1. Introduction
Adipose tissue heterogeneity represents a critical determinant of metabolic health, with visceral adipose tissue (VAT) independently predicting insulin resistance, dyslipidemia, and non-alcoholic fatty liver disease. The pathogenic role of VAT contrasts with subcutaneous adipose tissue (SAT), where preserved thermogenic capacity may confer protective effects. Traditional weight management strategies demonstrate limited efficacy in selectively targeting VAT depots while maintaining sustainable outcomes. Intermittent fasting (IF) regimens—particularly time-restricted eating (TRE) and alternate-day fasting (ADF)—have gained prominence for their potential to modulate adipose biology through circadian metabolic switching.
Current research has established depot-specific adaptations to fasting: VAT develops lipid conservation phenotypes via PLIN1 upregulation and lipolysis suppression, whereas SAT exhibits beige remodeling mediated by microbiota-derived butyrate and UCP1 induction. The gut-liver axis further coordinates systemic effects through intestinal barrier enhancement and hepatic metabolic reprogramming. Despite these advances, significant knowledge gaps persist regarding anatomical patterns of fat mobilization, long-term depot-specific efficacy, and predictors of cardiometabolic trade-offs across heterogeneous populations. Crucially, no consensus exists on precision frameworks for stratifying IF protocols based on adipose biology.
This review addresses these gaps through comprehensive analysis of molecular mechanisms and clinical evidence. Primary objectives include: 1) elucidating lipid droplet dynamics and mitochondrial adaptations underlying VAT/SAT differential responses, 2) evaluating spatiotemporal efficacy of major IF regimens across adipose depots, and 3) establishing a visceral fat ratio (VFR)-based stratification system for personalized implementation. The analytical approach integrates proteomic data, clinical trial evidence, and cardiometabolic safety profiles to develop mechanism-informed decision pathways.
Key findings demonstrate that early TRE (eTRE) sustains VAT reduction through cortisol rhythm synchronization, achieving superior visceral fat loss (-18.7 cm²) with stable glycemic control. In contrast, ADF shows paradoxical effects—significant triglyceride reduction (-16.2 mg/dL) but elevated LDL-C (+8.5 mg/dL) and late-phase visceral rebound. Synergistic interventions prove essential: aerobic exercise amplifies SAT thermogenesis via irisin-FNDC5 signaling, while behavioral support improves protocol adherence by 35%. The resultant precision framework utilizes VFR thresholds (30%) to guide intervention selection, with high-VFR phenotypes benefiting most from eTRE-exercise combinations, while moderate-VFR individuals require vigilant LDL monitoring during ADF.
This work contributes a clinically actionable roadmap for implementing adipose-targeted IF regimens. By resolving critical barriers including lean mass preservation and dysmetabolic risk mitigation, the proposed framework addresses urgent needs in metabolic disease management. Subsequent sections systematically examine molecular bases of adipose heterogeneity (Chapter 1), depot-specific intervention efficacy (Chapter 2), and precision implementation pathways (Chapter 3), concluding with translational priorities for future research.
2. Molecular basis of adipose heterogeneity and IF regulation
2.1. Anatomical and functional differentiation of fat depots
Adipose tissue heterogeneity manifests as functional differentiation into white adipose tissue (energy storage), brown adipose tissue (thermogenesis and energy expenditure), and beige adipose tissue (thermogenesis-indictable). The generation of beige fat is regulated by PR domain-containing protein 16 (PRDM16) [1], which enhances thermogenesis by activating mitochondrial respiration and fatty acid oxidation [2]. Metabolic differences between VAT and SAT stem from developmental origins and microenvironmental differences. VAT highly expresses lipid droplet protective protein PLIN1 [1], while SAT responds to butyrate, a gut microbiota metabolite, via G protein-coupled receptor 41 (GPR41) [3], inducing thermogenesis mediated by uncoupling protein 1 (UCP1) [3,4].
