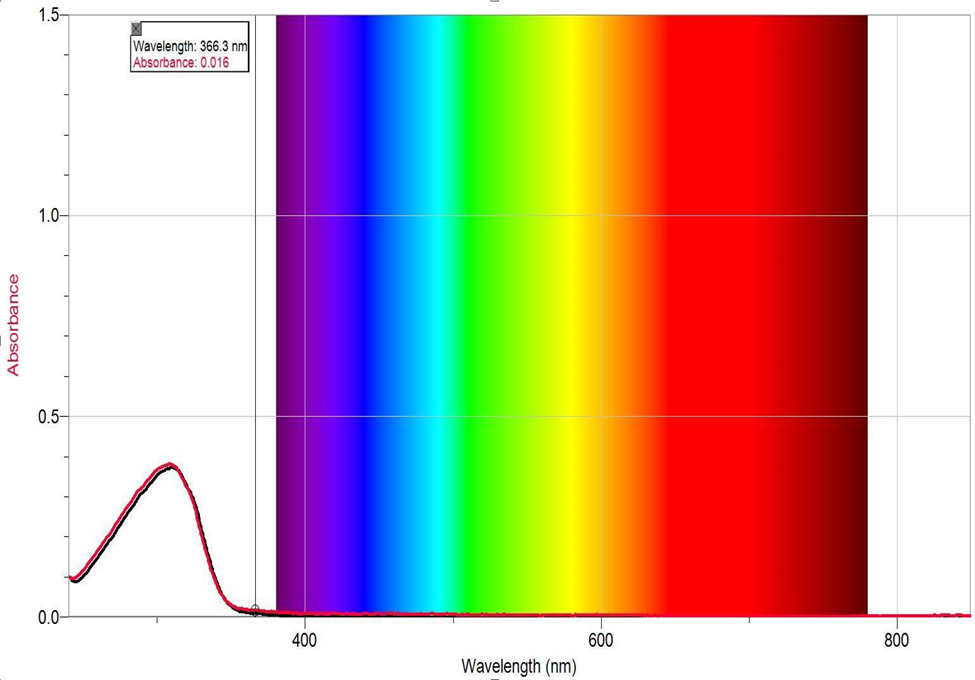
Figure 5. UV-VIS spectrum of Pyrylium.
The fluorescence spectrum of the synthesized pyrylium salt has a maximum emission peak at the approximate wavelength of 380 nm (λmaxem). As expected for pyrylium salt, this emission wavelength is slightly lower, indicating impurity and low concentration. Since the sample used for fluorescence is the same as that used to acquire the UV-Vis spectrum (Figure. 1), the improvement methods discussed for UV-Vis can also apply here.
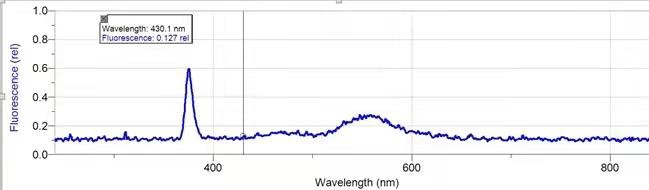
Figure 6. Fluorescence spectrum of Pyrylium.
The cyclic voltammetry trace of synthesized pyrylium salt has a ground state reduction potential of -0.5732 V (E1/2).
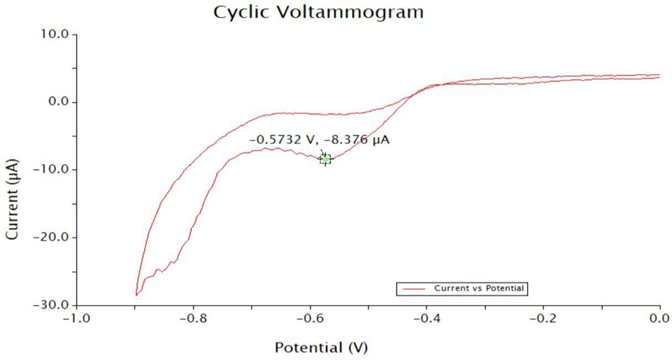
Figure 7. Cyclic voltammetry of Pyrylium.
