1. Introduction
Dasatinib is an oral multikinase inhibitor that used to treat Philadelphia chromosome (Ph)-positive leukemia [1]. Patients with Chronic Myelogenous Leukemia (CML) and Acute Lymphoblastic Leukemia (ALL) have a chromosomal defect called Ph. The body develops leukemia due to the lack of mature blood cells. A large number of leukemia cells in the body are capable of widespread and uncontrolled proliferation, which in turn appears in the bone marrow and many other organs and tissues and enters the peripheral bloodstream, seriously jeopardizing human health. With the advancement of medicine, alleviation of leukemia is now no longer the ultimate goal of treatment. Understanding the mechanism of action of the disease and how to target and improve the cure rate so that most leukemia patients can be cured have become the focus of today’s hematology research. In this process, the regulatory mechanisms, synthetic pathways and current status of drug applications are equally important. This article provides an in-depth analysis of the mechanism of action and synthesis route of dasatinib.
2. Leukemias and Dasatinib
2.1. CML
CML is a malignant multifunctional hematopoietic stem cell bone marrow abnormal proliferation of malignant neoplasms, which has a global incidence of 1.6-2.0 per 100,000 people, accounting for 15%-20% of all leukemias [2]. CML is brought on by the translocation of chromosomes 9 and 22 to create the Ph chromosome, which forms an abnormal fusion gene, BCR-ABL, which further causes the human organism to produce an abnormal fusion protein. This fusion protein regulates the body’s production of excessive, uncontrolled proliferation of granulocytes, which ultimately leads to an increase in abnormal granulocytes in the blood. Therefore, the Ph chromosome and the BCR-ABL fusion gene are confirmatory indicators of CML.
2.2. ALL
ALL is a type of cancer brought on by the aberrant multiplication of primitive lymphocytes from the B or T lineage of lymphocytes in the bone marrow. Primitive cells that are abnormally proliferating can infect tissues outside of the bone marrow, such as the meninges, lymph nodes, gonads, and liver, and aggregate in the bone marrow to prevent normal hematopoiesis. ALL and lymphoma are considered to be two modes of presentation of the same disease. In the former, most of the cancer cells are found in the blood and bones, while in the latter they are found in the lymph nodes.
In China, a study on the prevalence of leukemia found that the incidence of ALL was roughly 0.67 per 100,000 people, and that it was substantially higher in oil fields and other contaminated locations than it was overall. ALL can occur in all ages and the incidence increases with age, according to statistics, patients between the ages of 18 and 35 account for roughly 12 to 30% of ALL cases, patients aged 36-50 years account for about 40-45%, and patients between the ages of 60 and above account for roughly 50% [3]. Philadelphia chromosome-like B-ALL, similar to Philadelphia chromosome-positive ALL, occurs in approximately 12% of pediatric B-ALL cases [4].
Numerous studies have demonstrated that protein tyrosine-kinases promote tumor cell proliferation and tumor angiogenesis, which are closely related to leukemogenesis. Among them, the BCR-ABL gene fusion formed by the translocation of chromosomes 9 and 22 produces the fusion protein p-210, which can continuously activate tyrosine-kinases and activate a series of downstream signaling pathways, allowing tumor cells to proliferate in the absence of growth factors, which is the pathogenesis of CML and Ph+ [5].
The body develops leukemia due to lack of mature blood cells. The quantity of healthy cells in the bone marrow declines as the leukemia cell count rises. Red blood cells’ role is to guarantee that the lungs acquire oxygen and release it for cellular use in capillaries throughout the body. And a low number of red blood cells in the body leads to anemia. Anemia causes compensatory increased heart rate and respiration, and severe anemia is often associated with neurological manifestations such as headache, dizziness, tinnitus, poor concentration and memory loss. Platelets help clot blood to slow or stop bleeding. Too few platelets can cause bleeding and predispose to bleeding disorders and bruising of the skin. White blood cells defend against and destroy invading pathogenic microorganisms by, among other things, phagocytosis and antibody production. If there are too few white blood cells, the body may develop an infection. Whether the infection is mild or severe, this can lead to death.The lymph nodes, spleen, liver, spine, and brain are a few areas outside of the bone marrow where cancerous cells in ALL might spread. In rare cases, it can even spread to the skin, mouth, kidneys and testicles. Once spread to other organs, leukemia cells will continue to eat normal cells and cause organ failure.