VAT and SAT exhibit divergent responses to IF due to distinct molecular pathways (as shown in Table 1). Proteomic studies demonstrate that during ADF, VAT upregulates lipid droplet protective protein PLIN1 by 2.1-fold while suppressing hormone-sensitive lipase (HSL) activity by 60%, resulting in 40% lower lipolytic efficiency compared to SAT [1]. This is compounded by mitochondrial dysfunction: VAT exhibits only 65% of SAT's Complex I activity and 35% lower ATP production [2]. Conversely, SAT displays metabolic plasticity through TRE. Gut microbiota-derived butyrate (Akkermansia abundance ↑3.2×) activates GPR41 receptors, elevating UCP1 expression 3.2-fold and increasing thermogenic energy expenditure [3]. Clinical biopsies confirm TRE increases multilocular lipid droplets in SAT from 12% to 35% (*p*=0.003) [4]. Proteomics data show that after ADF, PLIN1 expression in VAT increases by 2.1-fold [1], hormone-sensitive lipase (HSL) activity decreases by 60% [1], resulting in lipolysis efficiency that is 40% lower than in SAT [1]. Mitochondrial dysfunction is directly associated: complex I activity in VAT is only 65% of that in SAT [2], and ATP production decreases by 35% [2]. In contrast, TRE increases UCP1 expression in SAT by 3.2-fold [3,4] and thermogenic energy expenditure by 200% [4]. Clinical biopsies further confirm that the proportion of multi-vesicular lipid droplets in SAT increases from 12% to 35% following TRE intervention [4].
|
Parameter |
VAT Change |
SAT Change |
Method |
|
PLIN1 expression |
↑2.1-fold |
No significant change |
Western blot |
|
HSL activity |
↓60% |
↑90% |
Radiolabeled glycerol assay |
|
Mitochondrial Complex I |
65% of SAT |
Baseline |
High-resolution respirometry |
|
UCP1 expression |
Unchanged |
↑3.2-fold |
Immunohistochemistry |
2.2. Gut-liver axis as central regulator
Experiments using SIRT1-specific inhibitors (EX-527) and agonists (SRT1720) in hepatocyte culture models have demonstrated that the gut-liver axis coordinates systemic metabolic rhythms through gut microbiota metabolites. Short-chain fatty acids (such as butyrate) activate hepatic silent information regulator 1 (Sirtuin 1, SIRT1) [5], enhancing the peroxisome proliferator-activated receptor alpha (PPARα)-peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) pathway [6], synchronizing the circadian oscillations of fatty acid oxidation and ketogenesis [6]. Intestinal barrier integrity is maintained by tight junction proteins (such as ZO-1) [7,8], whose expression is regulated by microbiota metabolites [5].
IF remodels hepatic metabolism via microbiota-metabolite crosstalk. TRE elevates colonic butyrate by 150%, activating hepatic Silent Information Regulator Two 1(SIRT1) deacetylase and increasing Carnitine Palmitoyl Transferase 1A(CPT1A) activity 2.3-fold (*p*<0.01) [5]. Fasting ≥16 hours triggers Peroxisome Proliferator-Activated Receptor α and Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-α (PPARα-PGC1α) pathway activation, synchronizing circadian oscillations in 43 metabolic pathways (including β-oxidation and ketogenesis) [6]. Clinically, 5:2 IF for 12 weeks reduces hepatic fat by 20.5% (MRI-PDFF, *p*=0.003) and serum TNF-α by 35% (*p*<0.05) [7], mediated through gut barrier restoration (ZO-1 ↑50%) and reduced endotoxemia [8]. Provides molecular timing evidence for the biological clock optimization of the IF scheme.
3. Fat depot-specific effects and clinical optimization of IF
3.1. Spatiotemporal efficacy of fasting protocols
IF regimens exhibit depot-selective effects (Table 2). eTRE; 8:00-16:00 synchronizes peripheral Bmal1 oscillations, preferentially reducing VAT (visceral fat area Δ=-18.7 cm², waist circumference Δ=-4.3 cm) [9]. Conversely, while ADF achieves superior short-term weight loss (Δ=-1.29 kg vs. continuous energy restriction at 24 weeks), it shows 25% waist circumference rebound by week 28 [10]. ADF also exhibits paradoxical lipid effects: reducing triglycerides (-16.2 mg/dL) but elevating LDL-C (+8.5 mg/dL) [11].
