1. Introduction
Cancer is a malignant disease characterized by disorderly proliferation of cells, which has become a major problem that needs to be dealt with urgently in the field of global public health [1]. Its root is that gene mutation disrupts the cell cycle regulation network, resulting in unbalanced cell division and accelerating the development of tumors [1]. Such a pathological process will not only cause local tissue damage, but also spread to other organ systems through metastasis. Then it leads to multiple organ failure and even life-threatening [1]. Because cancer has obvious heterogeneity, its clinical manifestations are complicated and its prognosis is quite different, which reflects the necessity of adopting individualized and precise medical strategies [1]. In recent years, the development of new anti-tumor drugs has become the main breakthrough to solve this problem [2]. Traditional chemotherapy drugs, such as paclitaxel, mostly kill the rapidly proliferating cancer cells by inducing apoptosis [3], while the molecular targeted drug imatinib focuses on specific gene mutation, thus achieving precise intervention [4].
2. An overview of the global cancer burden
2.1. Symptoms and its global burden
Cancer has diversified characteristics due to its complex clinical manifestations. Its most typical symptoms are inexplicable weight loss, persistent fatigue, palpable mass on the body surface, abnormal bleeding or subcutaneous bruising, which often delay diagnosis and worsen the condition [5]. Cancer is one of the most important causes of death in the world. It is estimated that there will be nearly 20 million new cases and more than 9.7 million deaths in 2022 [6]. Moreover, it is estimated that with the acceleration of population aging and the change of people's lifestyle, the burden caused by cancer will be about 60% more than now by 2040, and the mortality rate of cancer is very high, which will not only make many lives disappear, but also bring huge economic burden and social psychological pressure, thus increasing the consumption of medical resources and affecting the quality of life of patients [6].
There is an obvious spatial imbalance in the global cancer burden, and the cancer mortality rate in low-income and middle-income countries is generally higher than that in high-income countries [7], which is mostly caused by the lack of access to prevention, diagnosis and treatment resources in these places [7]. China is a typical example, which occupies a key position in the world cancer situation, and the newly-added cancer patients and deaths account for about a quarter of the global total every year [8]. After the interaction of some specific environmental factors and living habits (including air pollution, smoking and diet structure), lung cancer, gastric cancer and liver cancer are more common in China [8], and China has become the main area of international anti-cancer research, so it is necessary to innovate intervention methods to deal with the grim situation [8].
2.2. Cancer types
Breast cancer and chronic myeloid leukemia (CML) are two completely different disease models, and there are obvious differences in their clinical manifestations and treatment methods [9]. Breast cancer is a solid tumor disease with high incidence all over the world, and it has become an important topic in the health field, and the number of new patients has reached 2.3 million in 2022 [6]. Cytotoxic chemotherapy is often regarded as the main way in the treatment scheme of this disease. As for CML, it belongs to the category of hematological malignancies, and there will be 1-2 new patients per million people every year [10]. This disease was once considered as one of the most intractable problems, but now it can achieve long-term and stable results to control the development of the disease by relying on standardized diagnosis and treatment. A variety of treatment schemes for solid tumors and hematological malignancies reflect the specific embodiment of personalized medical concept in clinical practice. Relevant cases show that the design of diagnosis and treatment path for malignant tumors should fully consider the pathological characteristics and biological attributes of patients [9].
The course of drug development shows that there has been obvious progress in cancer treatment in recent decades, from the traditional broad-spectrum cytotoxic chemotherapy to the new targeted therapy [2]. Paclitaxel (as shown in Figure 1) is one of the typical drugs, which originated from the extract of Pacific yew bark and was identified as having pharmacological activity in the compound screening project carried out by the National Cancer Institute of the United States [11]. Paclitaxel controls the cell cycle process by stabilizing the microtubule structure and preventing the spindle formation [12].
Take imatinib (Gleevec) as an example, as shown in Figure 1. It is a landmark new drug in tumor treatment [4]. This kind of new drugs can identify some molecular abnormalities in cancer cells, and make efficient and specific inhibitory drugs by chemical synthesis, so as to disable cancer-causing protein [13]. Compared with traditional broad-spectrum antimetabolites such as vinblastine and small-dose antineoplastic drugs such as imatinib, this kind of anticancer drug has many differences in mechanism, use and curative effect, which provides good basic information for further discussion of these two drugs [2].
