1. Introduction
The popular adage, “you are what you eat” has been proven time and again to be true in matters of health and wellbeing. This includes physical and mental health conditions. Particularly, Alzheimer’s disease has been related to being impacted by one’s nutrition. Alzheimer’s disease (AD) refers to the health condition that causes loss of mental ability following the deterioration of neurons in the brain. It features memory loss, personality alteration, and eventually death. While 50 million AD patients exist globally, AD is only manageable and incurable. AD risk factors include, but are not limited to aging, head injuries, lifestyle, diabetes, chemical exposure, and genetics [1]. The authors assert that nutritional behaviors can aggravate or relieve AD. It indicated that a high-carbohydrates diet might affect the brain to some extent. The high-carbohydrates diet is a dietary pattern in which carbohydrates make up a high percentage of a diet. Divided into three main groups for carbohydrates- oligosaccharides (short-chain carbs) (DP 3-9), sugars (DP 1-2) and polysaccharides (DP> or =10) [2]. Therefore, this diet is also considered the high-sugar diet. Additionally, the high-carbohydrate diet is linked to several diseases. For instance, after a comparatively short exposure to a high-fat, high-sugar diet, hippocampus damage can be detected, and cognitive deficits can occur rapidly [3]. It indicated that the high-carbohydrates diet is related to AD. Therefore, this paper will focus on the relationship between the high-carbohydrates diet and AD and the therapeutic methods. It could help people know more about the relationship between them. Furthermore, the paper will be sorted into five parts-the means of high-carbohydrates diets, AD forming, and biomarkers, the relationship between high- carbohydrates diet and AD, treatments, and other derived diseases detailly.
2. Risk factors of AD
Family history, aging, head injury, or having the epsilon 4 allele of the apolipoprotein E gene (APOE-4) are all nonmodified risk factors for AD. Familial forms of Alzheimer's disease (FAD) are rare and account for 7-10% of cases. Early-onset AD (EOAD) is brought on by mutations in a number of different genes, including those encoding the amyloid precursor protein (APP) at chromosome 21 and the presenilin (PS) 1 and 2 (chromosomes 14 and 1), which enhance the production of -amyloid (A) from APP [4]. Other possible risks include oxidative damage, neuroinflammation and brain insulin resistance can be altered through diets, such as Mediterranean diet and ketogenic diet [5]. Moreover, there are numbers of factors that can lead to AD. Prion plays a role in AD. Prion is a folded protein which developed from un-folded prion protein by misfolding. When more and more misfolding prions gather, they will destroy the nerve cell and leave that empty. According to the pathological peculiarity in Aβamyloid, it transmitted to patients by injecting of human growth hormone that was polluted with Aβamyloid, and transmission of misfolded Aβ aggregates, which has been proved in experimental animals and cell cultures [6]. It also indicated that Aβ aggregates will lead to AD. But the most important is, that neuroinflammation and amyloidosis are the leading causes of AD, which means amyloidosis are hard to lead to AD without neuroinflammation. Some studies indicated that glial activation and the outcome of pro-inflammatory cytokines (including IL-1β, IL-6, IL-8, and TNF) could be attracted by different species of Aβ aggregates [7]. Besides, there are two distinct phenotypes, M1 and M2, for microglia, M1 has pro-inflammatory features, whereas that of the M2 has antiphlogistic characteristics [8]. Hence, in M1, microglia will be in the activated state and produce proinflammatory cytokine, which will increase in the risk of AD. As for the amyloidosis and inflammatory roles in AD, they set up a circulation. The amyloidosis leading to increased Aβ concentration causes tauopathies and subsequent NFTs, and also according to the M1 phenotypes by microglia, neuroinflammation increases, which can further mount amyloidosis and tauopathies resulting in cognitive reduction [8]. To sum up, it is complex to cause AD; however, neuroinflammation and amyloidosis' circulation is the leading cause to AD.
