1. Introduction
1.1. Distribution of breast cancer
Breast cancer is one of the most common cancers worldwide, with an estimated 2.3 million new occurrences in 2024 [1].Its incidence is strongly correlated with the Human Development Index (HDI), with higher rates in developed countries. However, over half of new cases and two-thirds of deaths occur in developing countries. In developed countries, higher incidence is linked to longer life expectancy and higher HDI, while in developing countries, a younger population and shorter life expectancy result in a lower average age at diagnosis.
1.2. Symptoms of breast cancer
Breast cancer symptoms include breast lumps, skin changes, abnormal nipple discharge, nipple inversion, breast or nipple pain, and changes in breast contour [2]. However, early-stage breast cancer may present without obvious symptoms, emphasizing the significance of regular screening and self-examination.
1.3. Global impact of breast cancer
Globally, breast cancer is the leading cause of cancer-related deaths among women, resulting in roughly 685,000 female fatalities in 2020 [3], with almost two-thirds occurring in developed countries. In developed regions, over 80% 5-year survival rates are achieved due to early detection and effective treatments. In contrast, 5-year survival rates are below 70% in India and below 50% in South Africa. Late presentation in developed regions leads to poorer survival outcomes and strains healthcare systems. Breast cancer incidence and mortality rates among women have been rising in China as well. In 2022, breast cancer ranked top among female malignancies and accounted for 15.6% of all female cancers, with estimated 357,200 cases [4]. Thus, in 2021, the World Health Organization started the “Global Breast Cancer Initiative” with the goal of raising survival rates by health promotion, prompt diagnosis and comprehensive treatment.
1.4. Molecular mechanisms of breast cancer
The molecular biological mechanisms of breast cancer are complex and diverse, primarily involving gene mutations, estrogen stimulation and human epidermal growth factor receptor 2 (HER2) overexpression [5].
1.4.1. Gene mutations
Genetic mutations are one of the important factors that trigger the formation of breast cancer. BRCA1/BRCA2 are highly expressed in the breast, and their core function is the repair of DNA double-strand breaks. When they mutate, the homologous recombination repair function is lost, leading to genomic instability and significantly raising the chance of developing breast cancer [6]. This is particularly relevant to triple-negative breast cancer and estrogen receptor positive (ER+) breast cancer. In addition, TP53 gene mutations are common in triple-negative breast cancer and are linked to tumor aggressiveness and poor prognosis.
1.4.2. Estrogen stimulation
Under long-term high-level estrogen stimulation, E2 binds to ER, activating Cyclind1 and MYC overexpression, leading to uncontrolled cell proliferation. This is especially relevant to ER+ breast cancer.
1.4.3. HER2 positive
The progression of breast cancer is also intimately linked to aberrant signaling pathway activation. HER2 is a tyrosine kinase receptor and an important member of the epidemal growth factor receptor (EGFR) family. HER2 itself does not have a direct binding ligand but conducts signal transduction by forming homodimers itself or forming heterodimers with other EGFR family members (such as HER1, HER3, HER4).
As shown in Figure 1, when HER2 homodimerizes or heterodimerizes with HER3 that has bound the EGF ligand, PI3K is activated, generating PIP3, which in turn activates AKT, releasing the cell cycle block, enhancing transcription and translation, promoting cell proliferation, and inhibiting apoptosis [7]. In Figure 2, when HER2 is overexpressed, the PI3K/AKT signaling pathway is overly activated, causing uncontrolled cell growth and leading to HER2-positive breast cancer.