2.3. Mechanisms of dasatinib modulation in CML and ALL
ABL tyrosine-kinases gene and Breakpoint Cluster Region (BCR) gene fusion results in chimeric oncogenes and constitutive active BCR-ABL tyrosine. Imatinib, a first-generation small-molecule protease tyrosine kinase inhibitor before the advent of the second-generation tyrosine kinase inhibitors (TKIs) dasatinib, has been shown to bind to the catalytic center site of the kinase by competing with the substrate or ATP to cut off the phosphate group toward the tyrosine residue in patients with CML and ALL. And the fusion protein of BCR-ABL is able to express in patients with CML and ALL by competing with the substrate or ATP, competing for the binding to the catalytic center of the kinase, cutting off the movement of the phosphate group towards tyrosine residues, which results in the inability to phosphorylate the BCR-ABL, and then cutting off the signaling from the tumor cells, and thus achieving the specific inhibition of the expression of ABL, as well as the infinite proliferation of BCR-ABL cells and the induction of apoptosis [6]. Without altering the functionality of healthy cells, imatinib can specifically block the growth of malignant cells. However, imatinib is often a major cause of treatment failure due to its drug resistance. However, unlike imatinib, another tyrosine kinase used for the treatment of CML and Ph-positive ALL, dasatinib can be linked to the active site of the ABL kinase structural domain and to its inactive site, making it more potent than imatinib [3].
3. Properties of Dasatinib
3.1. Physicochemical properties of dasatinib
Dasatinib character is white powder, melting point is 280-286 ℃, easy to absorb water, generally stored in a dry environment, need to be long-term storage should be placed in -20 ℃ in the low temperature. The solubility of Dasatinib is low, almost insoluble in water and ethanol, slightly soluble in methanol and acetone, with good solubility in DMF, DMA and DMSO [5]. The structure of Dasatinib is shown in Figure 1.
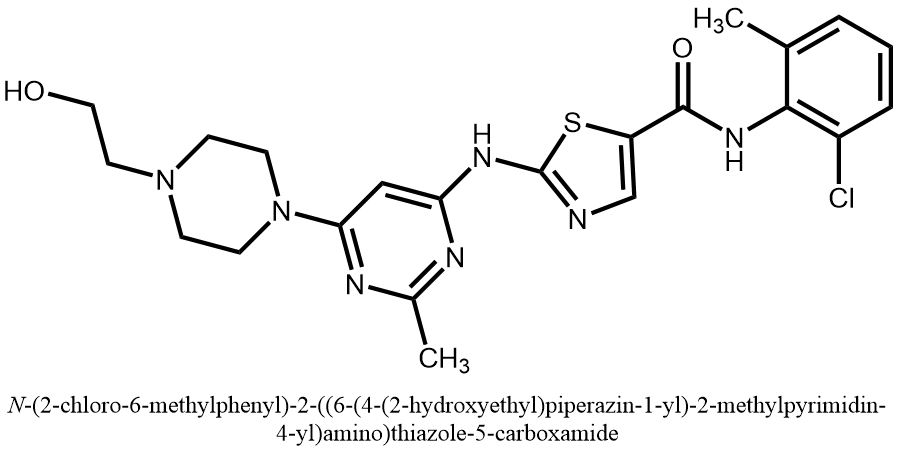
Figure 1. The structure of dasatinib.
