The current applications and potential advantages of the Neuropixels
Jiuzhang Meng1,4 †, Surimanda2, †, Yongling Yi3, †
1School of Basic Medical Sciences, Capital Medical University, Beijing, China 100069
2Beijing ETown Academy, Sihe Road 12, Daxing District, Beijing, China
3Pinghe Bilingual School, Mingyue road 1029, Shanghai, China
4mengjz@ccmu.edu.cn
†These authors contributed equally
Abstract. Neuropixels technology has revolutionized neuroscience research by allowing scientists to obtain physiological firing data from single neurons in the central nervous system with unprecedented temporal and spatial resolution. While the technology is already highly advanced, researchers continue to work on improving it further. In this article, the authors summarize several current studies on improvements and applications of Neuropixels probes. This technology is used to capture the characteristic waveforms of neurons in different brain regions, record particular types of neurons, and acquire detailed data. The improvement of the Neuropixels is the AMIE, which solves the problem of implanting the device in unrestrained mice and recycling, enriching the electrodes and the recording sites on each Neuropixels device. By discussion and investigation, the current conduct and some refinements of the Neuropixels are demonstrated in this brief review. However, fragility and ethical problems are still inevitable obstacles of Neuropixels, and their potential harm to human beings still deserves attention.
Keywords:Neuropixels,neural recording,neuroscience technique.
1. Introduction
For many years, neuroscientists have tried to find and invent new devices to record the electrophysiological activities of neurons. In the past, researchers and scientists had limited tools and technologies available to them to record and understand neuron firing, relying on Indium and tungsten electrodes because of their robustness and isolation [1-2]. Tetrode and electron-beam lithography brought a great breakthrough in Electrophysiological equipment, especially the latter one which facilitated the development of silicon probe devices such as the Utah array [3-5]. However, these techniques all have their limitations. Some of them might damage normal neurons and other devices cannot insert into the brain for a long time because of their complex structure [6].
In recent years, neurotechnology has gained significant attention from society due to its potential to advance brain research and the development of new treatments for neurological disorders. One of the most promising advancements in neurotechnology in recent years is Neuropixels technology, which has transformed neuroscience research by enabling scientists to obtain physiological firing data from single neurons in the brain in a highly efficient way. The Neuropixels technology is based on silicon probes that contain hundreds of tiny electrodes, each measuring just a few microns in diameter. These electrodes can be inserted into the brain with minimal damage to the surrounding tissue and are able to simultaneously collect electronic data from a mountain of neurons. This allows for more accurate and precise measurements of neural activity, enabling researchers to gain deeper insights into how the brain works. In this review, we briefly introduce the mechanism of Neuropixels and summarize several current studies on improvements and applications in labs. Some potential advantages of this technique are also provided in this article, intending researchers to have a broad investigation on Neuropixels.
2. Mechanism
Neuropixels technology has been developed over the past few years. It is the first digital neurobiological device that efficiently integrates CMOS materials. Compared to The Neurotrophic Electrode, which was initially invented by neurologist Kennedy et al. and was successfully implanted for the first time in a human patient in 1996 and utilized to record the electrical signals produced by the brain during information processing, Neuropixels were certainly the more innovative technology [7]. It is now considered a powerful tool in the field of neuroscience because of its relatively advanced designed construction.
A Neuropixels probe is a kind of invasive brain-computer interface. The official website shows the control system consists of three parts: a head stage, an interface cable, and a PXIe acquisition module. It provides 960 recording sites on an extremely narrow shank and 384 parallel, configurable, low-noise recording channels, with onboard amplification, digitization, and multiplexing. The spikes from multiple neural cells in different parts of the brain cortexes can be detected by these delicate and sensitive sites. Then the device will translate those neuron signals into digital codes [8].
3. Applications
One of the major advantages of Neuropixels technology is its great number of channels, which enables each probe to simultaneous collecting samples from numerous neurons. In addition, the transmission of low-noise data is appropriate for this device because of its powerful capabilities to amplify and digitalize the signals. By reason of the tiny size of this device, multiple probes can be used to probe different brain functional regions simultaneously. Most researchers choose Neuropixels as the recording device in their experiments.
