1. Introduction
Renal cell carcinoma (RCC) is a common malignant tumor originating from the renal parenchyma or renal cortex, with a rising incidence globally [1]. Papillary renal cell carcinoma (pRCC) is the second most common subtype of RCC, accounting for approximately 15%–20% of all RCC cases [2]. Due to its relatively rare pathology, current research on the clinicopathological characteristics and survival prognosis of pRCC patients remains limited both domestically and internationally. Most domestic studies rely on small samples from single centers. The Surveillance, Epidemiology, and End Results (SEER) program of the U.S. National Cancer Institute collects incidence, mortality, and morbidity data from approximately 35% of the U.S. population across multiple centers [3]. This study extracted a large dataset of clinicopathological data on patients with pRCC from the SEER database, identified variables associated with cancer-specific survival (CSS), and developed a nomogram to predict postoperative CSS in patients with pRCC. The goal is to provide clinicians with a more effective clinical assessment tool and to improve the diagnosis and treatment of pRCC.
2. Materials and methods
2.1. General information
Clinical data of patients diagnosed with pRCC were obtained from the SEER database using SEER*Stat version 8.4.0. Inclusion criteria: 1) Pathologically confirmed diagnosis of papillary renal cell carcinoma; 2) Diagnosed between 2010 and 2015. Exclusion criteria: 1) Age over 85 years; 2) Tumor diameter greater than 500 mm; 3) Incomplete clinicopathological features or follow-up data.
2.2. Analyzed variables
Variables included in the study were: age at diagnosis, sex, race, laterality of the tumor, surgical method (including no surgery, cryoablation, nephroureterectomy involving unilateral nephrectomy, partial nephrectomy, and radical nephrectomy), histological grade, tumor size, and TNM staging based on the 7th edition of the American Joint Committee on Cancer (AJCC).
2.3. Statistical analysis
The dataset was randomly split into a training cohort and a validation cohort in a 7:3 ratio using the "caret" package in R version 4.2.2. Univariate and multivariate Cox regression analyses were conducted on the training cohort using the "survival" package to identify variables affecting survival time and outcomes. Significant prognostic factors were incorporated into a Cox proportional hazards model, and a nomogram was constructed using the "rms" package. Time-dependent receiver operating characteristic (ROC) curves were generated using the "timeROC" package. The discriminative ability and accuracy of the prognostic prediction model were evaluated by the area under the ROC curve (AUC) and calibration plots.
3. Results
3.1. Clinicopathological characteristics of patients
A total of 1,788 patients with pRCC were included and randomly assigned into a training cohort (n = 1,252) and a validation cohort (n = 536) in a 7:3 ratio. Among them: Patients over 60 years old accounted for a relatively high proportion (73.7%); Male patients comprised the majority (75.8%); Most patients underwent surgery (97.9%), primarily partial nephrectomy (48.9%) and radical nephrectomy (38.0%). There were no significant statistical differences in baseline characteristics between the training and validation cohorts (P ≥ 0.05; see Table 1), indicating that the training cohort could be reliably used to construct the model, with the validation cohort used for verification.