3.2. Mechanism of action of dasatinib
Dasatinib is a tyrosine kinase inhibitor (TKI) that works by blocking the activity of specific proteins, particularly BCR-ABL, SRC family kinases, and other receptor tyrosine kinases. Dasatinib interferes with signaling pathways that support the growth and survival of cancer cells by blocking these kinases.
The recognition site of dasatinib is the ABL kinase with which it forms three hydrogen bonds: 1) between the hydrogen on the ammonia of the amide bond of dasatinib and the oxygen atom on the carbonyl group of Met318, 2) between the nitrogen on the thiazole ring and the hydrogen atom on the amino group of Met318 in the structure of dasatinib, and 3) between the hydrogen of the amide ring between the pyrimidine ring and the thiazole ring and the oxygen on the hydroxyl group of the amino group on the Thr315 amino acid in dasatinib, as shown in Figure 2 [6].
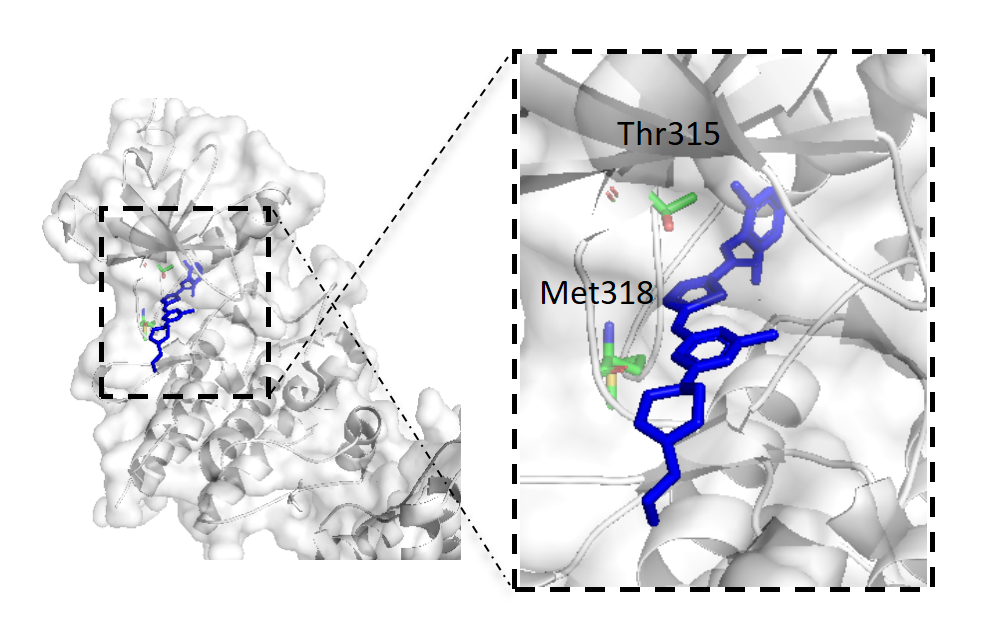
Figure 2. Structure of activated ABL kinase domain in complex with dasatinib.
4. Synthesis of Dasatinib
4.1. 2-Bromothiazole-5-carboxylic acid as raw material
As shown in Figure 3, 2-bromothiazole-5-carboxylic acid (b) was used as starting material, lithium carbonate as the acid-binding agent and N, N-dimethylformamide (DMF) as the solvent, and two substitution reactions took place under the same conditions to obtain the compound 2-({6-[4-(2-acetyloxyethyl)-1-piperazinyl]-2-methyl4-pyrimidinyl}amino)-5-thiazolecarboxylic acid (f) [7]. (f) undergoes a chlorination reaction in the presence of sulfoxide chloride to obtain the chloride intermediate, then undergoes an amidation reaction in the presence of the acid-binding agent potassium carbonate, and finally undergoes a hydrolysis reaction in alkaline conditions to remove the acetyl protecting group to obtain the final product dasatinib (a), with an overall yield of about 82.5%.