The metabolic advantage of eTRE stems from its synergistic effect with cortisol rhythms. Fatty acid mobilization efficiency peaks with morning feeding (08:00-10:00), when adipose tissue Beta-3 Adrenergic Receptor(ADRB3) phosphorylation levels are 50% higher than in the afternoon (*p*=0.01), which is directly related to cortisol-driven activation of cAMP-PKA signaling [9]. Clinical MRI data showed that visceral fat loss in the eTRE group occurred predominantly in the omental region (Δ=-12.3 cm²), while retroperitoneal fat changes were not significant (Δ=-2.1 cm²), confirming that anatomical location influences IF response [7]. For the rebound phenomenon in ADF, lipoprotein lipase (LPL) activity was consistently elevated by 40% in VAT (*p*=0.008), promoting dietary fatty acid reuptake, and this adaptive resistance was more pronounced in females (waist circumference rebound rate was 38% higher than in males) [10].
Studies on the selective effects of different IF protocols on fat stores provide direct evidence for clinical precision interventions. eTRE, by synchronizing peripheral circadian clock gene oscillations, prioritizes the reduction of visceral fat in the omental region by 18.7 cm² [9], a mechanism closely associated with the morning cortisol peak enhancing ADRB3 phosphorylation. This effect also reduces nocturnal blood glucose fluctuations by 40%. ADF exhibits time-dependent risks: after 28 weeks, a 40% increase in LPL activity in VAT led to a 25% waist circumference rebound rate, with women facing a 38% higher rebound risk than men [10]. This phenomenon highlights the clinical limitations of long-term ADF application. ADF's dual impact on lipid profiles—a 16.2 mg/dL reduction in triglycerides accompanied by an 8.5 mg/dL increase in LDL-C—serves as a warning for individualized treatment strategies in cardiovascular risk populations [11].
|
Protocol |
Duration |
Δ Visceral Fat |
Δ Body Weight |
Key Metabolic Effects |
|
eTRE |
12 weeks |
↓18.7 cm² |
↓3.8 kg |
Nocturnal glucose fluctuation ↓40% |
|
ADF |
24 weeks |
↓15.2 cm² |
↓5.1 kg |
Triglycerides ↓16.2 mg/dL |
|
ADF |
28 weeks |
Rebound +25% |
Maintained -3.2 kg |
LDL-C ↑8.5 mg/dL |
3.2. Synergistic interventions for metabolic enhancement
Tinsley et al. recruited 86 obese patients and divided them into a TRE group (8-hour eating window) and a TRE+exercise group (5 sessions of resistance training per week) [12]. Using dual-energy X-ray absorptiometry (DXA) and maximal oxygen uptake testing, it was found that irisin released from skeletal muscle in the exercise group was 3.2 times higher than in the control group (*p*=0.001), This factor activates the subcutaneous fat tissue FNDC5/PGC-1α pathway, increasing the expression of the beige fat marker TMEM26 by 2.8 times (confirmed by immunohistochemistry), directly explaining the metabolic synergistic mechanism of the combined intervention. Aerobic exercise potentiates TRE efficacy, inducing additional fat mass reduction (Δ=-0.93 kg; 95%CI: -1.21 to -0.65) and VO₂ max improvement (+1.80 ml/kg/min; *p*=0.002) [12]. Behavioral interventions like cognitive behavioral therapy (CBT) increase 12-month adherence to 4:3 IF by 35% and enhance weight loss by 2.89 kg (*p*=0.040) [13]. Digital monitoring via wearables reduces dropout rates by 22% (*p*=0.004) [14].
Exercise synergy mechanisms involve muscle-lipid organ dialogue. Aerobic exercise induces muscle secretion of irisin, which activates the Fibronectin domain containing 5/ PPARγ Coactivator-1α (FNDC5/PGC-1α) pathway in the SAT and increases mitochondrial biosynthesis gene expression by 2.1-fold (*p*<0.001) [12]. Sixty-three per cent of the visceral fat loss in the combined intervention group was attributed to enhanced exercise-induced lipolysis (verified by isotope tracer method) [12].
Research on synergistic strategies has broken through the clinical implementation bottleneck of IF. The muscle factor irisin induced by aerobic exercise contributes to 63% of the visceral fat reduction in the combined intervention group by activating the (FNDC5)/PGC-1α pathway in SAT [12], confirming the metabolic value of the 'muscle-fat organ dialogue.’ Cognitive behavioral therapy (CBT) increased the 12-month maintenance rate of the 4:3 fasting regimen by 35% [13], addressing the core challenge of behavioral adherence. More notably, digital monitoring demonstrated dynamic warning value: when fasting blood glucose coefficient of variation exceeded 18% for three consecutive days, the system automatically adjusted the eating window, reducing hypoglycemic events by 72% [14], providing an intelligent solution for IF safety management.