The pyrylium salt’s excited state energy, E0,0, is calculated using its maximum absorption wavelength (320 nm) determined from the UV-Vis spectrum (Figure. 1) and its maximum emission wavelength (380 nm) determined from the fluorescence spectrum (Figure. 2). The ground state reduction potential, E1/2, is determined from the CV trace (Figure. 3). The corresponding excited state reduction potential, E*1/2, is calculated using both E0,0 and E1/2, and is used to quantify the ability of electron transferring of pryrlyum as an oxidizing agent.5
Sample calculation for pyrylium salt synthesized in this experiment:
\( E0,0 = (1230 Vxnm)/((λ abs+ λ em)/2)=(1230 Vxnm)/((320 nm+380 nm)/2)=3.514 V \)
\( E*1/2 = E1/2 + E0.0 = -573.2 mV + 3.514 V = -0.5732 V + 3.514 V = 2.941 V \)
The absorption absorbance, the emission absorbance, and the potential reduction values are determined for the following six pyrylium salts (A1 to A3, B1 to B3) and for the pyrylium salt synthesized in this experiment. The structure of 2-fluorophenyl-4-chlorophenyl-6-phenylpyrylium is shown using ChemDraw.
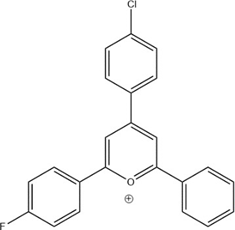
Figure 8. Novel Pyrylium product.
The 1H NMR diagram of the reaction mixture after the alcohol oxidation reaction is obtained at 60 MHz in HMDSO.
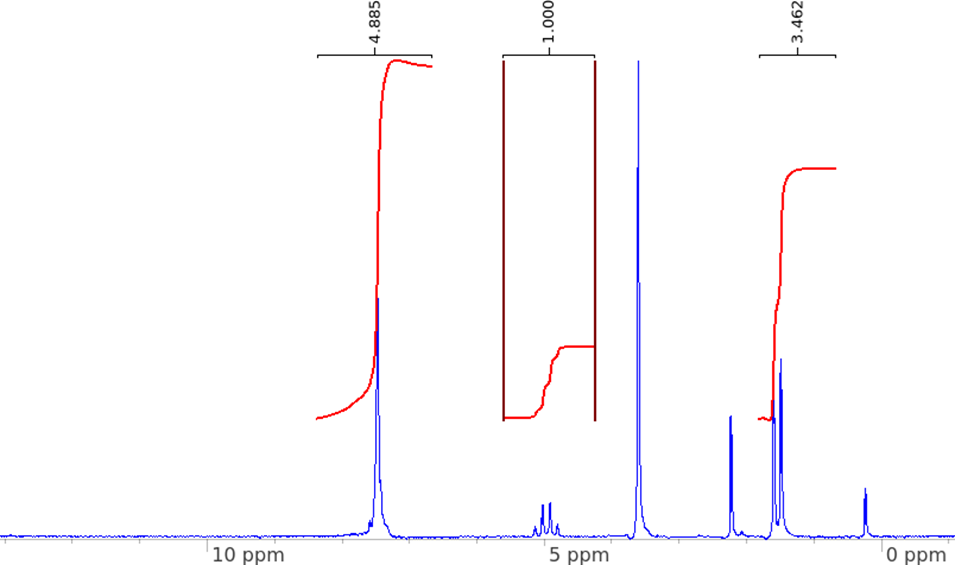
Figure 9. 1H NMR of post-alcohol oxidation mixture.
To assess the efficacy of the synthesized pyrylium, it is used as a photoredox catalyst in the oxidation of 1-phenylethanol to acetophenone. However, the resulted NMR diagram does not show any yield of the product acetophenone, because the 3Hs on acetophenone's methyl group should have a singlet shown at 2.60 ppm, according to the literature.6 However, this NMR shows a singlet at the aromatic region, a multiplet at five ppm, and a doublet at the methyl region, indicating the presence of the starting material, 1-phenylethanol. The reaction mixture should have been heated for more time. It is also possible that the pyrylium catalyst solution is not effective enough due to impurities, which are also observed in the NMR. More recrystallizations or other purification methods could have been applied to purify the pyrylium. The pyrylium synthesis and the alcohol oxidation reaction could be repeated more times to reduce uncertainties caused by systematic errors, such as inaccurate reading, loss of chemicals during transferring or heating, and contaminations of experimental instruments.
