1. Introduction
1.1. The definition of hypertension
Hypertension is both a distinct disease and a significant risk factor for other conditions. The international guidelines for management hypertension primarily adopt two approaches to define hypertension: either basing on the blood pressure (BP) threshold requiring treatment initiation, or determined by blood pressure cut-off level associated with elevated cardiovascular risk events. Current guidelines determine hypertension criteria based on blood pressure thresholds where the proven benefits of antihypertensive treatment surpass the treatment risks in interventional clinical trials. The threshold for defining hypertension based on this approach is 140/90 mmHg, consistent with standard clinical measurement methods [1]. Guidelines also emphasize ranges of hypertension severity by stratifying blood pressure level exceeding these thresholds into some tiers. Based on it, hypertension is defined into three stages, ranging from stage 1 to stage 3 hypertension (Table 1) [2,3].
Epidemiological studies demonstrate that the occurrence of cardiovascular incidents—including heart attack, stroke, heart failure, etc.—rises progressively with increasing blood pressure. This association follows an exponential trend and is more pronounced for systolic than diastolic pressure.
|
Stage |
Systolic blood pressures(SBP) (mmHg) |
Diastolic blood pressures(DBP) (mmHg) |
Degree of Target Organ Damage |
|
Normal blood pressure |
<120 |
<80 |
/ |
|
Elevated blood pressure |
120~139 |
80~89 |
/ |
|
Stage 1 hypertension |
140~159 |
90~99 |
No organ damage |
|
Stage 2 hypertension |
160~179 |
100~109 |
Compensated organ injury |
|
Stage 3 hypertension |
≥180 |
≥110 |
Decompensated dysfunction of the injured organ |
1.2. Global epidemiology
According to an analysis of 135 population-based studies encompassing 0.9 million adults, the worldwide age-standardized prevalence of hypertension in 2010 was approximated at 31.1% (95% confidence interval 30.0-32.2%). The finding showed a modestly higher rate among males (about 32%) compared to females (about 30%) [4]. When analyzed by national income levels, HICs exhibited a lower prevalence than LMICs (Table 2) [4]. Current evidence indicates that racial disparities in hypertension risk cannot be explained by genetic factors. Environmental exposures, sociodemographic characteristics, and behavioral patterns are more probable responsible for ethnic variations in mean blood pressure and hypertension prevalence [5]. Furthermore, multiple factors like obesity, high sodium intake, physical inactivity, and unhealthy dietary patterns—demonstrate consistent associations with elevated hypertension risk.
|
Population |
Men (Age-standardized prevalence %) (95% CIs) |
Women (Age-standardized prevalence %) (95% CIs) |
|
Global |
31.9 (30.3–33.5) |
30.1 (28.5–31.6) |
|
HICs |
31.6 (29.6–33.6) |
25.3 (23.9–26.7) |
|
LMICs |
31.7 (29.7–33.6) |
31.2 (29.3–33.1) |
Hypertension is strongly linked to increased global incidence of cardiovascular disease. Data from 2015 demonstrated that SBP ≥115 mmHg contributed to about 10.7 million deaths from all causes accounting for 19.2% of all deaths. About 7.8 million deaths for 14.0% of all deaths were associated with SBP ≥140 mmHg. The most kinds of deaths that were related to SBP ≥115 mmHg, were included ischemic heart disease and hemorrhagic stroke and so on. When using the higher threshold (SBP≥140 mmHg), the corresponding mortality figures were 3.6 million and 1.4 million. Consistent with hypertension prevalence trends, there was a notable rise in blood pressure-related cardiovascular mortality between 1990 and 2015, particularly pronounced in LMICs [6].
Randomized clinical trials showed that conventional antihypertensive regimens (including angiotensin receptor blockers, diuretics, angiotensin-converting enzyme inhibitors (ACEIs), and calcium channel blockers) effectively reduce cardiovascular disease mortality. Meta-analyses show a positive correlation between the extent of BP reduction and the reduction of relative risk for both cardiovascular outcomes and all-cause death [7].
Scaling up coverage of blood pressure-lowering interventions to reduce associated incidence rate and mortality rate should constitute a worldwide public health priority.