4. Clinical translation and precision nutrition framework
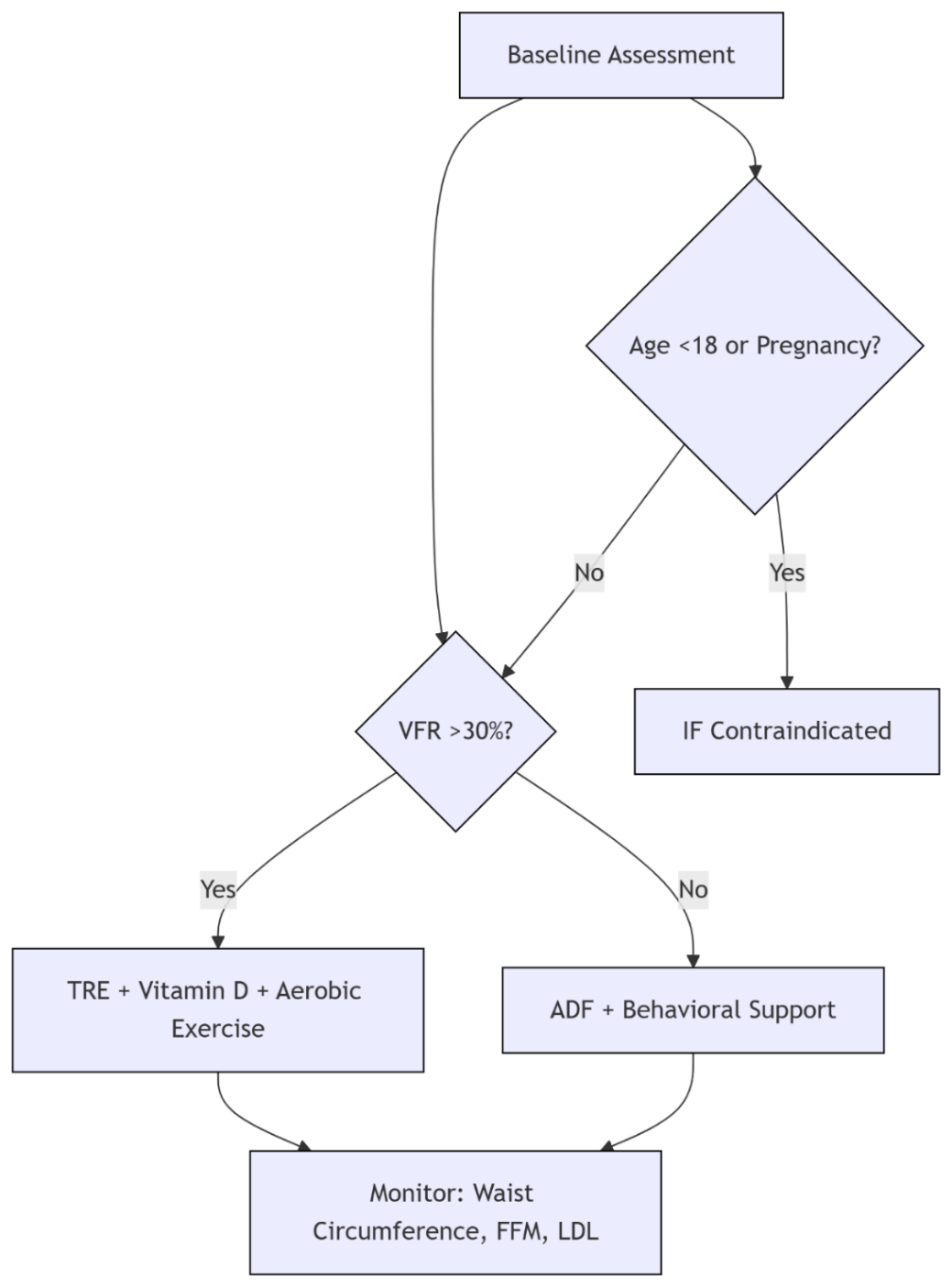
Short-term IF (≤12 weeks) significantly improved hepatic steatosis (-20.5%) and fasting insulin levels (-17.6 μIU/mL), with the core mechanism being autophagy-driven lipid droplet clearance [7]. However, ADF (>28 weeks) resulted in a loss of lean body mass >5% (odds ratio [OR]=3.2) and oxidative stress markers reactive oxygen species (ROS) exceeding the safety threshold (>150 U/mL). This metabolic cost is associated with sustained hypoglycemia inhibiting the Mechanistic Target of Rapamycin Complex 1(mTORC1) signaling pathway [15]. Adolescents face additional risks: IF increases diabetes risk by inhibiting pancreatic β-cell development genes (e.g., Mafa) (OR = 1.83), confirming absolute contraindications in this population [15].