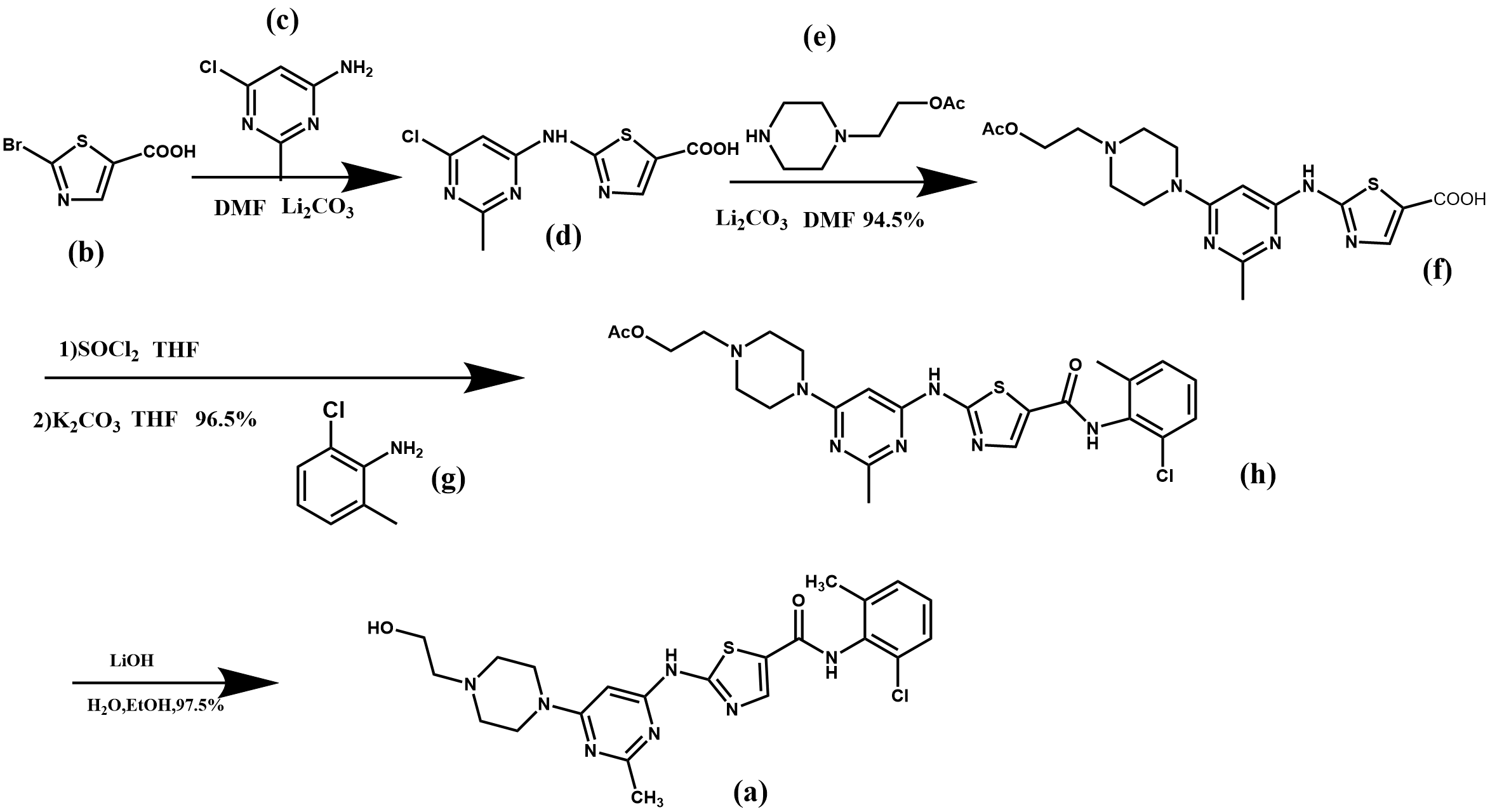
Figure 3. Synthesis route 1 of dasatinib.