1.3. Principles of antihypertensive agent therapy
The primary approach to managing hypertension involves pharmacological therapy combined with dietary sodium restriction. The primary goal of antihypertensive medication is to maintain blood pressure within a safe range, thereby preventing complications and reducing mortality risk. In clinical practice, most hypertensive patients requiring pharmacotherapy present with comorbidities such as hypercholesterolemia, obesity, or diabetes. For these individuals, treatment objectives extend beyond achieving blood pressure levels below 140/90 mmHg to include targeted management of concurrent conditions. Given the diverse mechanisms of action and patient-specific indications among antihypertensive drugs, individualized therapeutic regimens are essential.
• Targeted treatment principle: Due to variations in patients’ age, underlying diseases, and tolerance levels, the selection of medications should consider complications and blood pressure values.
• Minimizing side effects principle: There are numerous medications available for the treatment of hypertension, each with distinct adverse reactions. Typically, hypertensive patients are treated with a combination of two or more drugs; however, the increased number of medications in combination therapy raises the risk of adverse reactions [8]. Therefore, patients should be instructed to promptly inform their physicians of any discomfort, and physicians—guided by pharmacists—should adjust the treatment regimen to minimize adverse drug effects.
1.4. The mechanism of primary hypertension
The fundamental factors determining arterial blood pressure (ABP) consist of two components: Cardiac output and vascular resistance. Cardiac output is primarily influenced by cardiac function, venous return, and blood volume, while peripheral resistance is associated with the constriction of arterioles. The regulation of arterial blood pressure occurs mainly through two mechanisms: neural regulation mediated by the sympathetic nervous system, and humoral regulation via the renin-angiotensin-aldosterone system (RAAS).
1.4.1. RAAS dysregulation
Current research consensus indicates that a significant proportion of hypertension cases result from dysfunction of the RAAS. To address this pathophysiological mechanism, regulating water-sodium metabolism serves as a crucial therapeutic target. Maintaining optimal water-sodium balance prevents relative hypervolemia, thereby contributing to blood pressure control. This principle forms a fundamental basis for clinical hypertension management [9].
Hepatic production of angiotensinogen, encoded by the AGT gene, initiates the RAAS [10]. Under renin’s catalytic action, angiotensinogen is cleaved into angiotensin I (Ang I), which is later converted by angiotensin-converting enzyme (ACE) to angiotensin II (Ang II). Ang II binds to AT receptors, inducing vasoconstriction of arteriolar smooth muscle and elevating microcirculatory resistance, thereby increasing blood pressure (Figure 1). Besides, angiotensin II encourages aldosterone secretion from the adrenal cortex’s zona glomerulosa, promoting renal sodium and water retention while enhancing potassium excretion—mechanisms that further exacerbate hypertension [11].Therefore, water-sodium retention is the direct causative factor of hypertension, while angiotensin II plays a crucial mediating role.
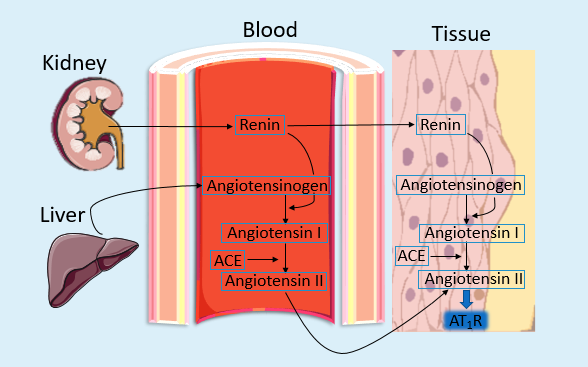
1.4.2. Sympathetic nerve activity(SNA) contributions
The sympathetic nervous system regulates (ABP) through functional modulation of the vascular system, kidneys, and heart. Dysfunction in sympathetic activity is both a causative factor and a pathophysiological component in various cardiovascular diseases, including hypertension [12]. Manifestations of sympathetic overactivity in human hypertension include increased norepinephrine spillover, and an exaggerated blood pressure-lowering response following acute ganglionic blockade [13].