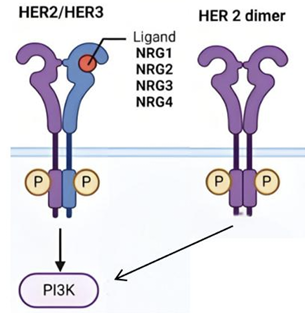
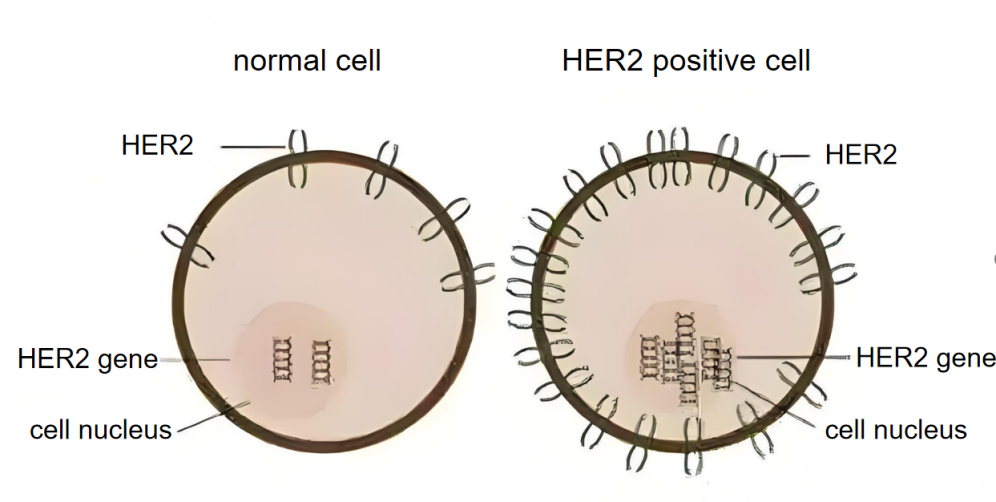
Approximately 20-30% of breast cancers have HER2 overexpression [8], and such cancer cells expand rapidly and are prone to metastasis. Therefore, the paper mainly focuses on HER2-positive breast cancer.
2. Paclitaxel
2.1. Chemical properties of paclitaxel
Paclitaxel is a broad-spectrum anticancer drug, primarily extracted from the bark of the Pacific yew tree. It is later produced on a large scale through semi-synthetic technologies and can also be extracted from parts of plants such as hazelnuts [9]. Chemically speaking, it belongs to the diterpenoid taxane class, with a molecular formula of C47H51NO14 . As exhibited in Figure 3, its structure consists of a taxane ring, a homochiral ester side chain at position C13, anda four-membered oxetane side ring at positions C4 and C5. The ester side chain and complete taxane ring are critical for its cytotoxic activity.
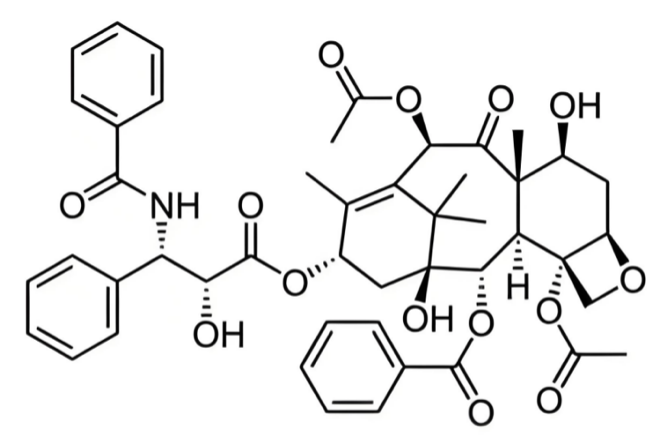
Paclitaxel is a hydrophobic drug with low water solubility, which limits its dissolution in water. However, its application can be improved by binding to albumin or using specific nanodelivery systems.
2.2. Pharmacology of paclitaxel
2.2.1. Drug targets
The mechanism of paclitaxel in treating breast cancer mainly involves mitotic arrest and induction of cell death, which is illustrated in Figure 4.
![Figure 4. Mechanism of paclitaxel [10]](https://file.ewadirect.com/press/media/markdown/document-image4_6Eihvaw.png)
The main target is microtubules. Cell division depends on the dynamic changes of microtubules. Paclitaxel can tightly bind to it, promote microtubule polymerization, stabilize the microtubule structure, and inhibit the normal dynamic depolymerization of microtubules, leading to abnormal spindle function, thereby stopping mitosis at the M phase and late G2 phase of the cell cycle and inducing cancer cell death [10].
In addition, paclitaxel can also activate the signaling pathways related to apoptosis, induce apoptosis; act on NLRP3 protein to induce pyroptosis, and act on LC3B protein to induce autophagy.
2.2.2. Mode of delivery
Traditional paclitaxel formulations use polyoxyethylated castor oil and ethanol as solvents but have side effects such as allergic reactions and toxicity. Currently, nanomedicine delivery systems are the focus, including nanoparticle albumin-bound paclitaxel (Nab-PTX) and paclitaxel polymer micelles [11], which can enhance drug concentration in tumor tissue, reduce toxicity to normal tissues, and prolong drug circulation in the body.