In this synthetic route, the cost of raw materials used is low, and the substitution and hydrolysis reactions are easy to operate, the whole route has a high yield. However, there are drawbacks: the use of chlorinated reagent sulfoxide chloride brings difficulties in industrial production, which requires high equipment, and the environment is more polluted, which needs to be further improved.
From the perspective of green environmental protection, solid phosgene can be used to replace the chlorosulfoxide in this synthetic route, with a simpler process, better product quality, lower cost, and more convenient environmental treatment.
4.2. Ethyl formate and ethyl chloroacetate as raw materials
As shown in Figure 4, ethyl formate (i) and ethyl chloroacetate (j) were used as starting materialsto generate haloaldehyde intermediates under alkaline conditions with methyl tert-butyl ether (MTBE) as a solvent, followed by Hantzsch thiazole synthesis with thiourea (k) under acidic conditions, after which two substitution reactions took place to give the compound 2-({6-[4-(2-hydroxyethyl) piperazin-1-yl]-2-methylpyrimidin-4-yl}amino)thiazole-5-carboxylic acid ethyl ester (p) [7]. (p) underwent amidation reaction under alkaline conditions to obtain the final product dasatinib, with an overall yield of 20.3 %.
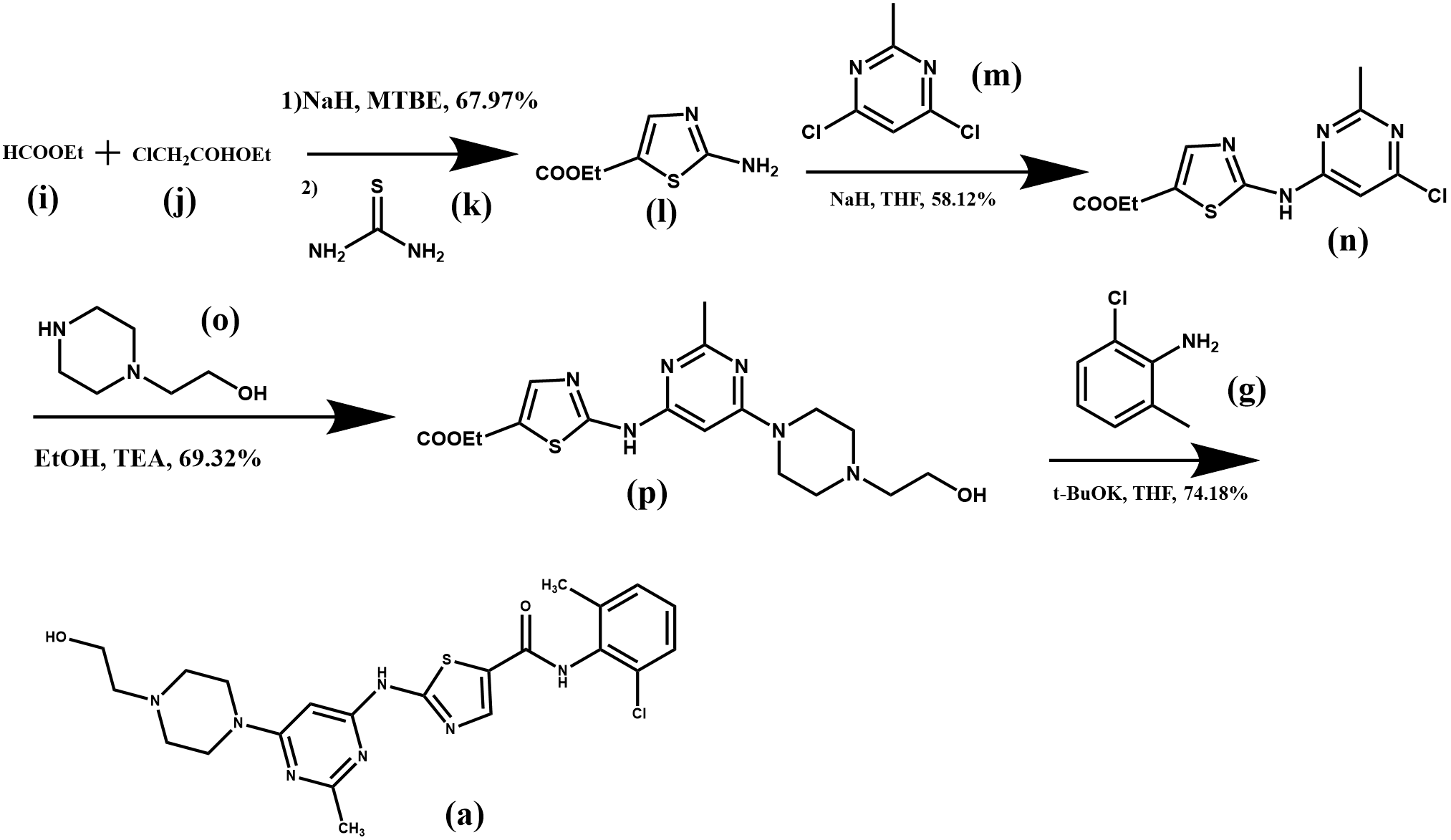
Figure 4. Synthesis route 2 of dasatinib.
The use of chlorinated reagents, which are volatile and have a high environmental impact, was avoided in this synthetic route. Ethyl formate (i) and ethyl chloroacetate (j) were used to synthesize the more expensive compound (l), which reduced the production cost. However, during the synthesis of compound (n), the hazardous reagent sodium hydride was used, which is prone to produce double-substituted by-products, resulting in a yield of only 58.12% for this step of the reaction. On the other hand, the post-treatment of this synthesis reaction is also complicated, not easy to operate and produces more industrial wastewater. Therefore, the total yield of this method is low and not suitable for industrial production.