The pathogenesis of hypertension is predominantly linked to increased SNA. The regulation of SNA involves multiple brain nuclei, including the paraventricular nucleus (PVN), organum vasculosum of the lamina terminalis (OVLT), caudal ventrolateral medulla (CVLM), arcuate nucleus (Arc), subfornical organ (SFO), rostral ventrolateral medulla (RVLM), nucleus of the solitary tract (NTS), median preoptic nucleus (MnPO), and orexin neurons [14-16]. These nuclei constitute central sympathetic neural circuits via synaptic connections, collectively modulating blood pressure regulation and feedback control. The SFO and OVLT can sense angiotensin II, aldosterone, plasma osmolality, and certain cytokines, thereby activating the PVN-RVLM-SPGN pathway, which enhances SNA and increases blood pressure [17]. Elevated levels of insulin and leptin can be detected by the arcuate nucleus (Arc), leading to the activation of the PVN-RVLM-SPGN pathway [18]. The ARC can also directly activate the RVLM or SPGN, thereby enhancing SNA activity [19]. Carotid baroreceptors may activate the NTS, which in turn activates the CVLM. Once stimulated by upstream signals, the CVLM exerts inhibitory effects on the RVLM, leading to blood pressure reduction [20]. These sympathetic-related nuclei form intricate interactions, and dysfunction at any node within this feedback regulatory neural circuit may shift the homeostatic set point of blood pressure control—a potential mechanism underlying hypertension pathogenesis (Figure 2).
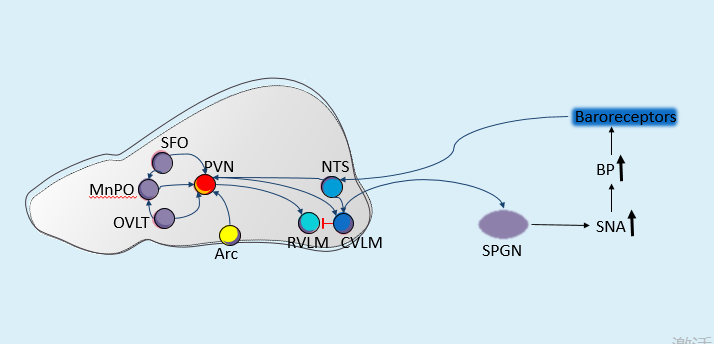
1.5. Drugs used to treat the hypertension
Hypertension is commonly managed using various categories of antihypertensive drugs. Diuretics (e.g. thiazides like hydrochlorothiazide) reduce blood volume by promoting natriuresis. ACEIs (e.g. Enalapril) and angiotensin II receptor blockers (ARBs; e.g. losartan) modulate RAAS to induce vasodilation. Calcium channel blockers (CCBs; e.g. Nifedipine) inhibit vascular smooth muscle contraction, reducing peripheral resistance. Beta-blockers (e.g. Propranolol) decrease cardiac output via β-adrenergic receptor antagonism. This paper will conduct a comprehensive investigation into two representative antihypertensive drugs.
2. Entresto (LCZ696)
2.1. Chemical structure
Entresto (LCZ696) is an inhibitor targeting both angiotensin II receptor and neprilysin. This complex unimolecular compound consists of valsartan and AHU377 molecular moieties in a 1:1 stoichiometric ratio. With a molecular weight of 843.99 and molecular formula of C48H55N6O8, the structural configuration is depicted in Figure 3.
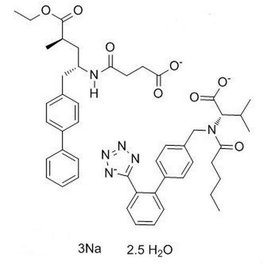
2.2. Pharmacology of LCZ696
As one of the primary active components of LCZ696, Angiotensin Receptor Blockers (ARBs) are currently recognized internationally as first-line antihypertensive medications. Sodium-water retention and activation of the RAAS are considered central factors in the pathogenesis of hypertension, with the latter being closely associated with both hypertension development and target organ damage. When the RAAS system is activated due to various causes, angiotensin II exerts multiple pathophysiological effects through binding to AT1 receptors, including promoting vascular smooth muscle contraction, stimulating aldosterone synthesis and exacerbating sodium-water retention, as well as inducing myocardial remodeling.