3. The relationship between high-carbohydrates diet and AD
Brain neuroinflammation, one of the diagnostic biomarkers of AD, is proved to be associated with the growth of AD at the onset and progression of the disease. Study suggests that inflammation poses a component of AD pathogenesis, causing AD neurodegeneration; inflammatory molecules significantly surge in AD patients [9]. Evidence indicates that continual intake of anti-inflammatory drugs can decrease the risk of AD and postpone the onset of AD [10]. A high carbohydrate diet, with an excessive amount of sugar intake, negatively affects brain health and aggravates brain inflammation by releasing “pro-inflammatory cytokines that promote nitric oxide, facilitating anxious states in humans and rodents” [11]. A high carbohydrate diet causes low-density lipoprotein cholesterol to surge, which leads to more C-reactive protein. This has been shown to cause inflammation [12]. In general, since AD is linked is characterized by brain inflammation and a high-carbohydrate diet is proved to activate brain inflammation, intaking excessive sugar and glucose in a long-term diet can promote the progression of AD. Among AD patients, reliance on high-carbohydrates diets has the potential to influence gene expression and AD outcome. Butler et al.’s research on rats with AD reveals the need for concern about carbohydrates’ permanent effects. The study finds that people who consume highly processed carbohydrate foods risk exacerbating their hippocampus’ cognitive inability. Further, the older an individual is, the greater the extent to which inflammatory gene is expressed in the DNA. Refined carbohydrates, however, do not cause neuroinflammatory gene expression and diminished mental abilities among the young. These patterns of gene expression gravely affect memory functions that are regulated by the amygdala as well as the hippocampus parts of the brain [13]. Based on this revelation, the side effects of high carbohydrate diets are definitely affirmed to present a danger to AD patients’ health. Knowing that extreme refinement of carbohydrates predisposes elderly persons to permanent gene alteration at the fundamental level underpins the need for research that seeks ways to overturn such effects. Other studies have also related the level of carbohydrates diet to glycemic regulating biomarkers. For example, Yang, Eun Ju, et al.’s work on the same establishes that consuming diets with low-carbohydrates content heightens the quantity and rate at which basal insulin is secreted in the body of disease-free adults and that such individuals exhibit a high level of Serum C-peptide[14]. This means that limiting the amount of carbohydrate intake ensures that a person’s glycemic biomarkers are not impeded –an aspect that is beneficial for healthy blood glucose regulation. Otherwise, high carbohydrate diets would diminish Serum C-peptide hence worsening insulin secretion and resultantly exacerbating predisposition to AD and worsening cognitive performance.
Glycation, a process that impairs serum proteins when they are exposed to high glucose levels, results in the emergence of a group diversified from terminally misfolded proteins known as advanced glycation end products (AGEs). High amounts of AGEs are found in the brains of AD patients, and these AGEs aid in amyloid plaques formation [15]. The oxidative stress speeds up this formation. The effects of AGEs and intermediate glycated isoforms on the body are varied. They have the potential to cause the buildup of harmful amyloid fibrils or to trigger the irreversible cross-linking of extracellular matrix proteins, but more importantly, they are in charge of activating the AGE-receptor for AGE (RAGE) signaling pathway, which in turn causes a variety of cellular reactions, such as apoptosis and inflammation [16]. The early stage of the Alzheimer's cascade may involve glycation of ApoE, and the increased affinity of ApoE-4 may significantly increase the risk of developing AD. Also, glycation may be an early sign of AD because it has been demonstrated that the Aβ plaque is one of the characteristics of AD that associated with AGEs and that these damaged proteins can be found even in micro-neural slices where Aβ is absent [17].
On the other hand, high carbohydrate diet also affects insulin and glucose metabolism, contributing to the deterioration of AD. How is carbohydrate related to glucose metabolism impairment? One way that deduced glucose metabolism can speed up AD is that it leads to dysfunctional mitochondria which ultimately causes the blockade of energy sources. In addition, impaired glucose metabolism negatively impacts brain health through metabolic diseases and property change of essential proteins like tau. Besides, some evidence also shows that in rats, treating by high sucrose diet, found learning impairment while spontaneous locomotor activity remained unchanged [18].
4. High- carbohydrates diet affects cognitive function
Consumption of diets that are high in carbohydrate diminish cognitive ability and in the process cause and worsen Alzheimer’s disease symptoms. Empirical studies by Beilharz Maniam & Morris on the relationship between participants’ source of energy affirm that consumption of large quantities of carbohydrates causes and worsen AD. Particularly, people who rely on carbohydrates for their dietary sugars often experience brain damage in the hippocampus area, the brain portion which is responsible for memory. Simple sugars from carbohydrates are posited to have some of the most dangerous effects on the functioning of the hippocampus. These effects are asserted to be worse than the ramifications caused by proteins and fats on cognitive abilities [19]. Humans’ ability to remember short-term or episodic events: like recollecting the food that is already taken, the ability to react to feelings of hunger, and satisfaction from food already eaten are all performed by the hippocampus.
The brain’s inability to sense such episodic events and signals thus implies that one would not remember their feeding behaviors and would thus consume food in undesirable patterns, in excessive quantity, and without knowing when to stop feeding [19]. As FIGURE 1 demonstrates, the main source of hippocampal and memory damage, the carbohydrate-sourced simple sugars, would over-accumulate in the body and worsen the same symptoms without the person knowing. Consequently, the dangerous pattern would aggravate AD and related health complications like type 2 diabetes. Large doses of dietary carbohydrates lead to the [19, 20]. Generally, the main challenge posed by carbohydrate-rich diets on cognitive function is the strain caused by simple sugars upon the hippocampus.