Since sodium cyanide is toxic and easy to react violently with nitrate, nitrite, etc., causing an explosion, it is a very dangerous chemical, so it can be replaced by lime sulphur and alkali catalytic compound in the synthesis, which is non-toxic and non-polluting, and basically has no negative impact on the environment.
4.3. N-hydroxyethylpiperazine as raw material
N-hydroxyethyl piperazine (o) was used as starting material and amidation reaction followed by amidation in the presence of trimethylaluminum (TMA) hexane solution gave the compound N-(2-chloro-6-methylphenyl)-2-{3-[4-(2-hydroxyethyl) piperazin-1-yl]-3-oxopropionamido} thiazole- 5-carboxamide (t). The cycloaddition reaction of (t) with acetamidine hydrochloride (u) under alkaline conditions gave the end product dasatinib (a) in 82.7% overall yield (Figure 5) [7].
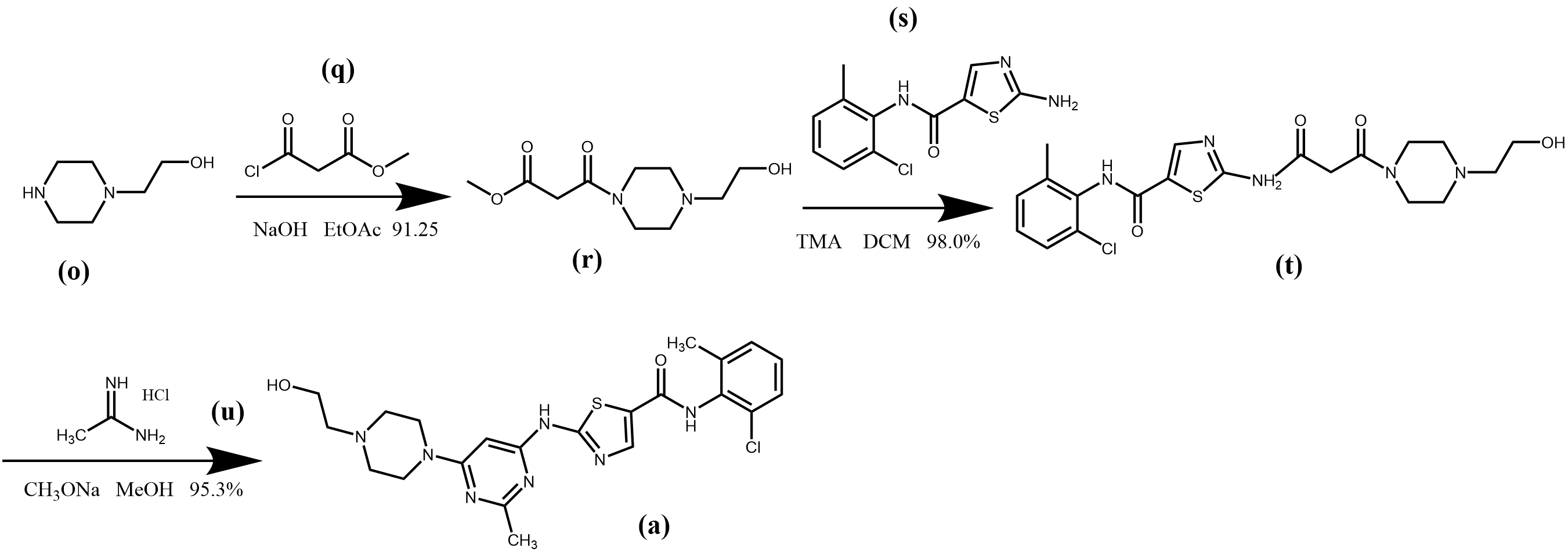
Figure 5. Synthesis route 3 of dasatinib.
This synthetic route does not require the introduction of pyrimidine in the synthesis and has fewer synthetic steps, moreover, the reaction conditions are milder, more experimentally tractable and in higher yields. However, the compounds (s) and trimethylaluminum hexane solution were more expensive, which relatively increased the production cost.
5. Applications Status of Dasatinib
Dasatinib, as a second-generation oral tyrosine kinase inhibitor with a novel molecular structure, exerts a dual inhibitory effect on the SCR-ABL and SRC families [3], which not only inhibits the active and inactive conformations of the structural domains of the ABL kinase, but also inhibits a different kinase profile overlapping with that of the kinase sequences inhibited by imatinib [8], which can broadly overcome the problem of drug resistance to imatinib.