Nab-PTX has demonstrated excellent efficacy and safety in clinical trials and practical applications. Paclitaxel polymer micelles, however, due to their relatively short market availability, still require the accumulation of long-term efficacy and side effect data.
3. Trastuzumab
3.1. Structure of trastuzumab
Trastuzumab is a humanized monoclonal antibody made using recombinant DNA technology. As shown in Figure 5, it consists of two Fab segments and one Fc segment. The Fab segments can specifically bind to the HER2 receptor, while the Fc segment can activate the immune system [12], such as complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC), to attack HER2-positive tumor cells.
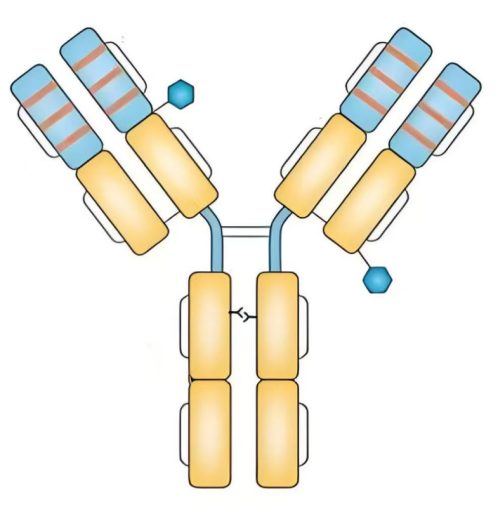
3.2. Pharmacology of trastuzumab
3.2.1. Drug targets
The primary target of trastuzumab is HER2. HER2 is overexpressed in various tumors, especially breast cancer, and is closely correlated with tumor cell proliferation, differentiation, apoptosis and other processes. Thus, it's a key target for treating breast cancer.
In breast tumor cells, HER2 is overexpressed. In Figure 6, trastuzumab specifically binds to HER2 [13], disrupting the synthesis of HER-2 homodimers and HER2-HER3 heterodimers, thereby preventing the PI3K/AKT signaling pathway from being activated, inhibiting uncontrolled cell growth, and exerting anti-tumor effects.
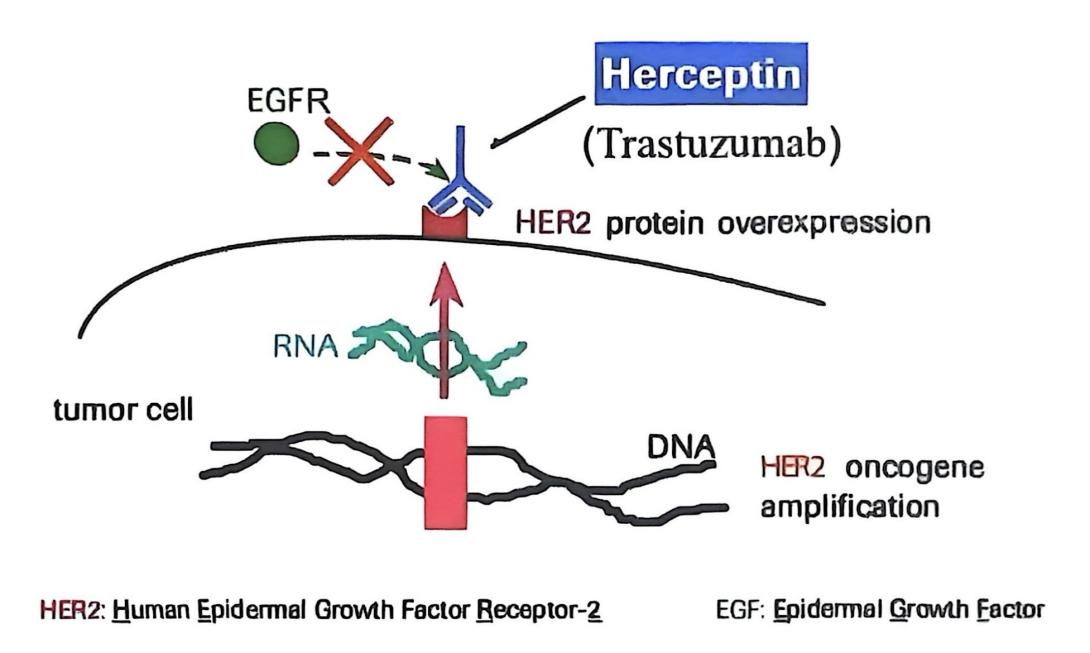
Trastuzumab can also activate ADCC, increase the activity of tumor-associated B cells [14], and enable immune effector cells to secrete granzymes and perforin, inducing tumor cell apoptosis.