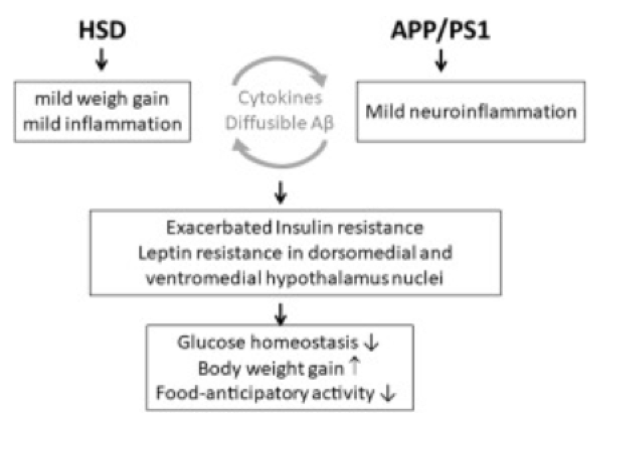
Figure 1. High sugar diet and AD worsening [21].
5. Relationship between a high-carbohydrate diet with age, and AD
Besides, the quantity and type of carbohydrates consumed by a person influence their AD outcomes in relation to their age. Empirical studies have linked the effect of high carbohydrate diets on whether the same would cause AD in younger or older people. For example, it is noted that although high carbohydrate diets affect the elderly more, it does not impair memory challenges in children in any significant way. However, when young adults and children consume glycemia-rich carbohydrates, their memory capabilities deteriorate significantly [19]. Another study affirms that feeding on high-carbohydrate foods worsens glycemia levels in older adults whose genotype is the “apolipoprotein E (APOE) έ4 allele” and this subsequently leads to serious memory and attention deficit among Alzheimer’s Disease patients [20].
When individuals eat foods high in carbohydrates, the body breaks down the carbohydrates into simple sugars. The high sugar content subsequently accumulates in the blood –this in turn leads to hyperglycemia. Hyperglycemia otherwise referred to as extreme blood glucose saturation, is worse among older adults who are suffering from AD, especially when the consumed carbohydrates are extremely processed. The study establishes that while older adults who are non-carriers of APOE έ4 suffer from worse verbal memory due to high carbohydrate intake APOE έ4 carriers with AD suffer most from declined ability to pay attention due to carbohydrate-rich diets [20]. It is thus apparent that genetic aspects such as alleles interact with age and resultantly influence the kind of cognitive impairment that becomes more pronounced following a carbohydrate-rich diet. It can be noted that in as much as carbohydrate-rich foods negatively impacts AD symptoms, the amount of glycemia and whether the carbohydrate is processed, as well as the subject’s age, can be used to regulate AD onset and symptoms.
6. Carbohydrate-rich diets prevent brain repair by cyclically destroy the brain
Although the brain is naturally self-repairing, carbohydrate-rich diets prevent the brain from achieving these activities, hence predisposing affected people to AD, and also worsening the risk of irreparable brain damage and the worst AD outcome. According to Yeh et al.’s study, foods high in sucrose limit the brain’s ability to produce leptin hormone for brain cell repair and growth [22]. Since leptin is responsible for the creation, growth, and protection of brain cells, reduced brain signaling for leptin secretion tends to impede leptin production in the nervous system. As a consequence, a low amount of leptin hormone reduces the brain’s capacity to naturally facilitate memory areas of the brain from developing, growing, repairing, and/or protecting itself from degenerating [23]. FIGURE 2 simplifies the relationship between leptin and AD.
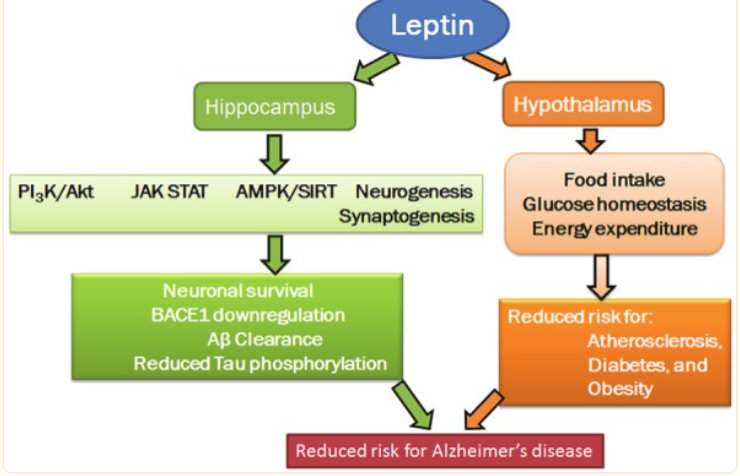
Figure 2. The relationship between sugar intake, leptin action, and AD risk factors [23].