Table 1. Comparison of clinicopathological characteristics between training and validation cohorts (n, %)
Variable | Training Cohort (N=1252) | Validation Cohort (N=536) | Total (N=1788) | P-value |
Age | 0.599 | |||
≤60 | 335 (26.8) | 137 (25.6) | 472 (26.3) | |
>60 | 917 (73.2) | 399 (74.4) | 1316 (73.7) | |
Sex | 0.924 | |||
Male | 948 (75.7) | 407 (75.9) | 1355 (75.8) | |
Female | 304 (24.3) | 129 (24.1) | 433 (24.2) | |
Race | 0.681 | |||
White | 888 (70.9) | 373 (69.6) | 1261 (70.5) | |
Black | 297 (23.7) | 129 (24.1) | 426 (23.8) | |
Other races | 67 (5.4) | 34 (6.3) | 101 (5.6) | |
Tumor laterality | 0.144 | |||
Left | 631 (50.4) | 268 (50.0) | 899 (50.3) | |
Right | 620 (49.5) | 265 (49.4) | 885 (49.5) | |
Bilateral | 1 (0.1) | 3 (0.6) | 4 (0.2) | |
Surgical method | 0.404 | |||
No surgery | 27 (2.2) | 10 (1.9) | 37 (2.1) | |
Cryoablation | 37 (3.0) | 20 (3.7) | 57 (3.2) | |
Partial nephrectomy | 620 (49.5) | 255 (47.6) | 875 (48.9) | |
Nephroureterectomy | 89 (7.1) | 51 (9.5) | 140 (7.8) | |
Radical nephrectomy | 479 (38.3) | 200 (37.3) | 679 (38.0) | |
Histological grade | 0.379 | |||
1 | 142 (11.3) | 60 (11.2) | 202 (11.3) | |
2 | 670 (53.5) | 281 (52.4) | 951 (53.2) | |
3 | 417 (33.3) | 178 (33.2) | 595 (33.3) | |
4 | 23 (1.8) | 17 (3.2) | 40 (2.2) | |
M stage | 0.061 | |||
0 | 1230 (98.2) | 519 (96.8) | 1749 (97.8) | |
1 | 22 (1.8) | 17 (3.2) | 39 (2.2) | |
N stage | 0.063 | |||
0 | 1208 (96.5) | 507 (94.6) | 1715 (95.9) | |
1 | 44 (3.5) | 29 (5.4) | 73 (4.1) | |
T stage | 0.763 | |||
1 | 968 (77.3) | 412 (76.9) | 1380 (77.2) | |
2 | 135 (10.8) | 58 (10.8) | 193 (10.8) | |
3 | 146 (11.7) | 63 (11.8) | 209 (11.7) | |
4 | 3 (0.2) | 3 (0.6) | 6 (0.3) | |
Tumor size (mm) | 0.850 | |||
≤50 | 842 (67.3) | 358 (66.8) | 1200 (67.1) | |
>50 | 410(32.7) | 178 (33.2) | 588 (32.9) |
3.2. Univariate and multivariate Cox regression analysis
A Cox proportional hazards model was employed for univariate and multivariate analyses on the training set using the competing risks framework. The results indicated that age at diagnosis, surgical method, Fuhrman grade, and TNM stage were independent prognostic factors for papillary renal cell carcinoma (pRCC) patients (P < 0.05; see Table 2). Race, tumor laterality, and tumor size were not significantly associated with overall survival (P ≥ 0.05). However, sex was included in the multivariate model due to its clinical relevance in prognosis.
Table 2. Univariate and multivariate Cox regression analysis for prognostic factors in pRCC patients
Variable | Univariate Analysis | Multivariate Analysis | |||
HR (95%CI) | P Value | HR (95%CI) | P Value | ||
Age | |||||
≤60 | 1 | 1 | |||
>60 | 1.371(1.154~1.562) | <0.001 | 1.293(1.098~1.452) | <0.001 | |
Sex | |||||
Male | 1 | 1 | |||
Female | 0.890(0.678~1.168) | 0.401 | 0.801(0.611~1.063) | 0.123 | |
Race | |||||
White | 1 | ||||