Since dasatinib has no interactions with some of the residues linked to these mutations and has a rapid onset of action and a broad therapeutic range, the therapeutic efficacy is far superior to that of imatinib, with a reduction in adverse effects compared to imatinib [9-11], and dasatinib has a higher rate of overcoming resistance compared to other alternative TKIs. Therefore, the use of this drug has become a therapeutic alternative for cancer patients who have developed resistance to imatinib [8].
Dasatinib has a manageable safety profile, but adverse reactions such as myelosuppression, fluid retention, and cardiovascular events have been reported, making regular monitoring and dose adjustments necessary to minimize these risks.
6. Conclusion
This paper focuses on the current research on dasatinib, the diseases that can be treated by the drug, the process synthesis route of the drug and the application status. Two diseases that can be treated by this drug are selected and outlined in terms of incidence, pathogenesis and harm. The emerging synthetic routes of Dasatinib was summarized and compared their advantages and disadvantages in terms of cost, total yield and environmental friendliness, and suggests improvements accordingly. It can be seen that these three emerging synthetic routes have more or less shortcomings in terms of both total yield and environmental friendliness, and more efficient and green methods need to be further explored. Finally, by investigating the current application of dasatinib, it can be found that the drug has a higher resistance rate than other TKIs in the treatment of CML and ALL, which confirms the feasibility of dasatinib for the therapy of imatinib-resistant or intolerant CML and ALL. However, at the same time, it is also necessary to pay attention to the potential adverse reactions caused by this drug in the course of treatment and adjust the dosage in time. Overall, further studies are needed in the future to fully exploit the potential of dasatinib in the treatment of these hematologic malignancies.