Taking high-carbohydrate meals increases sucrose in the body, -this consequently paves way for AD aggravation due to limited leptin secretion and function. Insel, Turner, & Ross underpin that carbohydrates exist mainly as either simple or complex sugars. The authors further distinguish simple sugars as monosaccharides and disaccharides. Among the complex sugars is sucrose. The disaccharides, which are basically a combination of two bonded monosaccharides, include sucrose and are found in table sugar, and honey, among other carbohydrates [24]. The categories of carbohydrate-derived sugars, their sources, and examples are summarized in FIGURE 3 and 4. Besides the knowledge that consuming high-carbohydrate meals is harmful to individuals suffering from Alzheimer’s Disease, it is even much more crucial to be wary of the worst outcome that could happen over time to the vulnerability of the brain cells to permanent destruction following sucrose-induced leptin non-secretion and ineffectiveness.
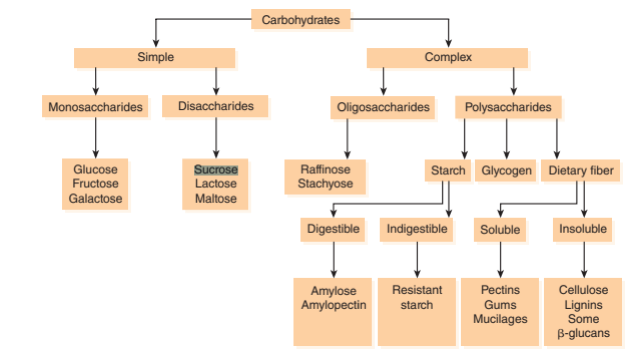
Figure 3. Structure and examples of carbohydrate (sucrose) [24].
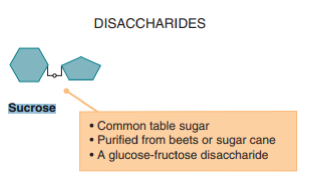
Figure 4. Structure and examples of carbohydrate (sucrose) [24].
7. Therapeutic method
7.1. Low carbohydrate diet-ketogenic diets
A high carbohydrate diet has a strong relationship with increasing the risk of AD. Hence, a low carbohydrate diet, such as a ketogenic diet, can be helpful in the healing of AD. The ketogenic diet (KD) is a diet that consists of very low carbohydrate intake and high fat intake, which is adequate to increase the generation of ketone bodies. In particular, the KD has also been shown to have prominent effects in preventing and alleviating cognitive impairment. 11 human and 11 animal studies were included in a translational review that supplementation with ketogenic diets significantly improved overall cognition, ability of memorizing information, and executive function in humans and improved cognitive function in mouse models of AD and aging animals [25]. Moreover, one of the recent research projects suggests that a KD containing 20 g of medium-chain triglycerides (MCTs) improved their memory when they are working, more sensitive to the visual senses, and feel free when exchanging task in non-demented older individuals [26]. Additionally, mild to moderate AD patients experienced substantial to take a turn for the better logical memory tests and processing speed compared to baseline scores at 8 and 12 weeks after starting the MCT-based nutritional ketogenic formulation [27]. Moreover, one of the symptoms of AD is restraining brain glucose metabolism. A ketogenic diet can produce ketone body (KB); it can supply brain glucose which helps heal AD and KB can rise the efficiency of mitochondria, which issue in an improve in the capacity of brain’s normal reliance on glucose [28] . In some experiments, from the comparison of the effect of using standard diet (SD) comprising of high carbohydrate and low fat and a ketogenic diet (KD) consisting of the low carbohydrate & high saturated fat diet to mice, more evidence shows that feeding KD can reduce around 25% of total brain Aβ levels [29]. Nevertheless, although the ketogenic diet brings numerous benefits to healing AD, some points also need to be noticed. TABLE 1 below shows the effects of eating the low carbohydrate diet or extremely low carbohydrate in the long term, it could cause amounts of diseases and harm such as lack of variety of nutrients.
Table 1. Advantages and disadvantages of a low-carb diet [30].
Merits | Pitfalls |
Helps short term weight loss | Lack of some items of nutrients from the diet. |
Helps glycemic control in people with diabetes | Might related to higher risk of mortality. |
When extreme carbohydrate restriction (50g carbohydrate per day) induces ketosis, hunger decreases. | Halitosis, nausea, weariness, an inability to exercise, and an electrolyte and volume imbalance are among the symptoms of ketosis. |
Potentially harmful long-term health effects, including gout, kidney stones, and bone loss. |
7.2. Effects of caffeine in AD
A purine alkaloid known as caffeine (1,3,7-trimethylxanthine), caffeine is always found in coffee beans, tea, cola, and nuts. It exhibits variable favorability in different age groups, with young people favoring cola more than others [31]. Caffeine consumption in several studies reduced cognitive decline and memory deficits in the APPswe transgenic mice model and displayed neuroprotective action [31]. Caffeine's neuroprotective properties and decreased Aβproduction were caused by the suppression of BACE-1 and γ-secretase, reduced caspase-3 activity, cAMP/PKA (cyclic adenosine monophosphate/phosphokinase A) signaling pathway activation, and stimulation of cAMP response element-binding protein (CREB) phosphorylation in the striatum all work together to prevent neuronal cell death [31]. The trial, however, also demonstrates that caffeine consumption in pregnant rats may increase the likelihood of developing AD in its early stages [31]. Additionally, cilostazol (CSZ) and caffeine normalized the buildup of Amyloid beta (Aβ-42) and phosphorylated tau protein (p-tau) positive cells in the brain of T2D mice. Additionally, some data suggests that CSZ + caffeine will have a more significant impact than either drug alone [32]. Generally, caffeine can improve cognitive function, which is helpful for AD patients, but pregnant women should reduce taking caffeine to prevent the risk of suffering AD.