Black | 0.813(0.610~1.085) | 0.160 | |||
Other races | 1.019(0.637~1.629 | 0.939 | |||
Tumor laterality | |||||
Left | 1 | ||||
Right | 0.954(0.759~1.235) | 0.690 | |||
Surgical method | |||||
No surgery | 1 | 1 | |||
Cryoablation | 0.383(0.163~0.902) | 0.028 | 0.452(0.202~1.044) | 0.063 | |
Partial nephrectomy | 0.180(0.095~0.338) | <0.001 | 0.231(0.133~0.415) | <0.001 | |
Nephroureterectomy | 0.510(0.252~1.031) | 0.061 | 0.713(0.372~1.366) | 0.308 | |
Radical nephrectomy | 0.631(0.343~1.161) | 0.139 | 0.636(0.365~1.082) | 0.095 | |
Histological grade | |||||
1 | 1 | 1 | |||
2 | 1.293(0.813~2.058) | 0.278 | 1.205(0.778~1.897) | 0.419 | |
3 | 2.502(1.580~3.963) | <0.001 | 1.762(1.125~2.760) | 0.015 | |
4 | 5.379(2.863~10.10) | <0.001 | 2.389(1.223~4.627) | 0.011 | |
M stage | |||||
0 | 1 | 1 | |||
1 | 17.84(11.75~27.08) | <0.001 | 7.492(4.183~11.43) | <0.001 | |
N stage | |||||
0 | 1 | 1 | |||
1 | 9.632(6.921~13.41) | <0.001 | 2.253(1.378~3.701) | <0.001 | |
T stage | |||||
1 | 1 | 1 | |||
2 | 1.680(1.142~2.472) | 0.008 | 0.892(0.621~1.899) | 0.548 | |
3 | 3.341(2.551~4.375) | <0.001 | 1.401(1.012~1.943) | 0.046 | |
4 | 23.80(9.650~58.72) | <0.001 | 0.881(0.293~2.675) | 0.819 | |
Tumor size (mm) | |||||
≤50 | 1 | ||||
>50 | 1.072(0.898~1.176) | 0.765 | |||
3.3. Construction of the nomogram model
Based on the five significant prognostic factors identified above, a nomogram model was constructed to predict cancer-specific survival (CSS) in patients with papillary renal cell carcinoma. Each subcategory of the prognostic variables is assigned a score, and the sum of these scores corresponds to a total point score at the bottom of the nomogram. By aligning the total score with the predicted overall survival (OS) values, the 1-, 3-, and 5-year survival probabilities can be estimated. (See Figure 1)
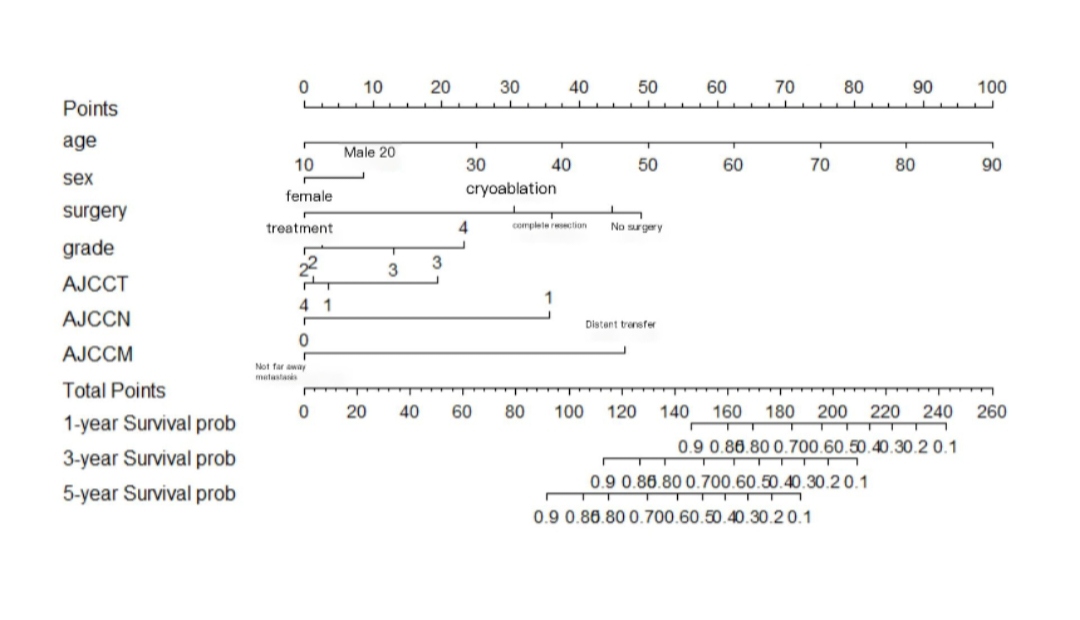
Figure 1. Nomogram for predicting cancer-specific survival in pRCC patients based on the training set
3.4. Validation of the nomogram model
A time-dependent receiver operating characteristic (ROC) curve was plotted with the false positive rate on the x-axis and the true positive rate on the y-axis. The results showed that the area under the curve (AUC) values and 95% confidence intervals (CI) for predicting 1-, 3-, and 5-year survival in the training set were 0.7978 (0.7823–0.8133), 0.7813 (0.7656–0.8030), and 0.7542 (0.7379–0.7705), respectively. For the validation set, the AUC values for the 1-, 3-, and 5-year predictions were 0.6793 (0.6541–0.7045), 0.7114 (0.6856–0.7372), and 0.7174 (0.6918–0.7430), respectively (Figure 2). These results indicate that the model has a certain level of predictive accuracy. The calibration curves demonstrated good agreement between predicted and observed survival probabilities, suggesting that the nomogram provides reliable survival predictions (Figure 3).