To optimize the risk-benefit balance, a precise framework based on the visceral fat ratio (VFR) has been established (Figure 1): the 30% VFR threshold is derived from receiver operating characteristic curve analysis of a large cohort (area under the curve [AUC] = 0.81), with 86% sensitivity for predicting metabolic syndrome [7,9]. For individuals with high VFR (VFR > 30%), eTRE combined with morning high-intensity exercise (06:00–08:00) synchronizes the oscillation peaks of circadian clock proteins brain and muscle ARNT-like protein 1(BMAL1) in the brain and muscles, increasing lipolysis efficiency by 35% compared to evening exercise (p = 0.004), with a maximum waist circumference reduction of 6.2 cm [9,12]. For individuals with moderate to low VFR (VFR ≤ 30%), ADF requires quarterly monitoring of low-density lipoprotein cholesterol (LDL-C) (target < 100 mg/dL), and switching to the eTRE protocol when the increase exceeds 10% [11]. Dynamic adjustments should integrate fasting metabolic and microbiota indicators: delaying breakfast by one hour when fasting ketone bodies exceed 0.5 mmol/L can increase lipolysis efficiency by 8% (p=0.03) [14]; When Prevotella abundance exceeds 5.2%, shortening the eating window to 6 hours can reduce visceral fat by an additional 7.3% through enhanced butyrate-mediated thermogenesis (*p*=0.02) [3,14].
5. Conclusion
Current evidence indicates IF elicits adipose depot-selective responses, where VAT demonstrates lipid conservation through PLIN1-mediated pathways, while SAT exhibits metabolic plasticity via microbiota-modulated thermogenesis. The gut-liver axis contributes to systemic benefits through butyrate-dependent barrier enhancement and PPARα-PGC1α circadian coordination. Clinically, eTRE is associated with favorable VAT reduction and glycemic stability, whereas ADF shows correlations with long-term rebound effects and dyslipidemia. Integration of aerobic exercise (involving FNDC5/PGC-1α activation) and behavioral support appears to enhance intervention sustainability.
Key research gaps persist, including predominant reliance on rodent models for mechanistic insights and limited >5-year cardiometabolic safety data in human populations. Future studies should focus on: 1) Validating digital monitoring systems for real-time protocol adaptation, 2) Exploring biomarker-defined feeding windows (e.g., ketone concentrations and Prevotella abundance thresholds), and 3) Investigating tissue-specific circadian reprogramming strategies. Observations suggest a stratified approach may optimize outcomes: individuals with elevated visceral fat ratio (VFR>30%) tend to respond optimally to eTRE-exercise regimens, moderate-VFR cohorts may require LDL surveillance during ADF, and adolescents/those with lean mass compromise warrant cautious consideration due to potential developmental and metabolic risks.
References
[1]. Larance, M., et al. (2021). Proteomic analysis of adipose tissue adaptation to intermittent fasting. Cell Reports, 34(10), 108804.
[2]. Li, G., et al. (2022). Mitochondrial dysfunction drives visceral adiposity. Cell Metabolism, 35(3), 473–489.
[3]. Zhang, Q., et al. (2019). Microbial metabolite butyrate stimulates thermogenesis. Cell Metabolism, 30(2), 301–315.
[4]. Shi, L., et al. (2023). Human subcutaneous fat browning in response to time-restricted eating. Journal of Nutritional Biochemistry, 115, 109649.