A trial was conducted to evaluate the effectiveness and safety of LCZ696 in Asian patients with hypertension. 364 Asian patients with hypertension randomly receive 100 mg, 200 mg, or 400 mg of LCZ696 or placebo for 8 weeks. Compared with the placebo group, patients receiving LCZ696 demonstrated significant reductions in diastolic blood pressure (7.84 mmHg, 7.29 mmHg, and 8.76 mmHg, respectively; P < 0.0001). Similarly, pulse pressure decreased by 4.01 mmHg, 5.4 mmHg, and 6.73 mmHg (P < 0.001). These findings indicate that one of the antihypertensive mechanisms of LCZ696 may involve the attenuation of sodium retention [21].
One of the active ingredients in LCZ696 is the angiotensin receptor antagonist valsartan. By inhibiting the AT₁ receptor, it blocks the harmful effects of Ang II mediated through AT₁ binding, thereby slowing the advancement of heart failure caused by activation of the RAAS. Additionally, due to the blockade of AT₁ receptors, the concentration of Ang II increases. The elevated Ang II binds to the AT₂ receptor, producing effects opposite to those of AT₁ activation, such as inhibiting cardiomyocyte growth, promoting cardiomyocyte differentiation, and enhancing nitric oxide synthesis [22]. In the randomized controlled trial, the antihypertensive efficacy of LCZ696 was comparison with valsartan monotherapy. The study enrolled about one thousand subjects ranging in age from 18 to 75 years with mild hypertension to moderate hypertension, who were designated to receive 100 mg, 200 mg, or 400 mg of LCZ696; 80 mg, 160 mg, or 320 mg of valsartan; 200 mg of AHU377; or placebo. Among them, 1,215 participants completed the trial. Results demonstrated that patients receiving LCZ696 exhibited substantially greater reduction in mean seated DBP in comparison to those receiving equivalent doses of valsartan. Notably, the blood pressure-lowering difference was more pronounced between LCZ696 compared to valsartan (Table 3). This trial confirmed that LCZ696 provides superior antihypertensive efficacy compared to equivalent doses of valsartan [23].
|
Dose (mg) |
Additional reduction with LCZ696 (mmHg) |
95% CI |
p-value |
|
Overall LCZ696 vs Valsartan |
-2.17 |
-3.28 to -1.06 |
p<0.0001 |
|
200 mg LCZ696 vs 160 mg Valsartan |
-2.97 |
-4.88 to -1.07 |
p=0.0023 |
|
400 mg LCZ696 vs 320 mg Valsartan |
-2.70 |
-4.61 to -0.80 |
p=0.0055 |
3. Captopril
3.1. Chemical structure
Captopril, an ACEI, is a small-molecule compound whose chemical name is (2S)-1-[(2S)-2-methyl-3-sulfanyl-1-oxopropyl]-L-proline. It has a molecular weight of 217.29 and a molecular formula of C9H15NO3S. The key structural features of captopril include a sulfhydryl (-SH) functional group, a proline-derived scaffold, and a stereogenic center (S-configuration). Its structural configuration is depicted in Figure 4.