3.2.2. Mode of delivery
Trastuzumab is a lyophilized powder that ranges from white to pale yellow. In order to administer it by intravenous transfusion, it must be diluted with sterile water for injection first, creating the colorless to pale yellow and transparent to slightly opalescent solution.
4. Combined use of paclitaxel and trastuzumab
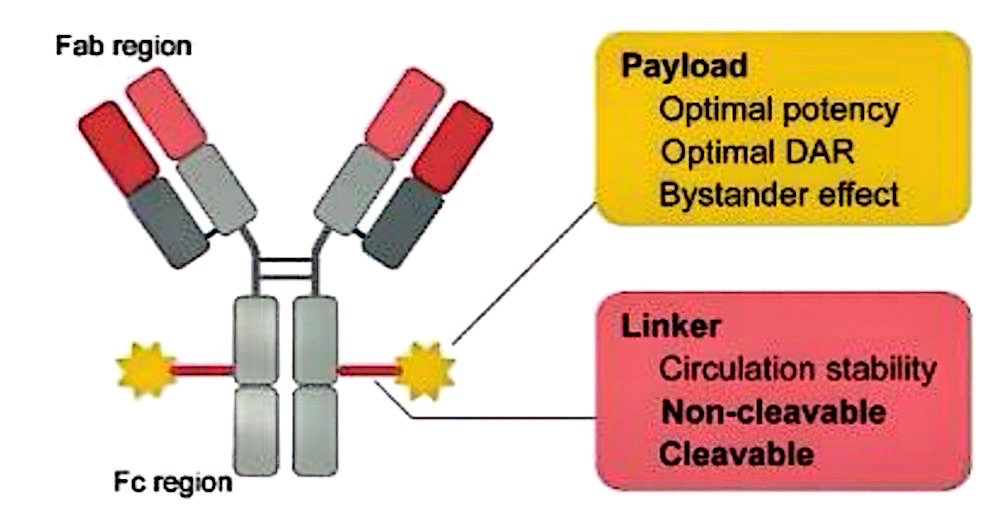
In the clinical treatment of cancer, combined medication is often used to enhance efficacy and reduce side effects. When treating HER2 positive breast cancer, it may be possible to combine chemotherapy drug paclitaxel with the targeted drug trastuzumab to form an antibody-drug conjugate (ADC) as shown in Figure 7, allowing paclitaxel to selectively target HER2-positive tumor cells, inhibiting their proliferation and promoting their apoptosis [15]. This approach offers higher precision, reduces harm to normal cells, and also enhances the body's immune response to tumor cells through trastuzumab, thereby improving efficacy and safety.
In one study, patients with HER2 were split into two groups: the combination group and the control group. For three cycles, each lasting 21 days, both groups got continuous treatment. The control group received an intravenous infusion of paclitaxel every day, while the combination group had an intravenous infusion of trastuzumab once a week in addition to the paclitaxel injection. According to the study, the combination group's tumor marker levels were lower than those of the control group, and their breast cancer control rate was greater [16]. This experimentally indicates that the combined use of paclitaxel and trastuzumab is more effective than the chemotherapy with paclitaxel alone.
Nevertheless, no ADC medication that combines paclitaxel and trastuzumab has been authorized for the clinical treatment of breast cancer as of yet [17]. The conjugated drug's structure, effectiveness, and safety require further research. ADC consists of an antibody, a cytotoxic payload and a linker. The design of the drug that conjugates trastuzumab and paclitaxel needs to consider the ratio of the drug to the antibody (DAR), the bystander effect (whether paclitaxel can kill adjacent tumor cells that do not express HER2), as well as the stability of the linker and whether the linker can be cleaved, etc.