4. Conclusion
Overall, the results of this project were not very successful. The percent yield of chalcone was not very high, with a 28.2% yield. Also, although pyrylium was generated, it was very little, with only a 10% yield. Additionally, although the percent recovery was higher (38%), it was still not very high. The UV-Vis and fluorescence spectra indicated that this value was likely to be lower as they showed the presence of impurities.
Furthermore, the oxidation of the alcohol could not be completed due to low amounts of pyrylium and the short reaction time, resulting in 0% yield of acetophenone. Although the results of these chemical reactions were insignificant, some things can be learned from the synthesized pyrylium. The pyrylium synthesized in this project had lower conjugation and oxidizing activity based on the spectra; however, its E*1/2 was relatively high, suggesting that it might be a more potent oxidizing agent. These contrasting results most likely came from the skewed analysis due to impurities and the low yield of pyrylium. Broadly, pyrylium salts with EWGs should result in a more potent oxidizing agent as these have high electronegativity values and make the center of the pyrylium more unstable.
Since barely any pyrylium was generated and no alcohol was oxidized, future experiments would include changes to synthesize more pyrylium and acetophenone. This can include increasing the reagents for the synthesis of pyrylium, repeating the recrystallization step for better purification, and heating the pyrylium and the alcohol more during the alcohol oxidation step. Taking these changes would allow for successful oxidation and, therefore, a better analysis of the pyrylium regarding how substituents change its properties and efficacy. Successful analysis and oxidation would then provide this pyrylium as an alternative to metal catalysts used in oxidation reactions, as the substituents were halogens, which are EWGs.
References
[1]. Department of Chemistry of University of North Carolina at Chapel Hill. Chemistry 262L Laboratory in Organic Chemistry; Hayden McNeil, Spring/Summer 2022, pp 22.
[2]. Huff, C. A.; Cohen, R. D.; Dykstra, K. D.; Streckfuss, E.; DiRocco, D. A.; Krska, S. W. Photoredox-Catalyzed Hydroxymethylation of Heteroaromatic Bases. The Journal of Organic Chemistry 2016, 81 (16), 6980–6987.
[3]. Riener, M.; Nicewicz, D. A. Synthesis of Cyclobutane Lignans via an Organic Single Electron Oxidant–Electron Relay System. Chemical Science 2013, 4 (6), 2625.
[4]. Padías, A. B. Making the Connections: A How -To Guide for Organic Chemistry Lab Techniques; Hayden McNeil, 2007; pp 90.
[5]. Department of Chemistry of University of North Carolina at Chapel Hill. Chemistry 262L Laboratory in Organic Chemistry; Hayden McNeil, Spring/Summer 2022, pp 21-27.
[6]. Acetophenone. SDBSWeb. National Institute of Advanced Industrial Science and Technology. https://sdbs.db.aist.go.jp (accessed 2022-03-25)
Cite this article
Wu,J. (2023). Two-step synthesis and oxidizing power assessment of novel pyrylium. Theoretical and Natural Science,6,1-7.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the International Conference on Modern Medicine and Global Health (ICMMGH 2023)
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Department of Chemistry of University of North Carolina at Chapel Hill. Chemistry 262L Laboratory in Organic Chemistry; Hayden McNeil, Spring/Summer 2022, pp 22.
[2]. Huff, C. A.; Cohen, R. D.; Dykstra, K. D.; Streckfuss, E.; DiRocco, D. A.; Krska, S. W. Photoredox-Catalyzed Hydroxymethylation of Heteroaromatic Bases. The Journal of Organic Chemistry 2016, 81 (16), 6980–6987.
[3]. Riener, M.; Nicewicz, D. A. Synthesis of Cyclobutane Lignans via an Organic Single Electron Oxidant–Electron Relay System. Chemical Science 2013, 4 (6), 2625.
[4]. Padías, A. B. Making the Connections: A How -To Guide for Organic Chemistry Lab Techniques; Hayden McNeil, 2007; pp 90.
[5]. Department of Chemistry of University of North Carolina at Chapel Hill. Chemistry 262L Laboratory in Organic Chemistry; Hayden McNeil, Spring/Summer 2022, pp 21-27.
[6]. Acetophenone. SDBSWeb. National Institute of Advanced Industrial Science and Technology. https://sdbs.db.aist.go.jp (accessed 2022-03-25)