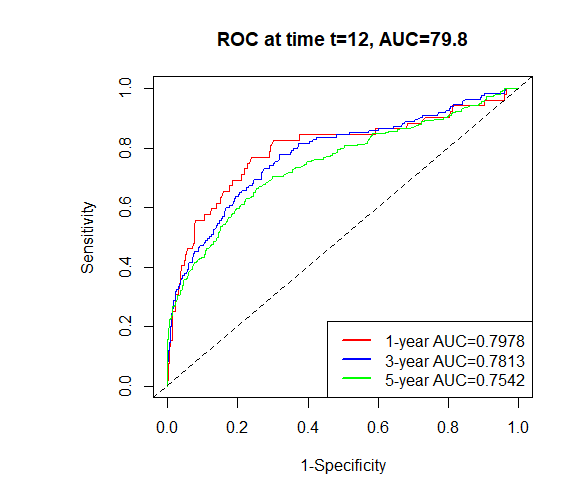
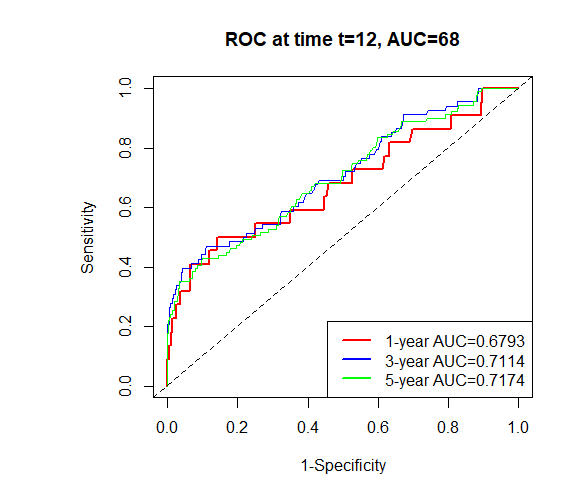
Figure 2. Time-dependent ROC curves for predicting pRCC patient survival in training and validation sets
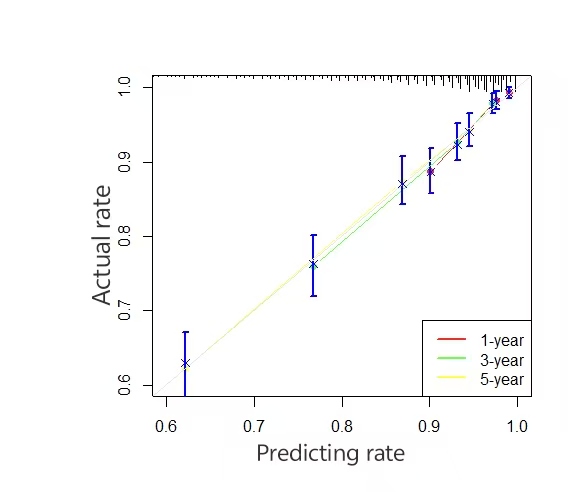
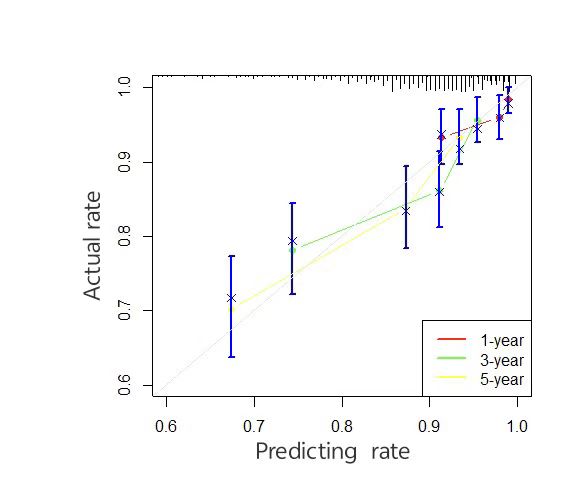
Figure 3. Calibration curves for the nomogram model predicting cancer-specific survival (CSS) in training and validation sets
4. Discussion
Papillary renal cell carcinoma (PRCC) is relatively rare in clinical practice, accounting for approximately 18.5% of renal epithelial tumors [4, 5]. Due to limited sample sizes at single centers, it is often difficult to establish a prognostic prediction model for PRCC. Currently, there are relatively few studies on PRCC prognostic models in China. Yan et al. [6], using a large multicenter sample from the SEER database, first identified seven factors (age, T stage, N stage, M stage, surgery/lymph node dissection, and insurance status) that were significantly associated with overall survival, and constructed a prognostic prediction model involving 4,859 patients diagnosed with PRCC between 2010 and 2014. Hu et al. [7] developed a nomogram for predicting postoperative overall survival in patients who underwent either partial or radical nephrectomy, and constructed a new risk assessment system to analyze the differences in survival outcomes between the two procedures. Their findings indicated that patients undergoing partial nephrectomy had better overall survival compared to those receiving radical nephrectomy, possibly due to differences in tumor stage at the time of diagnosis. Zhanghuang et al. [8], recognizing advanced age as an independent prognostic factor for PRCC, constructed a prognostic model for elderly patients using SEER database data. Their univariate and multivariate Cox regression analyses showed that age, tumor size, histological grade, TNM stage, surgery, radiotherapy, and chemotherapy were all independent prognostic factors, which had a particularly significant impact on elderly patients with PRCC. Haddad et al. [9] found that, among 173 patients with T1a RCC treated with percutaneous cryoablation (PCA), cryoablation was a feasible treatment option for those with clinical stage T1a-pRCC. Image-guided percutaneous ablation may be a favorable treatment strategy, particularly for PRCC. Nabavizadeh et al. [10] reported outcomes of 10 cases involving radical nephrectomy and nephroureterectomy for malignant tumors of transplanted allograft kidneys, showing good long-term survival after surgery. There was also a case report of a patient who underwent nephroureterectomy and experienced recurrent bladder PRCC metastasis, though prognosis was poor due to distant