5. Discussion
5.1. Paclitaxel resistance
In the treatment of breast cancer, resistance to paclitaxel may develop. The enhancement of the drug efflux mechanism is one of the important reasons: overexpression of ABC transporter family members such as P-glycoprotein in tumor cells can actively pump paclitaxel out of cells, reduce intracellular drug concentration, and weaken its killing effect. Furthermore, paclitaxel inhibits cell division by stabilizing microtubules [18]. However, when the expression of microtubulin subtypes (such as β-III tubulin) in tumor cells is abnormal, or the function of microtubule dynamic regulation-related proteins is dysfunctional, the sensitivity of microtubules to paclitaxel decreases. Consequently, the medication is unable to efficiently block the cell cycle. At the same time, fibroblasts in the tumor stroma secrete cytokines (such as IL-6 and TGF-β), may promote the drug-resistant phenotype of tumor cells and further aggravate the development of paclitaxel resistance.
5.2. Trastuzumab-induced cardiotoxicity
Trastuzumab is a commonly used targeted therapy drug for patients with HER2 positive breast cancer, but it can cause cardiac toxicity, posing a threat to patients' health. The mechanisms of its cardiac toxicity mainly include: first, weakening the activity of the neuroregulatory factor-1/tyrosine kinase receptor signaling pathway; second, increasing the expression of genes related to myocardial oxidative stress and nitrosative stress; third, inhibiting the signal transduction of human transmembrane receptor protein 1; fourth, activating the angiotensin receptor 1 signal pathway and causing myocardial damage. Therefore, when using trastuzumab for treatment, techniques such as cardiac magnetic resonance(CMR) can be adopted for monitoring, so as to timely and accurately detect cardiotoxicity and carry out appropriate intervention.
6. Conclusion
HER2 positive breast cancer has drawn much attention due to its strong invasiveness and high metastasis risk. Chemotherapy drug paclitaxel and monoclonal antibody trastuzumab can both effectively treat the cancer and reduce the risk of death to a certain extent when used alone. Additionally, the combination of these two drugs for targeted therapy against HER2 positive tumor cells may bring new hope to this subtype of breast cancer. Nevertheless, challenges such as paclitaxel resistance and cardiotoxicity of trastuzumab are also encountered. Future investigation can focus on optimizing the combined treatment mechanism, overcoming drug resistance, and reducing side effects to support the precision-driven advancement of treatments for HER2 positive breast cancer.
References
[1]. F. Zhao, Y. Cai, X. Guo, C. Xu, and B. Ma, “Impact and trend analysis of low physical activity on the disease burden of breast cancer among Chinese women, ” Clinical Education of General Practice, vol. 23, no. 07, pp. 623-628+673, Jul. 2025, doi: 10.13558/j.cnki.issn1672-3686.2025.007.012.
[2]. L. Wilkinson and T. Gathani, “Understanding Breast Cancer as a Global Health Concern, ” The British Journal of Radiology, vol. 95, no. 1130, Dec. 2021, doi: https: //doi.org/10.1259/bjr.20211033.
[3]. C. Katsura, I. Ogunmwonyi, H. K. Kankam, and S. Saha, “Breast Cancer: Presentation, Investigation and Management, ” British Journal of Hospital Medicine, vol. 83, no. 2, pp. 1–7, Feb. 2022, doi: https: //doi.org/10.12968/hmed.2021.0459.
[4]. B. Han et al., “Cancer incidence and mortality in China, 2022, ” Journal of the National Cancer Center, vol. 4, no. 1, pp. 47–53, Feb. 2024, doi: https: //doi.org/10.1016/j.jncc.2024.01.006.
[5]. X. Xiong et al., “Breast cancer: pathogenesis and treatments, ” Signal Transduction and Targeted Therapy, vol. 10, no. 1, Feb. 2025, doi: https: //doi.org/10.1038/s41392-024-02108-4.
[6]. Z. S. Lima, M. Ghadamzadeh, F. T. Arashloo, G. Amjad, M. R. Ebadi, and L. Younesi, “Recent advances of therapeutic targets based on the molecular signature in breast cancer: genetic mutations and implications for current treatment paradigms, ” Journal of Hematology & Oncology, vol. 12, no. 1, Apr. 2019, doi: https: //doi.org/10.1186/s13045-019-0725-6.
[7]. Kanwal Raghav and M. M. Moasser, “Molecular Pathways and Mechanisms of HER2 in Cancer Therapy, ” Clinical Cancer Research, vol. 29, no. 13, pp. 2351–2361, Dec. 2022, doi: https: //doi.org/10.1158/1078-0432.ccr-22-0283.