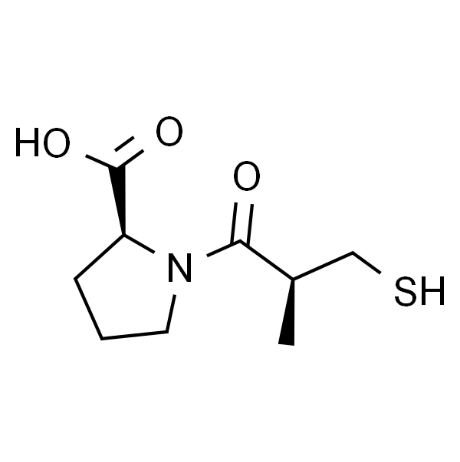
3.2. Pharmacology of Captopril
Captopril is one of the earliest and most well-established ACEIs. Captopril exerts its antihypertensive effects primarily through inhibition of the RAAS [24]. As an ACEI, it competitively blocks the enzymatic conversion of Ang I to Ang II. This suppression results in reduced plasma angiotensin II levels, as well as vasodilation and decreased aldosterone secretion. Consequently, captopril reduces peripheral arterial resistance. Within the cardiovascular system, ACEIs diminish cardiac preload through vasodilation and natriuresis, while reducing afterload via inhibition of angiotensin II production, thereby improving cardiac output and lowering blood pressure [25]. Additionally, since ACE also catalyzes the degradation of bradykinin — a potent vasodilator — ACE inhibitors prolong bradykinin activity, which contributes to both therapeutic vasodilation and the characteristic bradykinin-mediated cough. Notably, among ACE inhibitors, only captopril and lisinopril are pharmacologically active without requiring metabolic activation, whereas other agents in this class necessitate biotransformation to achieve therapeutic efficacy.
Captopril is effectively absorbed when orally taken and reaches peak plasma levels about one hour after administration. Food intake significantly reduces its bioavailability, thus administration 1 hour before meals is advised. The drug has a distribution volume of 0.8 L/kg and demonstrates approximately 25%–30% plasma protein binding. It easily crosses the blood-brain barrier and undergoes metabolic conversion to captopril dimer disulfide and cysteine-captopril disulfide in vivo [26]. The primary excretion pathway is renal elimination, with an elimination half-life of about 2 hours.
Captopril, while typically well-tolerated, exhibits several potential adverse effects.
• Renal failure, urinary frequency nephrotic syndrome: Frequently observed with renal artery stenosis or nephrosclerosis dilatation of the efferent arterioles occurs, reduced renal perfusion pressure, and impaired renal function. These effects are often reversible upon discontinuation [27].
• Sulfhydryl group-related effects: May induce neutropenia/agranulocytosis with bone marrow depression and dysgeusia (reversible and typically self-limiting).
• Angioedema: Rare but serious; associated with bradykinin and its metabolites though definitive evidence remains lacking. May occur in any region, but epiglottis or larynx may cause respiratory obstruction. Immediate intubation is required if airway compromise occurs until edema resolves.
• Dry cough: A common adverse reaction and major reason for discontinuation, potentially due to pulmonary bradykinin accumulation.
• First-dose hypotension.
• Hyperkalemia risk: ACE inhibitors may elevate serum potassium, particularly in renal impairment or diabetes with concomitant potassium-sparing diuretics. Fatal arrhythmias from hyperkalemia (e.g., cases combining ACE inhibitors with trimethoprim) have been reported [25].
4. Conclusion
The pharmacological profiles of LCZ696 and captopril demonstrate distinct mechanisms and clinical efficacies in hypertension management. LCZ696, as a ARNi, exhibits superior antihypertensive effects compared to valsartan monotherapy, attributed to synergistic RAAS inhibition and natriuretic peptide potentiation. This advantage is attributed to its synergistic RAAS inhibition and natriuretic peptides potentiation. In contrast, captopril, a classic ACE inhibitor, primarily targets angiotensin II suppression and bradykinin accumulation, though with a narrower therapeutic window and higher incidence of adverse effects (e.g., cough, hyperkalemia). While both drugs modulate RAAS, LCZ696’s dual-target action offers enhanced hemodynamic benefits, particularly in reducing peripheral resistance and sodium retention. However, captopril remains relevant for its rapid onset and cost-effectiveness in specific populations. Ultimately, the choice of therapy should balance efficacy, safety, and patient-specific factors. Looking ahead, individualized treatment strategies and the development of next-generation agents may further improve hypertension management and outcomes.