References
[1]. Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin R V, Shen Z, Cook L S, Doweyko A M, Pitt S, Pang S, Shen D R, Fang Q, de Fex H F, McIntyre K W, Shuster D J, Gillooly K M, Behnia K, Schieven G L, Wityak J and Barrish J C 2006 J. Med. Chem. 49 6819
[2]. Cortes J 2004 Hematol. Oncol. Clin. N. 18 569
[3]. Lindauer M and Hochhaus A 2018 Recent Results Cancer Res. 212 29
[4]. Pui C H, Roberts K G, Yang J J and Mullighan C G 2017 Cl. Lymph. Myelom. Leuk. 17 464
[5]. Luo X X, Huang L, Zhao H Y, Xue X C, Hu L, Yang C Q and Feng W Y 2018 Chin. J. New Drugs 27 7
[6]. Wang Q X 2017 Design and synthesis of dasatinib derivatives and study of their biological activities (Shanghai: Shanghai University of Applied Sciences)
[7]. Yan Y F, Jiang C F, Zhang B, Liu W T and Liu B 2023 Chin. J. Med. Chem. 33 315
[8]. Talpaz M, Shah N P, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O’Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir M A, Iyer V, Chen T T, Huang F, Decillis A P and Sawyers C L 2006 New Engl. J. Med. 354 2531
[9]. Tokarski J S, Newitt J A, Chang C Y, Cheng J D, Wittekind M, Kiefer S E, Kish K, Lee F Y, Borzillerri R, Lombardo L J, Xie D, Zhang Y and Klei H E 2006 Clin. Cancer Res. 66 5790
[10]. Levêque D, Becker G, Bilger K and Natarajan-Amé S 2020 Clin. Pharmacokinet. 59 849
[11]. Olivieri A and Manzione L 2007 Ann. Oncol. 18 42
Cite this article
Liu,X. (2023). Synthetic route of dasatinib and its application in leukemia. Theoretical and Natural Science,16,186-192.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Modern Medicine and Global Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin R V, Shen Z, Cook L S, Doweyko A M, Pitt S, Pang S, Shen D R, Fang Q, de Fex H F, McIntyre K W, Shuster D J, Gillooly K M, Behnia K, Schieven G L, Wityak J and Barrish J C 2006 J. Med. Chem. 49 6819
[2]. Cortes J 2004 Hematol. Oncol. Clin. N. 18 569
[3]. Lindauer M and Hochhaus A 2018 Recent Results Cancer Res. 212 29
[4]. Pui C H, Roberts K G, Yang J J and Mullighan C G 2017 Cl. Lymph. Myelom. Leuk. 17 464
[5]. Luo X X, Huang L, Zhao H Y, Xue X C, Hu L, Yang C Q and Feng W Y 2018 Chin. J. New Drugs 27 7
[6]. Wang Q X 2017 Design and synthesis of dasatinib derivatives and study of their biological activities (Shanghai: Shanghai University of Applied Sciences)
[7]. Yan Y F, Jiang C F, Zhang B, Liu W T and Liu B 2023 Chin. J. Med. Chem. 33 315
[8]. Talpaz M, Shah N P, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, O’Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir M A, Iyer V, Chen T T, Huang F, Decillis A P and Sawyers C L 2006 New Engl. J. Med. 354 2531
[9]. Tokarski J S, Newitt J A, Chang C Y, Cheng J D, Wittekind M, Kiefer S E, Kish K, Lee F Y, Borzillerri R, Lombardo L J, Xie D, Zhang Y and Klei H E 2006 Clin. Cancer Res. 66 5790
[10]. Levêque D, Becker G, Bilger K and Natarajan-Amé S 2020 Clin. Pharmacokinet. 59 849
[11]. Olivieri A and Manzione L 2007 Ann. Oncol. 18 42