3. Description of chemical structures of drugs
3.1. Overall introduction
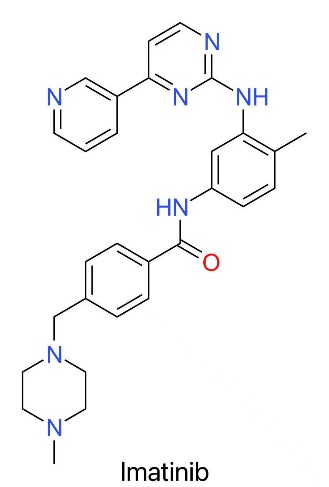
Imatinib (trade name Gleevec/Glivec) is recognized as a classic example in the field of drug research and development. As the structure shown in Figure 1, its formal name is 4-[(4-methyl-piperazine-1-yl) methyl]-N-[4-methyl-3-[(4-(pyridine-3-yl)-pyrimidine-2-yl)-amino]-phenyl]-benzamide monomethyl sulfonate as shown in Figure 1, which reveals the structure of the compound. Therefore, it shows its unique structural characteristics and precise regulation ability. This molecular structure includes several key structural panels, such as benzamide skeleton, methylpiperazine derivative and pyridyl pyrimidinamine group, and with the help of these elaborate designs, it achieves the close combination effect with specific target sites, which has shown obvious curative effect in the practical application of chronic myeloid leukemia [4].
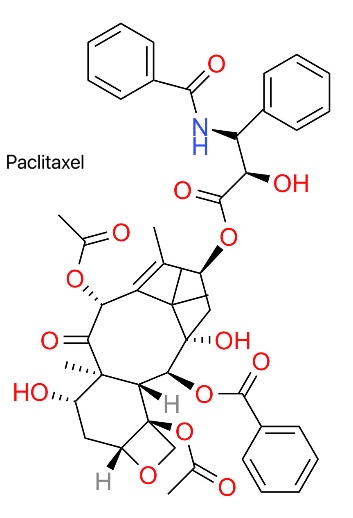
The structure of Paclitaxel is shown by Figure 2. Taxol (trade name: Taxol) is an anti-tumor drug screened from natural products, and its name has profound biological significance [11]. Taxol was originally extracted and isolated from the Pacific Echinacea (Taxus brevifolia) [11]. Unlike the well-designed molecular targeted drug imatinib, paclitaxel was discovered in a long-term screening program conducted by the National Cancer Institute in the 1960s [11]. Studies have shown that yew bark extract has a strong anti-cancer effect [11]. Later studies show that paclitaxel has unique chemical structure characteristics, and it contains a large cyclic skeleton system [11].
3.2. Chemical properties
The physical and chemical properties of the above two compounds are mostly determined by their molecular structure characteristics, and have obvious effects on their biological activities [2,11]. Imatinib mesylate is a small molecular drug with a molecular weight of 589.7g/mol, showing good pharmacokinetic properties [2]. It presents a white or white-like crystalline form, which is very suitable for making oral film-coated tablets [2]. Among them, piperazine group is the key structural part of it, which is easy to undergo dissociation reaction under physiological conditions, so that its water solubility can be greatly improved, and the absorption effect of gastrointestinal tract can be improved [2]. This design makes it a good choice for long-term outpatient treatment [2].
Paclitaxel is a high molecular weight macrocyclic diterpenoid, and its relative molecular weight is as high as 853.91g/mol [11]. This substance is white or white-like crystal with strong hydrophobic properties, which makes its solubility in water phase low [11]. Its molecular structure contains complex tetracyclic baccatin skeleton and ester bond side chain groups, which makes it have certain limitations in clinical application [11]. In order to solve these problems, it is often mixed with polyoxyethylene castor oil (Cremophor EL) and ethanol to form a micelle preparation suitable for intravenous injection [14].
4. Discussion of drug pharmacology
4.1. Description of drug targets and how their function is affected by the drugs
Imatinib and paclitaxel have unique pharmacological characteristics in cytology and histology, which indicates that the treatment of tumors is gradually moving from traditional cytotoxic chemotherapy to precise molecular targeted therapy [2].
Imatinib is the first high-efficiency signal transduction inhibitor widely used in clinic, which is a milestone in the field of tumor therapy. It can accurately target BCR-ABL protein, which exists in excess in chronic myeloid leukemia (CML) cells. When it is in the intracellular environment, imatinib will competitively bind with adenosine triphosphate (ATP), occupying the BCR-ABL kinase region, prompting this protein to be inactivated. Therefore, it can effectively inhibit the phosphorylation reaction of downstream substrate protein, which can block the important signal pathways that promote cell proliferation and inhibit cell apoptosis, so it shows a selective killing effect on CML patients [4].