References
[1]. Gabb, G. M., Mangoni, A. A., et al. (2016). Guideline for the diagnosis and management of hypertension in adults – 2016. Medical Journal of Australia, 205(2), 85–89. https: //doi.org/10.5694/mja16.00526
[2]. Williams, B., Mancia, G., et al. (2018). 2018 ESC/ESH guidelines for the management of arterial hypertension. European Heart Journal, 39(33), 3021-3104. https: //doi.org/10.1093/eurheartj/ehy339
[3]. Whelton, P. K., Carey, R. M., et al. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 71(6), e13-e115. https: //doi.org/10.1161/HYP.0000000000000065
[4]. Mills, K. T., Bundy, J. D., et al. (2016). Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation, 134(6), 441-450. https: //doi.org/10.1161/CIRCULATIONAHA.115.018912
[5]. Whelton, P. K., Einhorn, P. T., et al. (2016). Research needs to improve hypertension treatment and control in African Americans. Hypertension, 68(5), 1066–1072. https: //doi.org/10.1161/HYPERTENSIONAHA.116.07905
[6]. Forouzanfar, M. H., Liu, P., et al. (2017). Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA, 317(2), 165–182. https: //doi.org/10.1001/jama.2016.19043
[7]. Ettehad, D., Emdin, C. A., et al. (2016). Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. The Lancet, 387(10022), 957–967. https: //doi.org/10.1016/S0140-6736(15)01225-8
[8]. Zhang, P. (2016). Clinical observation and nursing analysis of side effects of antihypertensive drugs. China Health Standard Management, 7(02), 213-214. https: //doi.org/10.3969/j.issn.1674-9316.2016.02.154
[9]. Chen, D. (2020). Research progress on the pathogenesis of hypertension. Journal of Medicine and Philosophy, 33(22), 3722-3724+3727. https: //doi.org/10.19381/j.issn.1001-7585.2020.22.010
[10]. Wang, G., Zhang, Y., & Zhang, M. (2019). Research progress of RAAS gene and essential hypertension. Journal of Medical Forum, 40(3), 175-177. https: //doi.org/10.19613/j.cnki.1671-3141.2019.03.072
[11]. Te Riet, L., van Esch, J. H. M., et al. (2015). Hypertension: Renin-angiotensin-aldosterone system alterations. Circulation Research, 116(6), 960-975. https: //doi.org/10.1161/CIRCRESAHA.116.303587
[12]. Esler, M., Straznicky, N., et al. (2006). Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension, 48(5), 787–796. https: //doi.org/10.1161/01.HYP.0000242642.42177.49
[13]. Grassi, G., Mark, A., & Esler, M. (2015). The sympathetic nervous system alterations in human hypertension. Circulation Research, 116(6), 976–990. https: //doi.org/10.1161/CIRCRESAHA.116.303604
[14]. Guyenet, P. G., Stornetta, R. L., et al. (2020). Neuronal networks in hypertension: Recent advances. Hypertension, 76(2), 300-311. https: //doi.org/10.1161/HYPERTENSIONAHA.120.14521
[15]. Gerlach, D. A., Manuel, J., et al. (2021). Medullary and hypothalamic functional magnetic imaging during acute hypoxia in tracing human peripheral chemoreflex responses. Hypertension, 77(4), 1372-1382. https: //doi.org/10.1161/HYPERTENSIONAHA.120.16385
[16]. Dampney, R. A., Michelini, L. C., et al. (2018). Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. American Journal of Physiology-Heart and Circulatory Physiology, 315(5), H1200-H1214. https: //doi.org/10.1152/ajpheart.00216.2018
[17]. Stocker, S. D., Wenner, M. M., et al. (2022). Activation of the organum vasculosum of the lamina terminalis produces a sympathetically mediated hypertension. Hypertension, 79(1), 139–149. https: //doi.org/10.1161/HYPERTENSIONAHA.121.18117
[18]. Shi, Z., Wong, J., & Brooks, V. L. (2020). Obesity: Sex and sympathetics. Biology of Sex Differences, 11(1), 10. https: //doi.org/10.1186/s13293-020-00286-8
[19]. Wang, D., He, X., et al. (2015). Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Frontiers in Neuroanatomy, 9, 40. https: //doi.org/10.3389/fnana.2015.00040
[20]. Dampney, R. A. L. (2017). Resetting of the baroreflex control of sympathetic vasomotor activity during natural behaviors: Description and conceptual model of central mechanisms. Frontiers in Neuroscience, 11, 461. https: //doi.org/10.3389/fnins.2017.00461
[21]. Kario, K., Sun, N., et al. (2014). Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: A randomized, double-blind, placebo-controlled study. Hypertension, 63(4), 698–705. https: //doi.org/10.1161/HYPERTENSIONAHA.113.02002
[22]. Brandt, R. R., Redfield, M. M., et al. (1994). Clearance receptor-mediated control of atrial natriuretic factor in experimental congestive heart failure. American Journal of Physiology, 266(3), R936–R943.