7.3. Sugar regulation carbs and AD
Regulating the amount, pattern, and frequency of dietary sugars is a great way to avoid developing Alzheimer’s disease. Similar to previous studies that relate simple sugars to cognitive functions, Morris & Burns, and Akimoto et al. agree that minimizing the proportion of dietary sugar is imperative for AD prevention [33, 34]. The authors demonstrate this by affirming that on while excessive sugar consumption leads to overweight and subsequent development of type II diabetes. They augment the assertion that since AD often arises from insulin secretion failure and failed blood sugar regulation in T2D patients, preventing diabetes before it happens definitely eliminates a situation where the brain’s homeostatic activities impair. Lee et al. concur with this view, and they explain that repeated consumption of diabetes-causing foods, like processed sugars, over time induces hyperglycemia and consequently AD [35].
Beilharz, Maniam & Morris, and Hawkins et al. explain that it is vital to limit the amount of sugar consumed since this would minimize the calories one derives from dietary sugar [19, 20]. This would prevent inflammation of the central nervous system’s neurons and therefore ensure that the hippocampus and surrounding brain areas that are responsible for proper hippocampal activities remain healthy. Consequently, AD would not occur. Particularly, simple sugars, especially sucrose, must be avoided so as to prevent brain swelling and the related effect on negative biomarker effects [21]. As previously explained by Marwarha & Ghribi, the greater the quantity of sugar intake, the less the brain’s ability to signal for leptin release and function [23]. As such, minimizing the proportion of sugar ingestion assures insulin production and function in maintaining blood glucose. This way, no excessive blood sugar would bar leptin release [23]. The outcome would be that brain cell production, protection from damage, and healing would be sustained at par. The long-term outcome is that AD would not materialize in the first place.
8. Conclusion
AD is a devastating neurodegenerative and sporadic disease. Types of diet, such as high-carbohydrate diet, aging, head injuries and genetic are the major reasons that researchers have found influenced on AD. Although carbohydrates are the main source of energy that can fuel people’s brain and central nervous system, excessive intake of carbohydrates will have negative impact on human body, especially on brain and nervous system. In spite of considerable progress in the understanding of how the disease occurs at the molecular level, no effective therapeutic approaches are yet available. The studies that involve humans still have some restrictions. Studies of long-term case control are needed to see how high-carbohydrate diets influence on AD shown by the biomarkers, such as Aβ-42 plaques, t-Tau, and p-Tau. Some current research has focused on regulating the processing of APP proteins and correcting the imbalance between Aβ production and clearance. It’s still controversial that the main event that triggers the development of AD is the consumption of a high-carbohydrate diet. Collectively, a high-carbohydrate diet has either directly or indirectly negative effect on AD. According to this theory, relatively simple preventive measures like cutting back on starchy carbohydrates and increasing essential fatty acids intake might be helpful especially at the prodromal stage. More research is needed to demonstrate the effect of the HC diet on AD. Moreover, caffeine and other therapies like ketone body therapy may also be useful. It is hoped that more future studies will focus on the role of diet in AD and effective therapeutic prevention or treatment will discover in the future.
References
[1]. Z. Breijyeh,R. Karaman, "Comprehensive Review on Alzheimer's Disease: Causes and Treatment," Molecules, 25(24) (2020).
[2]. J. H. Cummings,A. M. Stephen, "Carbohydrate terminology and classification," European Journal of Clinical Nutrition, 61(S1), S5-S18 (2007).
[3]. T. Qiu, "The potential mechanism underlying hippocampus-dependent memory decline caused by high-fat and high-sugar diet and the interventions to combat diet-induced cognitive decline," AIP Publishing.
[4]. V. V. Giau, E. Bagyinszky, Y. S. Yang, et al., "Genetic analyses of early-onset Alzheimer’s disease using next generation sequencing," Scientific Reports, 9(1), 8368 (2019).
[5]. C.-Q. Chu, L.-l. Yu, G.-y. Qi, et al., "Can dietary patterns prevent cognitive impairment and reduce Alzheimer's disease risk: Exploring the underlying mechanisms of effects," Neuroscience & Biobehavioral Reviews, 135, 104556 (2022).