[8]. M. Park, D. Kim, S. Ko, A. Kim, K. Mo, and H. Yoon, “Breast Cancer Metastasis: Mechanisms and Therapeutic Implications, ” International Journal of Molecular Sciences, vol. 23, no. 12, p. 6806, Jun. 2022, doi: https: //doi.org/10.3390/ijms23126806.
[9]. Y. Choi et al., “Novel insights into paclitaxel’s role on tumor-associated macrophages in enhancing PD-1 blockade in breast cancer treatment, ” Journal for ImmunoTherapy of Cancer, vol. 12, no. 7, p. e008864, Jul. 2024, doi: https: //doi.org/10.1136/jitc-2024-008864.
[10]. H. Yu et al., “Paclitaxel anti-cancer therapeutics: from discovery to clinical use, ” Chinese Journal of Natural Medicines, vol. 23, no. 7, pp. 769–789, Jul. 2025, doi: https: //doi.org/10.1016/s1875-5364(25)60833-8.
[11]. J. Sharifi-Rad et al., “Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy, ” Oxidative Medicine and Cellular Longevity, vol. 2021, p. 3687700, Oct. 2021, doi: https: //doi.org/10.1155/2021/3687700.
[12]. S. M. Swain, M. Shastry, and E. Hamilton, “Targeting HER2-positive breast cancer: advances and future directions, ” Nature Reviews Drug Discovery, vol. 22, no. 2, pp. 101–126, Nov. 2022.
[13]. S. A. Hurvitz et al., “Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial, ” The Lancet, vol. 401, no. 10371, pp. 105–117, Dec. 2022, doi: https: //doi.org/10.1016/S0140-6736(22)02420-5.
[14]. T. P. DiPeri et al., “Adavosertib Enhances Antitumor Activity of Trastuzumab Deruxtecan in HER2-Expressing Cancers, ” Clinical Cancer Research, vol. 29, no. 21, pp. 4385–4398, Jun. 2023, doi: https: //doi.org/10.1158/1078-0432.ccr-23-0103.
[15]. Claudia von Arx et al., “The evolving therapeutic landscape of trastuzumab-drug conjugates: Future perspectives beyond HER2-positive breast cancer, ” Cancer Treatment Reviews, vol. 113, p. 102500, Feb. 2023, doi: https: //doi.org/10.1016/j.ctrv.2022.102500.
[16]. L. Zhang, “Observation on the Efficacy of Neoadjuvant Chemotherapy with Trastuzumab Combined with Paclitaxel in the Treatment of Advanced Breast Cancer, ” Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine, vol. 25, no. 04, pp. 19-22, Feb. 2025, doi: 10.13638/j.issn.1671-4040.2025.04.006.
[17]. S. M. Tolaney et al., “Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial, ” The Lancet Oncology, vol. 24, no. 3, pp. 273–285, Mar. 2023, doi: https: //doi.org/10.1016/s1470-2045(23)00051-7.
[18]. C. Ren et al., “Ubiquitination of NF-κB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and paclitaxel resistance, ” Cell Death & Differentiation, vol. 29, no. 2, pp. 381–392, Aug. 2021, doi: https: //doi.org/10.1038/s41418-021-00862-4.
Cite this article
Shen,Y. (2025). Treatment of HER2 Positive Breast Cancer with Paclitaxel and Trastuzumab. Theoretical and Natural Science,147,17-26.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. F. Zhao, Y. Cai, X. Guo, C. Xu, and B. Ma, “Impact and trend analysis of low physical activity on the disease burden of breast cancer among Chinese women, ” Clinical Education of General Practice, vol. 23, no. 07, pp. 623-628+673, Jul. 2025, doi: 10.13558/j.cnki.issn1672-3686.2025.007.012.
[2]. L. Wilkinson and T. Gathani, “Understanding Breast Cancer as a Global Health Concern, ” The British Journal of Radiology, vol. 95, no. 1130, Dec. 2021, doi: https: //doi.org/10.1259/bjr.20211033.
[3]. C. Katsura, I. Ogunmwonyi, H. K. Kankam, and S. Saha, “Breast Cancer: Presentation, Investigation and Management, ” British Journal of Hospital Medicine, vol. 83, no. 2, pp. 1–7, Feb. 2022, doi: https: //doi.org/10.12968/hmed.2021.0459.