[23]. Ruilope, L. M., Dukat, A., et al. (2010). Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study. The Lancet, 375(9722), 1255–1266. https: //doi.org/10.1016/S0140-6736(10)60696-1
[24]. Gan, Z., Huang, D., et al. (2018). Captopril alleviates hypertension-induced renal damage, inflammation, and NF-κB activation. Brazilian Journal of Medical and Biological Research, 51(11), e7338. https: //doi.org/10.1590/1414-431X20187338
[25]. Herman, L. L., Padala, S. A., et al. (2023, July 31). Angiotensin-converting enzyme inhibitors (ACEI). StatPearls Publishing. https: //www.ncbi.nlm.nih.gov/books/NBK431051/
[26]. Zisaki, A., Miskovic, L., & Hatzimanikatis, V. (2015). Antihypertensive drugs metabolism: An update to pharmacokinetic profiles and computational approaches. Current Pharmaceutical Design, 21(6), 806-822. https: //doi.org/10.2174/1381612820666141027112427
[27]. Lindle, K. A., Dinh, K., et al. (2014). Angiotensin-converting enzyme inhibitor nephrotoxicity in neonates with cardiac disease. Pediatric Cardiology, 35(3), 499–506. https: //doi.org/10.1007/s00246-013-0810-5
Cite this article
Chen,Y. (2025). Comparative Pharmacological Effects of LCZ696 and Captopril in Hypertension. Theoretical and Natural Science,144,142-150.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Gabb, G. M., Mangoni, A. A., et al. (2016). Guideline for the diagnosis and management of hypertension in adults – 2016. Medical Journal of Australia, 205(2), 85–89. https: //doi.org/10.5694/mja16.00526
[2]. Williams, B., Mancia, G., et al. (2018). 2018 ESC/ESH guidelines for the management of arterial hypertension. European Heart Journal, 39(33), 3021-3104. https: //doi.org/10.1093/eurheartj/ehy339
[3]. Whelton, P. K., Carey, R. M., et al. (2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 71(6), e13-e115. https: //doi.org/10.1161/HYP.0000000000000065
[4]. Mills, K. T., Bundy, J. D., et al. (2016). Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation, 134(6), 441-450. https: //doi.org/10.1161/CIRCULATIONAHA.115.018912
[5]. Whelton, P. K., Einhorn, P. T., et al. (2016). Research needs to improve hypertension treatment and control in African Americans. Hypertension, 68(5), 1066–1072. https: //doi.org/10.1161/HYPERTENSIONAHA.116.07905
[6]. Forouzanfar, M. H., Liu, P., et al. (2017). Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA, 317(2), 165–182. https: //doi.org/10.1001/jama.2016.19043
[7]. Ettehad, D., Emdin, C. A., et al. (2016). Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. The Lancet, 387(10022), 957–967. https: //doi.org/10.1016/S0140-6736(15)01225-8
[8]. Zhang, P. (2016). Clinical observation and nursing analysis of side effects of antihypertensive drugs. China Health Standard Management, 7(02), 213-214. https: //doi.org/10.3969/j.issn.1674-9316.2016.02.154
[9]. Chen, D. (2020). Research progress on the pathogenesis of hypertension. Journal of Medicine and Philosophy, 33(22), 3722-3724+3727. https: //doi.org/10.19381/j.issn.1001-7585.2020.22.010
[10]. Wang, G., Zhang, Y., & Zhang, M. (2019). Research progress of RAAS gene and essential hypertension. Journal of Medical Forum, 40(3), 175-177. https: //doi.org/10.19613/j.cnki.1671-3141.2019.03.072
[11]. Te Riet, L., van Esch, J. H. M., et al. (2015). Hypertension: Renin-angiotensin-aldosterone system alterations. Circulation Research, 116(6), 960-975. https: //doi.org/10.1161/CIRCRESAHA.116.303587