Taxol is a classic cytotoxic drug, and its lethal effect is mainly achieved by interfering with the microtubule network, a highly conservative biological target [12]. Microtubules are the key part of cytoskeleton, which are formed by α -and β -tubulin heterodimers and constantly perform the dynamic process of assembly and degradation in the process of cell cycle [12]. The special toxic mechanism of paclitaxel is to destroy this dynamic balance [12]. The compound can irreversibly bind to the β subunit inside microtubules [12], which will not affect the polymerization function of microtubules, but will cause tubulin to form stable super-long polymers and greatly delay their normal depolymerization process [12]. Studies have shown that paclitaxel can interfere with the tubulin polymerization network, thus obviously changing the dynamics of cytoskeleton during mitosis [12]. Its main mechanism is that paclitaxel promotes the spindle to form a stable but functionally limited microtubule structure, which hinders the traditional spindle assembly process [12], which leads to the stagnation of the cell cycle in the middle to late transition period (G2/M period), and finally promotes the rapid proliferation of tumor cells and other sensitive cells to die or produce programmed death [12].
4.2. Mode of delivery
The mode of administration of each drug is a direct and reasonable result of its chemical and pharmacological characteristics [2,14].
Because imatinib has good bioavailability and dissolution, it can achieve good drug absorption effect by oral administration [2]. At present, there have been two kinds of products in the market, namely 100 mg and 400 mg film-coated tablets [2]. For patients with chronic myeloid leukemia, the recommended daily dose is 800 mg (calculated by 400 mg film-coated tablets), preferably after meals, and drink enough water to reduce the possible gastrointestinal adverse reactions [2]. This simple and convenient medication method breaks the limitation of the previous treatment mode on the survival rate, promotes the change of disease management mode to home care mode, and greatly improves the quality of life and treatment compliance of patients [2,4].
Because the water solubility of paclitaxel is too low, it must rely on Cremophor EL as a carrier before intravenous injection [14]. Its conventional dose (according to different tumor types and treatment schemes, this value will be different, for example, the dose used for breast cancer is often 175 mg/m). Generally, intravenous injection is performed once every three weeks, and the single duration is about 3 to 24 hours. Moreover, Cremophor EL is closely related to severe allergic reactions, so this therapy is classified as a relatively invasive treatment and should be strictly monitored [2,14]. In order to reduce the possible adverse reactions, patients should receive the combined pretreatment of corticosteroids (such as dexamethasone), histamine H receptor blockers (such as diphenhydramine) and H receptor antagonists (such as cimetidine) at least 30 minutes in advance [14].
5. Conclusion
The comparative study of paclitaxel and imatinib has gone beyond the traditional research scope of drugs and become the most representative example in the field of tumor treatment in a new era. As a natural product extracted from yew plants, paclitaxel has stimulated the traditional chemotherapy methods by its unique biological characteristics. Although people still have disputes about its specific mechanism, there are still many opportunities for life to continue because of this magical treatment. At present, it has been widely used in various solid tumors, including but not limited to breast cancer, especially in the clinical treatment of breast cancer, which plays an important role that cannot be ignored by anyone. In contrast, the modern molecular targeted drug imatinib shows the development direction of a new treatment method at the molecular level, and embodies the core concept of "precision medicine", that is, by interpreting the genomic characteristics of diseases, a new type of small molecular substance with higher efficiency and safety can be designed, which can significantly improve the overall survival rate of patients with chronic myeloid leukemia and their living conditions. The road to cancer treatment is not a substitute for a single road, but a road that needs many roads to go together. Looking ahead, there will not be only one way to develop in the future, and we should think more about the interaction between various methods. Now there are still many big problems in our research. On the one hand, we should continue to give full play to the special advantages of traditional chemotherapy in cytotoxin cleaning, on the other hand, we should constantly improve the ultra-high accuracy of targeted therapy and immunotherapy. With the gradual understanding of personal tumor gene map, we can accurately find the most suitable targeted drugs and immunotherapy methods. The main purpose is to achieve the best results through the combination of many angles, postpone the stage of drug resistance and greatly improve the quality of life of patients, so that the treatment process can have both scientific basis and a sense of temperature.
References
[1]. National Cancer Institute. "Cancer Basics." National Institutes of Health, 2021.
[2]. Capdeville, R., et al. "Imatinib: A Paradigm of Targeted Therapy." Nature Reviews Cancer, vol. 2, no. 8, 2002, pp. 554-63.
[3]. Weaver, Beth A. "How Taxol/Paclitaxel Kills Cancer Cells." Molecular Biology of the Cell, vol. 25, no. 18, 2014, pp. 2677-81.