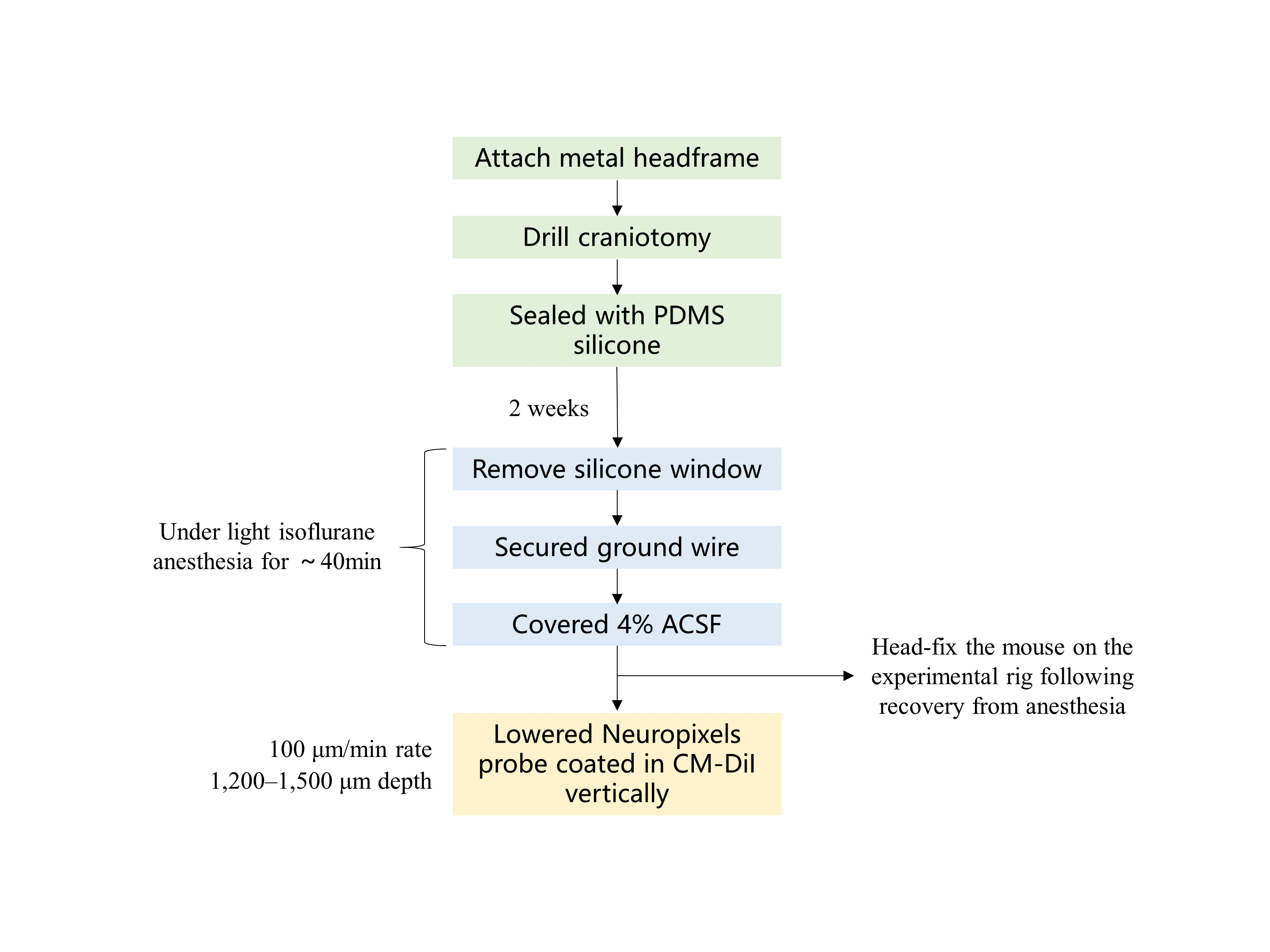
High-density recording can capture the characteristic waveforms from multiple neurons. Jia et al. distinguished different neural cells by their electrical properties using Neuropixels probes and five brain structures have been recorded [9]. Here is the process of the experiment (Figure 1). A metal headframe was fixed on mice’s heads by a metal bond. A craniotomy was drilled and a polydimethylsiloxane (PDMS) piece was put on it to seal the interspace. There were two weeks for mice to get used to it. Before the experiment began, the mice were anesthetized first and a ground wire was attached to their skulls after the PDMS silicone was taken off. Artificial cerebrospinal (ACSF) has to be smeared on the exposed brain. The whole span of anesthesia lasts about 40 minutes. As mice woke up, they were put on the device and the Neuropixels probe would be inserted into a specific position. It is worth noting that the probe had been covered with Digi, which is a Lipophilic material, aiming to detect tracks of the electrode histologically. Before the recording, the probe ought to settle for 30 min. These series of experiments demonstrate that it’s already possible for researchers to record large-scale electrical signals of neurons in living rodents.