[12]. Esler, M., Straznicky, N., et al. (2006). Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension, 48(5), 787–796. https: //doi.org/10.1161/01.HYP.0000242642.42177.49
[13]. Grassi, G., Mark, A., & Esler, M. (2015). The sympathetic nervous system alterations in human hypertension. Circulation Research, 116(6), 976–990. https: //doi.org/10.1161/CIRCRESAHA.116.303604
[14]. Guyenet, P. G., Stornetta, R. L., et al. (2020). Neuronal networks in hypertension: Recent advances. Hypertension, 76(2), 300-311. https: //doi.org/10.1161/HYPERTENSIONAHA.120.14521
[15]. Gerlach, D. A., Manuel, J., et al. (2021). Medullary and hypothalamic functional magnetic imaging during acute hypoxia in tracing human peripheral chemoreflex responses. Hypertension, 77(4), 1372-1382. https: //doi.org/10.1161/HYPERTENSIONAHA.120.16385
[16]. Dampney, R. A., Michelini, L. C., et al. (2018). Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. American Journal of Physiology-Heart and Circulatory Physiology, 315(5), H1200-H1214. https: //doi.org/10.1152/ajpheart.00216.2018
[17]. Stocker, S. D., Wenner, M. M., et al. (2022). Activation of the organum vasculosum of the lamina terminalis produces a sympathetically mediated hypertension. Hypertension, 79(1), 139–149. https: //doi.org/10.1161/HYPERTENSIONAHA.121.18117
[18]. Shi, Z., Wong, J., & Brooks, V. L. (2020). Obesity: Sex and sympathetics. Biology of Sex Differences, 11(1), 10. https: //doi.org/10.1186/s13293-020-00286-8
[19]. Wang, D., He, X., et al. (2015). Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Frontiers in Neuroanatomy, 9, 40. https: //doi.org/10.3389/fnana.2015.00040
[20]. Dampney, R. A. L. (2017). Resetting of the baroreflex control of sympathetic vasomotor activity during natural behaviors: Description and conceptual model of central mechanisms. Frontiers in Neuroscience, 11, 461. https: //doi.org/10.3389/fnins.2017.00461
[21]. Kario, K., Sun, N., et al. (2014). Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: A randomized, double-blind, placebo-controlled study. Hypertension, 63(4), 698–705. https: //doi.org/10.1161/HYPERTENSIONAHA.113.02002
[22]. Brandt, R. R., Redfield, M. M., et al. (1994). Clearance receptor-mediated control of atrial natriuretic factor in experimental congestive heart failure. American Journal of Physiology, 266(3), R936–R943.
[23]. Ruilope, L. M., Dukat, A., et al. (2010). Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study. The Lancet, 375(9722), 1255–1266. https: //doi.org/10.1016/S0140-6736(10)60696-1
[24]. Gan, Z., Huang, D., et al. (2018). Captopril alleviates hypertension-induced renal damage, inflammation, and NF-κB activation. Brazilian Journal of Medical and Biological Research, 51(11), e7338. https: //doi.org/10.1590/1414-431X20187338
[25]. Herman, L. L., Padala, S. A., et al. (2023, July 31). Angiotensin-converting enzyme inhibitors (ACEI). StatPearls Publishing. https: //www.ncbi.nlm.nih.gov/books/NBK431051/
[26]. Zisaki, A., Miskovic, L., & Hatzimanikatis, V. (2015). Antihypertensive drugs metabolism: An update to pharmacokinetic profiles and computational approaches. Current Pharmaceutical Design, 21(6), 806-822. https: //doi.org/10.2174/1381612820666141027112427
[27]. Lindle, K. A., Dinh, K., et al. (2014). Angiotensin-converting enzyme inhibitor nephrotoxicity in neonates with cardiac disease. Pediatric Cardiology, 35(3), 499–506. https: //doi.org/10.1007/s00246-013-0810-5