[6]. G. A. Carlson,S. B. Prusiner, "How an Infection of Sheep Revealed Prion Mechanisms in Alzheimer’s Disease and Other Neurodegenerative Disorders," International Journal of Molecular Sciences, 22(9), 4861 (2021).
[7]. F. Leng,P. Edison, "Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here?," Nature Reviews Neurology, 17(3), 157-172 (2021).
[8]. T. Li, L. Lu, E. Pember, et al., "New Insights into Neuroinflammation Involved in Pathogenic Mechanism of Alzheimer’s Disease and Its Potential for Therapeutic Intervention," Cells, 11(12), 1925 (2022).
[9]. J. Rogers, S. Webster, L. F. Lue, et al., "Inflammation and Alzheimer's disease pathogenesis," Neurobiol Aging, 17(5), 681-6 (1996).
[10]. Y. Yao, C. Chinnici, H. Tang, et al., "Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis," J Neuroinflammation, 1(1), 21 (2004).
[11]. J. A. S. Gomes, J. F. Silva, A. P. Marcal, et al., "High-refined carbohydrate diet consumption induces neuroinflammation and anxiety-like behavior in mice," J Nutr Biochem, 77, 108317 (2020).
[12]. M. S. Wu, P. Johnston, W. H. Sheu, et al., "Effect of metformin on carbohydrate and lipoprotein metabolism in NIDDM patients," Diabetes Care, 13(1), 1-8 (1990).
[13]. M. J. Butler, N. P. Deems, S. Muscat, et al., "Dietary DHA prevents cognitive impairment and inflammatory gene expression in aged male rats fed a diet enriched with refined carbohydrates," Brain Behav Immun, 98, 198-209 (2021).
[14]. E. J. Yang, J. M. Kerver, Y. K. Park, et al., "Carbohydrate intake and biomarkers of glycemic control among US adults: the third National Health and Nutrition Examination Survey (NHANES III)," Am J Clin Nutr, 77(6), 1426-33 (2003).
[15]. K. B. M P Vitek, J M Glendening , E Stopa, H Vlassara, R Bucala, K Manogue and A Cerami, Advanced glycation end products contribute to amyloidosis in Alzheimer disease., PNAS, 1994).
[16]. S. P. Banik, M. Bhattacharyya, R. Ghosh, et al., "Chapter 11 - Glycation-induced protein aggregation and cellular toxicity: an insight into the disease realm of high dietary sugar intake," in: Dietary Sugar, Salt and Fat in Human Health,edited by Preuss Harry G., Bagchi Debasis(Academic Press, 2020), pp. 251-275.
[17]. N. Sasaki, R. Fukatsu, K. Tsuzuki, et al., "Advanced Glycation End Products in Alzheimer's Disease and Other Neurodegenerative Diseases," The American Journal of Pathology, 153(4), 1149-1155 (1998).
[18]. N. Flores-Fuentes, C. Hernandez-Cruz, K. Bermeo, et al., "Motor learning impairment in rats under a high sucrose diet," Physiol Behav, 234, 113384 (2021).
[19]. J. E. Beilharz, J. Maniam,M. J. Morris, "Diet-Induced Cognitive Deficits: The Role of Fat and Sugar, Potential Mechanisms and Nutritional Interventions," Nutrients, 7(8), 6719-38 (2015).
[20]. M. A. W. Hawkins, N. G. Keirns,Z. Helms, "Carbohydrates and cognitive function," Curr Opin Clin Nutr Metab Care, 21(4), 302-307 (2018).
[21]. S. H. Yeh, F. S. Shie, H. K. Liu, et al., "A high-sucrose diet aggravates Alzheimer's disease pathology, attenuates hypothalamic leptin signaling, and impairs food-anticipatory activity in APPswe/PS1dE9 mice," Neurobiol Aging, 90, 60-74 (2020).
[22]. F. A. H. SARDÁ, E. B. GIUNTINI, J.-A. NAZARE, et al., "Effectiveness of carbohydrates as a functional ingredient in glycemic control," 38(4), 561-576 (2018).
[23]. G. Marwarha,O. Ghribi, "Leptin signaling and Alzheimer's disease," Am J Neurodegener Dis, 1(3), 245-65 (2012).
[24]. R. T. PM Insel, D Ross, Carbohydrates: Simple sugars and complex chains. Discovering Nutrition, 2006, pp. 103-133.
[25]. M. Lilamand, B. Porte, E. Cognat, et al., "Are ketogenic diets promising for Alzheimer’s disease? A translational review," Alzheimer's Research & Therapy, 12(1), 42 (2020).
[26]. M. Ota, J. Matsuo, I. Ishida, et al., "Effect of a ketogenic meal on cognitive function in elderly adults: potential for cognitive enhancement," Psychopharmacology, 233(21), 3797-3802 (2016).
[27]. M. Ota, J. Matsuo, I. Ishida, et al., "Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease," Neuroscience Letters, 690, 232-236 (2019).