Neuropixels can also be used to record a particular type of neuron and acquire detailed data [10]. Kostadinov et al. recorded complex spiking of Purkinje cells in rewards prediction circuits directly and complemented image experiments by Neuropixels electrode arrays. The process of the operation during the experiments resembles the process above. Similarly, the probe settled for 20 min before the recording started and it’s necessary to smear DiI on the probe. The array would then detect the signals distally on a micromanipulator. Neuropixels and their matched applications have shown significant utility in recording and analyzing complex waveforms. It would be a great adjunct to two-photon calcium imaging in this research.
Conventional detecting techniques sometimes seem limited to tracing neural activities of multiple structures in the cortex or the whole brain [11-12]. Considering that the Neuropixels probes can record different regions simultaneously, activations in subcortical areas and superior colliculus (SC) are detected by Musall et al. (2019) in one experiment. These two structures are both related to cognition and movement. The large scale of this device is an advantage for researchers to explore all the relevant neurons of one particular function. The electrophysiological data of this experiment was recorded through 348 channels, each spanned about 4mm. Data was collected by SpikeGLX and analyzed by MATLAB.
4. Improvements based on the original technique
4.1. Implanting in moving mice
Researchers need to investigate neural activities in uncommitted mice. In that case, many engineers were working to invent an efficient device of a small size. Static electrode arrays and microdrives previously allow neuroscientists to collect data on the discharge of neural cells in unconstrained mice. With the invention and the development of Neuropixels probes, researchers now manage to endow that property onto the Neuropixels technique. A progressive device, which is called the AMIE, has been invented to solve the problem of implanting the device in unrestrained mice and recycling [13]. The Neuropixels AMIE is mainly composed of three characteristic parts. In addition to being securely attached to the mice's heads, the AMIE is also able to record data reliably over long periods intended to ensure the record is consistent. The protocol for this technique has been published and provides technical support for experiments that need Neuropixels [14].
4.2. Neuropixels 2.0
It is hard to achieve recording and sample the signals from the same neural cell in a long term, especially for a large population of neurons. Nevertheless, with the improvement of the Neuropixels technique, this particular problem will be solved by more advanced technology, the most direct evidence of which is Neuropixels 2.0 [15]. In March 2021, the paper compiled by Steinmetz et al. is published in the journal Science. A probe in a smaller size, which was called the Neuropixels 2.0, was designed and texted by them (Table 1). This refined device has more recording sites and a lighter volume. It is also more convenient to connect to software on computers. The size of the probe and head stage on the Neuropixels 2.0 is three times smaller than the Neuronpixels 1.0 so the whole parts weigh only ~1.1 g. According to their tests, Neuropixels 2.0 with one particular algorithm guarantees > 80% success in tracking neural signals within two months [16].
Table 1. Comparison between Neuropixels and Neuropixels 2.0.
Geometry | Probe & Headstage | Channel | Sites pre Shank | Arrangement | |
Neuropixels | - | 1 | 384 | 960 | staggered |
Neuropixels 2.0 | denser, linearized | 1/3 | 384 | 1280 (single-shank) 5120 (4-shank) | Vertically aligned |
Compared with Neuropixels, Neuropixels 2.0 has a denser and linearized geometry but is only one-third in length than the first generation. Similarly, Neuropixels 2.0 also has 384 recording channels. One of the great breakthroughs is the sites on per shank. Neuropixels 2.0 has 1280 sites in a single-shank version, which is 320 more than Neuropixels. Moreover, the 2.0 has a 4-shank version and provides 5120 recording sites in total. Neuropixels 2.0 differs from the first generation in arrangements. The sites in the 2.0 are vertically aligned and they are staggered in Neuropixels.