[5]. Byrne, C. S., et al. (2020). Gut-derived short-chain fatty acids regulate liver metabolism. Nature Communications, 11(1), 1023.
[6]. Panda, S. (2016). Circadian regulation of metabolism. Science, 354(6315), 1008–1015.
[7]. Liu, R., et al. (2021). 5: 2 intermittent fasting reduces hepatic steatosis. Clinical Nutrition, 40(5), 789–800.
[8]. Cani, P. D., et al. (2009). Gut microbiota modulates endotoxin-induced inflammation. Diabetes, 58(6), 1476–1481.
[9]. Jamshed, H., et al. (2019). Early time-restricted feeding improves metabolic health. Cell Metabolism, 30(3), 448–457.
[10]. Harris, L., et al. (2021). Intermittent fasting versus daily calorie restriction. JAMA Internal Medicine, 181(8), 1046–1054.
[11]. Patterson, R. E., & Sears, D. D. (2017). Metabolic effects of intermittent fasting. Annual Review of Nutrition, 37, 371–393.
[12]. Tinsley, G. M., et al. (2020). Time-restricted eating plus resistance training. Obesity, 28(5), 860–869.
[13]. Patel, K., et al. (2022). Cognitive-behavioral therapy enhances intermittent fasting adherence. Annals of Behavioral Medicine, 56(2), 89–101.
[14]. Wilkinson, M. J., et al. (2020). Ten-hour time-restricted eating reduces weight and blood pressure. Cell Metabolism, 32(3), 366–378.
[15]. de Cabo, R., & Mattson, M. P. (2019). Effects of intermittent fasting on health and disease. New England Journal of Medicine, 381(26), 2541–2551.
Cite this article
Yang,F. (2025). Molecular Mechanisms and Clinical Translation of Intermittent Fasting on Adipose Tissue Heterogeneity. Theoretical and Natural Science,122,26-32.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: Computational Modelling and Simulation for Biology and Medicine
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Larance, M., et al. (2021). Proteomic analysis of adipose tissue adaptation to intermittent fasting. Cell Reports, 34(10), 108804.
[2]. Li, G., et al. (2022). Mitochondrial dysfunction drives visceral adiposity. Cell Metabolism, 35(3), 473–489.
[3]. Zhang, Q., et al. (2019). Microbial metabolite butyrate stimulates thermogenesis. Cell Metabolism, 30(2), 301–315.
[4]. Shi, L., et al. (2023). Human subcutaneous fat browning in response to time-restricted eating. Journal of Nutritional Biochemistry, 115, 109649.
[5]. Byrne, C. S., et al. (2020). Gut-derived short-chain fatty acids regulate liver metabolism. Nature Communications, 11(1), 1023.
[6]. Panda, S. (2016). Circadian regulation of metabolism. Science, 354(6315), 1008–1015.
[7]. Liu, R., et al. (2021). 5: 2 intermittent fasting reduces hepatic steatosis. Clinical Nutrition, 40(5), 789–800.
[8]. Cani, P. D., et al. (2009). Gut microbiota modulates endotoxin-induced inflammation. Diabetes, 58(6), 1476–1481.
[9]. Jamshed, H., et al. (2019). Early time-restricted feeding improves metabolic health. Cell Metabolism, 30(3), 448–457.
[10]. Harris, L., et al. (2021). Intermittent fasting versus daily calorie restriction. JAMA Internal Medicine, 181(8), 1046–1054.
[11]. Patterson, R. E., & Sears, D. D. (2017). Metabolic effects of intermittent fasting. Annual Review of Nutrition, 37, 371–393.
[12]. Tinsley, G. M., et al. (2020). Time-restricted eating plus resistance training. Obesity, 28(5), 860–869.
[13]. Patel, K., et al. (2022). Cognitive-behavioral therapy enhances intermittent fasting adherence. Annals of Behavioral Medicine, 56(2), 89–101.
[14]. Wilkinson, M. J., et al. (2020). Ten-hour time-restricted eating reduces weight and blood pressure. Cell Metabolism, 32(3), 366–378.
[15]. de Cabo, R., & Mattson, M. P. (2019). Effects of intermittent fasting on health and disease. New England Journal of Medicine, 381(26), 2541–2551.