[4]. B. Han et al., “Cancer incidence and mortality in China, 2022, ” Journal of the National Cancer Center, vol. 4, no. 1, pp. 47–53, Feb. 2024, doi: https: //doi.org/10.1016/j.jncc.2024.01.006.
[5]. X. Xiong et al., “Breast cancer: pathogenesis and treatments, ” Signal Transduction and Targeted Therapy, vol. 10, no. 1, Feb. 2025, doi: https: //doi.org/10.1038/s41392-024-02108-4.
[6]. Z. S. Lima, M. Ghadamzadeh, F. T. Arashloo, G. Amjad, M. R. Ebadi, and L. Younesi, “Recent advances of therapeutic targets based on the molecular signature in breast cancer: genetic mutations and implications for current treatment paradigms, ” Journal of Hematology & Oncology, vol. 12, no. 1, Apr. 2019, doi: https: //doi.org/10.1186/s13045-019-0725-6.
[7]. Kanwal Raghav and M. M. Moasser, “Molecular Pathways and Mechanisms of HER2 in Cancer Therapy, ” Clinical Cancer Research, vol. 29, no. 13, pp. 2351–2361, Dec. 2022, doi: https: //doi.org/10.1158/1078-0432.ccr-22-0283.
[8]. M. Park, D. Kim, S. Ko, A. Kim, K. Mo, and H. Yoon, “Breast Cancer Metastasis: Mechanisms and Therapeutic Implications, ” International Journal of Molecular Sciences, vol. 23, no. 12, p. 6806, Jun. 2022, doi: https: //doi.org/10.3390/ijms23126806.
[9]. Y. Choi et al., “Novel insights into paclitaxel’s role on tumor-associated macrophages in enhancing PD-1 blockade in breast cancer treatment, ” Journal for ImmunoTherapy of Cancer, vol. 12, no. 7, p. e008864, Jul. 2024, doi: https: //doi.org/10.1136/jitc-2024-008864.
[10]. H. Yu et al., “Paclitaxel anti-cancer therapeutics: from discovery to clinical use, ” Chinese Journal of Natural Medicines, vol. 23, no. 7, pp. 769–789, Jul. 2025, doi: https: //doi.org/10.1016/s1875-5364(25)60833-8.
[11]. J. Sharifi-Rad et al., “Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy, ” Oxidative Medicine and Cellular Longevity, vol. 2021, p. 3687700, Oct. 2021, doi: https: //doi.org/10.1155/2021/3687700.
[12]. S. M. Swain, M. Shastry, and E. Hamilton, “Targeting HER2-positive breast cancer: advances and future directions, ” Nature Reviews Drug Discovery, vol. 22, no. 2, pp. 101–126, Nov. 2022.
[13]. S. A. Hurvitz et al., “Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial, ” The Lancet, vol. 401, no. 10371, pp. 105–117, Dec. 2022, doi: https: //doi.org/10.1016/S0140-6736(22)02420-5.
[14]. T. P. DiPeri et al., “Adavosertib Enhances Antitumor Activity of Trastuzumab Deruxtecan in HER2-Expressing Cancers, ” Clinical Cancer Research, vol. 29, no. 21, pp. 4385–4398, Jun. 2023, doi: https: //doi.org/10.1158/1078-0432.ccr-23-0103.
[15]. Claudia von Arx et al., “The evolving therapeutic landscape of trastuzumab-drug conjugates: Future perspectives beyond HER2-positive breast cancer, ” Cancer Treatment Reviews, vol. 113, p. 102500, Feb. 2023, doi: https: //doi.org/10.1016/j.ctrv.2022.102500.
[16]. L. Zhang, “Observation on the Efficacy of Neoadjuvant Chemotherapy with Trastuzumab Combined with Paclitaxel in the Treatment of Advanced Breast Cancer, ” Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine, vol. 25, no. 04, pp. 19-22, Feb. 2025, doi: 10.13638/j.issn.1671-4040.2025.04.006.
[17]. S. M. Tolaney et al., “Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial, ” The Lancet Oncology, vol. 24, no. 3, pp. 273–285, Mar. 2023, doi: https: //doi.org/10.1016/s1470-2045(23)00051-7.
[18]. C. Ren et al., “Ubiquitination of NF-κB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and paclitaxel resistance, ” Cell Death & Differentiation, vol. 29, no. 2, pp. 381–392, Aug. 2021, doi: https: //doi.org/10.1038/s41418-021-00862-4.