5. Potential advantages
The most obvious benefit of Neuropixels is its relatively low cost and price performance. Neuropixels 1.0 costs about $1,400, and manufacturers are selling these sophisticated brain-signal monitors at that price. It provides an opportunity to achieve commercialization. Neuropixels 2.0 is also sold at a similar price to Neuropixels 1.0. This certainly gives lots of conveniences to brain-computer interface researchers [15].
A typical use of a large-scale recording probe is as an input device for a brain-computer interface. The device can obtain large amounts of electrophysiological data simultaneously, which means that it can quickly reflect the testee's mental activity to the machine. Moreover, it can achieve chronic implanting due to its simple structure. The use of neuron-detecting devices as brain-computer interfaces(BCI) has a long history, and researchers are constantly experimenting with new devices. Michigan arrays have been chronically implanted into primates in 2017. It recorded motion signals as the primate did a grasping task [17]. Last year, researchers used BCI to reflect the need of a patient with amyotrophic lateral sclerosis (ALS) [18]. These pieces of evidence suggest the possibility of patients using BCI to communicate and move and a device capable of efficiently recording neural activity is necessary. The Neuropixels probe is up to this job.
The usage of Neuropixels technology has significantly advanced our understanding of the brain, including its mechanisms for processing information, decoding electrical signals, and responding to treatments for neurological disorders. Considering that it has been conducted in human brains [19], this technology has the capacity to contribute to the update of new diagnostic schemes for various diseases in the brain.
6. Conclusion
In conclusion, Neuropixels technology has revolutionized neuroscience research by enabling the simultaneous recording of activity from thousands of neurons. Its extremely high temporal and spatial resolution brings great convenience to scientists when studying neural signals. The ability to record the wide range of neuron activities can perhaps allow us to refine the input equipment in the current brain-machine interface. While ethical limitations exist, ongoing technological innovations can also give us a further understanding of brain activations that respond to behaviors and develop new diagnoses and treatments for neurological disorders.
In addition to these potential advantages, the shortcomings of this equipment are also worthy of our attention. Because the probe has been overly attenuation, the device can still be damaged and broken even during normative conduct. This might lead to extra-economic costs and harm to the participants during the process of scientific research. Overall, Neuropixels technology represents a remarkable achievement in techniques of neuroscience research and opens up new frontiers of investigation. It presents valuable opportunities and challenges for neural devices.
References
[1]. Dowben, R. M. and Rose, J. E., “A metal-filled microelectrode,” Science 118(3053), 22-4 (1953).
[2]. Hubel, D. H., “Tungsten microelectrode for recording from single units,” Science 125(3247), 549-550 (1957).
[3]. Recce, M. and O’Keefe, J., “The tetrode: a new technique for multi-unit extracellular recording,” Soc. Neurosci. Abstr. 15, 1250 (1989).
[4]. Scholvin, J., Kinney, J. P., Bernstein, J. G., Moore, K. C., Kopell, N., Fonstad, C. G. and Boyden, E. S., “Close-Packed Silicon Microelectrodes for Scalable Spatially Oversampled Neural Recording,” IEEE Trans. Biomed. Eng. 63(1), 120-130 (2016).
[5]. Maynard, E. M., Nordhausen, C. T. and Normann, R. A., “The Utah intracortical Electrode Array: a recording structure for potential brain-computer interfaces,” Electroencephalogr. Clin. Neurophysiol. 102(3), 228-39 (1997).
[6]. Rios, G., Lubenov, E. V., Chi, D., Roukes, M. L. and Siapas, A. G., “Nanofabricated Neural Probes for Dense 3-D Recordings of Brain Activity,” Nano. Lett. 16(11), 6857-6862 (2016).
[7]. Dr. Kennedy, Senior Research Scientist, Neural Signals, interview, Sept. 30 2010
[8]. Kuhl, J., “How to make sense of the brain's billions of neurons,” Wellcome, 31 October 2018, <https://wellcome.org/news/how-make-sense-brains-billions-neurons> (31 March 2023).
[9]. Jia, X., Siegle, J. H., Bennett, C., Gale, S. D., Denman, D. J., Koch, C. and Olsen, S. R., “High-density extracellular probes reveal dendritic backpropagation and facilitate neuron classification,” J. Neurophysiol. 121(5), 1831-1847 (2019).
[10]. Kostadinov, D., Beau, M., Blanco-Pozo, M. and Häusser, M., “Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells,” Nat. Neurosci. 22(6), 950-962 (2019).