[28]. S. T. Henderson, "Ketone bodies as a therapeutic for Alzheimer’s disease," Neurotherapeutics, 5(3), 470-480 (2008).
[29]. I. Van Der Auwera, S. Wera, F. Van Leuven, et al., "A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer's disease," Nutrition & Metabolism, 2(1), 28 (2005).
[30]. A. D. Mooradian, "The Merits and the Pitfalls of Low Carbohydrate Diet: A Concise Review," The journal of nutrition, health & aging, 24(7), 805-808 (2020).
[31]. P. Londzin, M. Zamora, B. Kąkol, et al., "Potential of Caffeine in Alzheimer’s Disease—A Review of Experimental Studies," Nutrients, 13(2), 537 (2021).
[32]. A. A. Gomaa, H. S. M. Farghaly, A. M. Ahmed, et al., "Advancing combination treatment with cilostazol and caffeine for Alzheimer's disease in high fat-high fructose-STZ induced model of amnesia," Eur J Pharmacol, 921, 174873 (2022).
[33]. J. K. Morris,J. M. Burns, "Insulin: an emerging treatment for Alzheimer's disease dementia?," Curr Neurol Neurosci Rep, 12(5), 520-7 (2012).
[34]. H. Akimoto, A. Negishi, S. Oshima, et al., "Antidiabetic Drugs for the Risk of Alzheimer Disease in Patients With Type 2 DM Using FAERS," Am J Alzheimers Dis Other Demen, 35, 1533317519899546 (2020).
[35]. H. J. Lee, H. I. Seo, H. Y. Cha, et al., "Diabetes and Alzheimer's Disease: Mechanisms and Nutritional Aspects," Clin Nutr Res, 7(4), 229-240 (2018).
Cite this article
Wu,C.;Zhang,M.;Zhou,S.;Zhong,X. (2023). The Role of A High-Carbohydrates Diet on Alzheimer’s Disease. Theoretical and Natural Science,4,46-55.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Z. Breijyeh,R. Karaman, "Comprehensive Review on Alzheimer's Disease: Causes and Treatment," Molecules, 25(24) (2020).
[2]. J. H. Cummings,A. M. Stephen, "Carbohydrate terminology and classification," European Journal of Clinical Nutrition, 61(S1), S5-S18 (2007).
[3]. T. Qiu, "The potential mechanism underlying hippocampus-dependent memory decline caused by high-fat and high-sugar diet and the interventions to combat diet-induced cognitive decline," AIP Publishing.
[4]. V. V. Giau, E. Bagyinszky, Y. S. Yang, et al., "Genetic analyses of early-onset Alzheimer’s disease using next generation sequencing," Scientific Reports, 9(1), 8368 (2019).
[5]. C.-Q. Chu, L.-l. Yu, G.-y. Qi, et al., "Can dietary patterns prevent cognitive impairment and reduce Alzheimer's disease risk: Exploring the underlying mechanisms of effects," Neuroscience & Biobehavioral Reviews, 135, 104556 (2022).
[6]. G. A. Carlson,S. B. Prusiner, "How an Infection of Sheep Revealed Prion Mechanisms in Alzheimer’s Disease and Other Neurodegenerative Disorders," International Journal of Molecular Sciences, 22(9), 4861 (2021).
[7]. F. Leng,P. Edison, "Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here?," Nature Reviews Neurology, 17(3), 157-172 (2021).
[8]. T. Li, L. Lu, E. Pember, et al., "New Insights into Neuroinflammation Involved in Pathogenic Mechanism of Alzheimer’s Disease and Its Potential for Therapeutic Intervention," Cells, 11(12), 1925 (2022).
[9]. J. Rogers, S. Webster, L. F. Lue, et al., "Inflammation and Alzheimer's disease pathogenesis," Neurobiol Aging, 17(5), 681-6 (1996).
[10]. Y. Yao, C. Chinnici, H. Tang, et al., "Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis," J Neuroinflammation, 1(1), 21 (2004).
[11]. J. A. S. Gomes, J. F. Silva, A. P. Marcal, et al., "High-refined carbohydrate diet consumption induces neuroinflammation and anxiety-like behavior in mice," J Nutr Biochem, 77, 108317 (2020).
[12]. M. S. Wu, P. Johnston, W. H. Sheu, et al., "Effect of metformin on carbohydrate and lipoprotein metabolism in NIDDM patients," Diabetes Care, 13(1), 1-8 (1990).
[13]. M. J. Butler, N. P. Deems, S. Muscat, et al., "Dietary DHA prevents cognitive impairment and inflammatory gene expression in aged male rats fed a diet enriched with refined carbohydrates," Brain Behav Immun, 98, 198-209 (2021).