[4]. Druker, Brian J., et al. "Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia." New England Journal of Medicine, vol. 344, no. 14, 2001, pp. 1031-37.
[5]. National Cancer Institute. "Cancer Symptoms." National Institutes of Health, 29 Apr. 2015, www.cancer.gov/about-cancer/diagnosis-staging/symptoms.
[6]. Sung, Hyuna, et al. "Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries." CA: A Cancer Journal for Clinicians, vol. 74, no. 3, 2024, pp. 209-49.
[7]. World Health Organization. "Cancer." WHO, 2022, www.who.int/news-room/fact-sheets/detail/cancer.
[8]. Xia, Changfa, et al. "Cancer Statistics in China and United States, 2022: Profiles, Trends, and Determinants." Chinese Medical Journal, vol. 135, no. 5, 2022, pp. 584-90.
[9]. DeVita, Vincent T., et al. Cancer: Principles & Practice of Oncology. 11th ed., Lippincott Williams & Wilkins, 2018.
[10]. American Cancer Society. "Chronic Myeloid Leukemia." ACS, 2022.
[11]. Wani, Mansukh C., et al. "Plant Antitumor Agents. VI. The Isolation and Structure of Taxol, a Novel Antileukemic and Antitumor Agent from Taxus Brevifolia." Journal of the American Chemical Society, vol. 93, no. 9, 1971, pp. 2325-27.
[12]. Schiff, Peter B., et al. "Promotion of Microtubule Assembly In Vitro by Taxol." Nature, vol. 277, 1979, pp. 665-67.
[13]. Buchdunger, E., et al. "Inhibition of the Abl Protein-Tyrosine Kinase In Vitro and In Vivo by a 2-Phenylaminopyrimidine Derivative." Cancer Research, vol. 56, 1996, pp. 100-04.
[14]. Weiss, Raymond B., et al. "Hypersensitivity Reactions from Taxol." Journal of Clinical Oncology, vol. 8, 1990, pp. 1263-68.
Cite this article
Sun,C. (2025). From Cytotoxicity to Targeted Therapy: A Pharmacological Comparative Study of Paclitaxel and Imatinib. Theoretical and Natural Science,144,97-103.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. National Cancer Institute. "Cancer Basics." National Institutes of Health, 2021.
[2]. Capdeville, R., et al. "Imatinib: A Paradigm of Targeted Therapy." Nature Reviews Cancer, vol. 2, no. 8, 2002, pp. 554-63.
[3]. Weaver, Beth A. "How Taxol/Paclitaxel Kills Cancer Cells." Molecular Biology of the Cell, vol. 25, no. 18, 2014, pp. 2677-81.
[4]. Druker, Brian J., et al. "Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia." New England Journal of Medicine, vol. 344, no. 14, 2001, pp. 1031-37.
[5]. National Cancer Institute. "Cancer Symptoms." National Institutes of Health, 29 Apr. 2015, www.cancer.gov/about-cancer/diagnosis-staging/symptoms.
[6]. Sung, Hyuna, et al. "Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries." CA: A Cancer Journal for Clinicians, vol. 74, no. 3, 2024, pp. 209-49.
[7]. World Health Organization. "Cancer." WHO, 2022, www.who.int/news-room/fact-sheets/detail/cancer.
[8]. Xia, Changfa, et al. "Cancer Statistics in China and United States, 2022: Profiles, Trends, and Determinants." Chinese Medical Journal, vol. 135, no. 5, 2022, pp. 584-90.
[9]. DeVita, Vincent T., et al. Cancer: Principles & Practice of Oncology. 11th ed., Lippincott Williams & Wilkins, 2018.
[10]. American Cancer Society. "Chronic Myeloid Leukemia." ACS, 2022.
[11]. Wani, Mansukh C., et al. "Plant Antitumor Agents. VI. The Isolation and Structure of Taxol, a Novel Antileukemic and Antitumor Agent from Taxus Brevifolia." Journal of the American Chemical Society, vol. 93, no. 9, 1971, pp. 2325-27.
[12]. Schiff, Peter B., et al. "Promotion of Microtubule Assembly In Vitro by Taxol." Nature, vol. 277, 1979, pp. 665-67.
[13]. Buchdunger, E., et al. "Inhibition of the Abl Protein-Tyrosine Kinase In Vitro and In Vivo by a 2-Phenylaminopyrimidine Derivative." Cancer Research, vol. 56, 1996, pp. 100-04.
[14]. Weiss, Raymond B., et al. "Hypersensitivity Reactions from Taxol." Journal of Clinical Oncology, vol. 8, 1990, pp. 1263-68.