[11]. Musall, S., Kaufman, M. T., Juavinett, A. L., Gluf, S. and Churchland, A. K., “Single-trial neural dynamics are dominated by richly varied movements,” Nat. Neurosci. 22(10), 1677-1686 (2019).
[12]. Okun, M., Lak, A., Carandini, M. and Harris, K. D., “Long Term Recordings with Immobile Silicon Probes in the Mouse Cortex,” PLoS One 11(3), 171-180 (2016).
[13]. Juavinett, A. L., Bekheet, G. and Churchland, A. K., “Chronically implanted Neuropixels probes enable high-yield recordings in freely moving mice,” Elife 8, 165-188 (2019).
[14]. Juavinett, A. L., Bekheet, G. and Churchland, A. K., “Implanting and Recycling Neuropixels Probes for Recordings in Freely Moving Mice,” Bio-protocol 10(3), 35-42 (2020).
[15]. Steinmetz, N. A., Aydin, C., Lebedeva, A., Okun, M., Pachitariu, M., Bauza, M., Beau, M., Bhagat, J., Böhm,. C., Broux, M., Chen, S., Colonell, J., Gardner, R. J., Karsh, B., Kloosterman, F., Kostadinov, D., Mora-Lopez, C., O'Callaghan, J., Park, J., Putzeys, J., Sauerbrei, B., Van Daal, R. J. J., Vollan, A. Z., Wang, S., Welkenhuysen, M., Ye, Z., Dudman, J. T., Dutta, B., Hantman, A. W., Harris, K. D., Lee, A. K., Moser, E. I., O'Keefe, J., Renart, A., Svoboda, K., Häusser, M., Haesler, S., Carandini, M. and Harris, T. D., “Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings,” Science 372(6539), 1-11 (2021).
[16]. Singer, E., “A New Era in Neural Recording,” Simonsfoundation, 19 July 2021, <https://www.simonsfoundation.org/2021/07/19/a-new-era-in-neural-recording/> (31 March 2023).
[17]. Barz, F., Livi, A., Lanzilotto, M., Maranesi, M., Bonini, L., Paul, O. and Ruther, P., “Versatile, modular 3D microelectrode arrays for neuronal ensemble recordings: from design to fabrication, assembly, and functional validation in non-human primates,” J. Neural. Eng. 14(3), 1741-1749 (2017).
[18]. Chaudhary, U., Vlachos, I., Zimmermann, J. B., Espinosa, A., Tonin, A., Jaramillo-Gonzalez, A., Khalili-Ardali, M., Topka, H., Lehmberg, J., Friehs, G. M., Woodtli, A., Donoghue, J. P. and Birbaumer, N., “Spelling interface using intracortical signals in a completely locked-in patient enabled via auditory neurofeedback training,” Nat. Commun. 13(1), 1-9 (2022).
[19]. Chung, J. E., Sellers, K. K., Leonard, M. K., Gwilliams, L., Xu, D., Dougherty, M. E., Kharazia, V., Metzger, S. L., Welkenhuysen, M., Dutta, B. and Chang, E. F., “High-density single-unit human cortical recordings using the Neuropixels probe,” Neuron 110(15), 2409-2421. (2022).
Cite this article
Meng,J.;Surimanda,S.;Yi,Y. (2023). The current applications and potential advantages of the Neuropixels. Theoretical and Natural Science,6,394-399.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the International Conference on Modern Medicine and Global Health (ICMMGH 2023)
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Dowben, R. M. and Rose, J. E., “A metal-filled microelectrode,” Science 118(3053), 22-4 (1953).
[2]. Hubel, D. H., “Tungsten microelectrode for recording from single units,” Science 125(3247), 549-550 (1957).
[3]. Recce, M. and O’Keefe, J., “The tetrode: a new technique for multi-unit extracellular recording,” Soc. Neurosci. Abstr. 15, 1250 (1989).
[4]. Scholvin, J., Kinney, J. P., Bernstein, J. G., Moore, K. C., Kopell, N., Fonstad, C. G. and Boyden, E. S., “Close-Packed Silicon Microelectrodes for Scalable Spatially Oversampled Neural Recording,” IEEE Trans. Biomed. Eng. 63(1), 120-130 (2016).
[5]. Maynard, E. M., Nordhausen, C. T. and Normann, R. A., “The Utah intracortical Electrode Array: a recording structure for potential brain-computer interfaces,” Electroencephalogr. Clin. Neurophysiol. 102(3), 228-39 (1997).