[14]. E. J. Yang, J. M. Kerver, Y. K. Park, et al., "Carbohydrate intake and biomarkers of glycemic control among US adults: the third National Health and Nutrition Examination Survey (NHANES III)," Am J Clin Nutr, 77(6), 1426-33 (2003).
[15]. K. B. M P Vitek, J M Glendening , E Stopa, H Vlassara, R Bucala, K Manogue and A Cerami, Advanced glycation end products contribute to amyloidosis in Alzheimer disease., PNAS, 1994).
[16]. S. P. Banik, M. Bhattacharyya, R. Ghosh, et al., "Chapter 11 - Glycation-induced protein aggregation and cellular toxicity: an insight into the disease realm of high dietary sugar intake," in: Dietary Sugar, Salt and Fat in Human Health,edited by Preuss Harry G., Bagchi Debasis(Academic Press, 2020), pp. 251-275.
[17]. N. Sasaki, R. Fukatsu, K. Tsuzuki, et al., "Advanced Glycation End Products in Alzheimer's Disease and Other Neurodegenerative Diseases," The American Journal of Pathology, 153(4), 1149-1155 (1998).
[18]. N. Flores-Fuentes, C. Hernandez-Cruz, K. Bermeo, et al., "Motor learning impairment in rats under a high sucrose diet," Physiol Behav, 234, 113384 (2021).
[19]. J. E. Beilharz, J. Maniam,M. J. Morris, "Diet-Induced Cognitive Deficits: The Role of Fat and Sugar, Potential Mechanisms and Nutritional Interventions," Nutrients, 7(8), 6719-38 (2015).
[20]. M. A. W. Hawkins, N. G. Keirns,Z. Helms, "Carbohydrates and cognitive function," Curr Opin Clin Nutr Metab Care, 21(4), 302-307 (2018).
[21]. S. H. Yeh, F. S. Shie, H. K. Liu, et al., "A high-sucrose diet aggravates Alzheimer's disease pathology, attenuates hypothalamic leptin signaling, and impairs food-anticipatory activity in APPswe/PS1dE9 mice," Neurobiol Aging, 90, 60-74 (2020).
[22]. F. A. H. SARDÁ, E. B. GIUNTINI, J.-A. NAZARE, et al., "Effectiveness of carbohydrates as a functional ingredient in glycemic control," 38(4), 561-576 (2018).
[23]. G. Marwarha,O. Ghribi, "Leptin signaling and Alzheimer's disease," Am J Neurodegener Dis, 1(3), 245-65 (2012).
[24]. R. T. PM Insel, D Ross, Carbohydrates: Simple sugars and complex chains. Discovering Nutrition, 2006, pp. 103-133.
[25]. M. Lilamand, B. Porte, E. Cognat, et al., "Are ketogenic diets promising for Alzheimer’s disease? A translational review," Alzheimer's Research & Therapy, 12(1), 42 (2020).
[26]. M. Ota, J. Matsuo, I. Ishida, et al., "Effect of a ketogenic meal on cognitive function in elderly adults: potential for cognitive enhancement," Psychopharmacology, 233(21), 3797-3802 (2016).
[27]. M. Ota, J. Matsuo, I. Ishida, et al., "Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease," Neuroscience Letters, 690, 232-236 (2019).
[28]. S. T. Henderson, "Ketone bodies as a therapeutic for Alzheimer’s disease," Neurotherapeutics, 5(3), 470-480 (2008).
[29]. I. Van Der Auwera, S. Wera, F. Van Leuven, et al., "A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer's disease," Nutrition & Metabolism, 2(1), 28 (2005).
[30]. A. D. Mooradian, "The Merits and the Pitfalls of Low Carbohydrate Diet: A Concise Review," The journal of nutrition, health & aging, 24(7), 805-808 (2020).
[31]. P. Londzin, M. Zamora, B. Kąkol, et al., "Potential of Caffeine in Alzheimer’s Disease—A Review of Experimental Studies," Nutrients, 13(2), 537 (2021).
[32]. A. A. Gomaa, H. S. M. Farghaly, A. M. Ahmed, et al., "Advancing combination treatment with cilostazol and caffeine for Alzheimer's disease in high fat-high fructose-STZ induced model of amnesia," Eur J Pharmacol, 921, 174873 (2022).
[33]. J. K. Morris,J. M. Burns, "Insulin: an emerging treatment for Alzheimer's disease dementia?," Curr Neurol Neurosci Rep, 12(5), 520-7 (2012).
[34]. H. Akimoto, A. Negishi, S. Oshima, et al., "Antidiabetic Drugs for the Risk of Alzheimer Disease in Patients With Type 2 DM Using FAERS," Am J Alzheimers Dis Other Demen, 35, 1533317519899546 (2020).
[35]. H. J. Lee, H. I. Seo, H. Y. Cha, et al., "Diabetes and Alzheimer's Disease: Mechanisms and Nutritional Aspects," Clin Nutr Res, 7(4), 229-240 (2018).