[6]. Rios, G., Lubenov, E. V., Chi, D., Roukes, M. L. and Siapas, A. G., “Nanofabricated Neural Probes for Dense 3-D Recordings of Brain Activity,” Nano. Lett. 16(11), 6857-6862 (2016).
[7]. Dr. Kennedy, Senior Research Scientist, Neural Signals, interview, Sept. 30 2010
[8]. Kuhl, J., “How to make sense of the brain's billions of neurons,” Wellcome, 31 October 2018, <https://wellcome.org/news/how-make-sense-brains-billions-neurons> (31 March 2023).
[9]. Jia, X., Siegle, J. H., Bennett, C., Gale, S. D., Denman, D. J., Koch, C. and Olsen, S. R., “High-density extracellular probes reveal dendritic backpropagation and facilitate neuron classification,” J. Neurophysiol. 121(5), 1831-1847 (2019).
[10]. Kostadinov, D., Beau, M., Blanco-Pozo, M. and Häusser, M., “Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells,” Nat. Neurosci. 22(6), 950-962 (2019).
[11]. Musall, S., Kaufman, M. T., Juavinett, A. L., Gluf, S. and Churchland, A. K., “Single-trial neural dynamics are dominated by richly varied movements,” Nat. Neurosci. 22(10), 1677-1686 (2019).
[12]. Okun, M., Lak, A., Carandini, M. and Harris, K. D., “Long Term Recordings with Immobile Silicon Probes in the Mouse Cortex,” PLoS One 11(3), 171-180 (2016).
[13]. Juavinett, A. L., Bekheet, G. and Churchland, A. K., “Chronically implanted Neuropixels probes enable high-yield recordings in freely moving mice,” Elife 8, 165-188 (2019).
[14]. Juavinett, A. L., Bekheet, G. and Churchland, A. K., “Implanting and Recycling Neuropixels Probes for Recordings in Freely Moving Mice,” Bio-protocol 10(3), 35-42 (2020).
[15]. Steinmetz, N. A., Aydin, C., Lebedeva, A., Okun, M., Pachitariu, M., Bauza, M., Beau, M., Bhagat, J., Böhm,. C., Broux, M., Chen, S., Colonell, J., Gardner, R. J., Karsh, B., Kloosterman, F., Kostadinov, D., Mora-Lopez, C., O'Callaghan, J., Park, J., Putzeys, J., Sauerbrei, B., Van Daal, R. J. J., Vollan, A. Z., Wang, S., Welkenhuysen, M., Ye, Z., Dudman, J. T., Dutta, B., Hantman, A. W., Harris, K. D., Lee, A. K., Moser, E. I., O'Keefe, J., Renart, A., Svoboda, K., Häusser, M., Haesler, S., Carandini, M. and Harris, T. D., “Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings,” Science 372(6539), 1-11 (2021).
[16]. Singer, E., “A New Era in Neural Recording,” Simonsfoundation, 19 July 2021, <https://www.simonsfoundation.org/2021/07/19/a-new-era-in-neural-recording/> (31 March 2023).
[17]. Barz, F., Livi, A., Lanzilotto, M., Maranesi, M., Bonini, L., Paul, O. and Ruther, P., “Versatile, modular 3D microelectrode arrays for neuronal ensemble recordings: from design to fabrication, assembly, and functional validation in non-human primates,” J. Neural. Eng. 14(3), 1741-1749 (2017).
[18]. Chaudhary, U., Vlachos, I., Zimmermann, J. B., Espinosa, A., Tonin, A., Jaramillo-Gonzalez, A., Khalili-Ardali, M., Topka, H., Lehmberg, J., Friehs, G. M., Woodtli, A., Donoghue, J. P. and Birbaumer, N., “Spelling interface using intracortical signals in a completely locked-in patient enabled via auditory neurofeedback training,” Nat. Commun. 13(1), 1-9 (2022).
[19]. Chung, J. E., Sellers, K. K., Leonard, M. K., Gwilliams, L., Xu, D., Dougherty, M. E., Kharazia, V., Metzger, S. L., Welkenhuysen, M., Dutta, B. and Chang, E. F., “High-density single-unit human cortical recordings using the Neuropixels probe,” Neuron 110(15), 2409-2421. (2022).