metastasis [11]. In addition to traditional surgical approaches, prospective clinical trials have shown that immune checkpoint inhibitors exhibit certain therapeutic activity in PRCC [12]. However, although many of these surgical approaches have been clinically proven to improve prognosis in PRCC patients, no studies have yet developed a unified clinical prognostic prediction model to analyze long-term survival outcomes for patients undergoing different surgical treatments. Therefore, this study aims to establish a tool for evaluating disease-specific survival (CSS) outcomes among PRCC patients undergoing different surgical procedures, based on clinicopathological information from the SEER database. Despite some limitations of the SEER database—such as underrepresentation of Asian populations and partial missing clinical data—race is generally not considered to be significantly associated with CSS in PRCC. Moreover, the SEER database covers a broad patient population, which helps reduce bias compared to single-center or small-sample datasets. Thus, this study provides a potentially useful reference for clinicians and PRCC patients in making prognostic decisions. Nevertheless, as external validation was not performed in this study, the model’s generalizability may be limited. Further prospective studies are needed to validate the current findings.
Funding
The Key Joint Project of the Hunan Provincial Natural Science Foundation and Municipal Science Foundation (Grant No. 2024JJ7428).
References
[1]. Bahadoram, S., Davoodi, M., Hassanzadeh, S. (2022). Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G Ital Nefrol, 39(3).
[2]. Angori, S., Lobo, J., & Moch, H. (2022). Papillary renal cell carcinoma: current and controversial issues. Curr Opin Urol, 32(4), 344-351.
[3]. Doll, K. M., Rademaker, A., & Sosa, J. A. (2018). Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg, 153(6), 588-589.
[4]. Caraballo, B., Abdulla, M., Sham, S. (2022). Papillary Renal Cell Carcinomas Demonstrating Micropapillary Features: An Investigation Into the Diagnostic and Prognostic Implications. Cureus, 14(5), e24944.
[5]. Qian, X., Wan, J., Qian, C. (2021). Clinicopathological Features and Prognostic Outcomes of Papillary Renal Cell Carcinoma. Int J Gen Med, 14, 7523-7531.
[6]. Yan, H., Wei, X., Wu, A. (2020). Nomograms for predicting overall and cancer-specific survival in patients with papillary renal cell carcinoma: a population-based study using SEER database. Transl Androl Urol, 9(3), 1146-1158.
[7]. Hu, Y., Xu, S., Qi, Q. (2022). A novel nomogram and risk classification system predicting the overall survival of patients with papillary renal cell carcinoma after nephrectomy: A population-based study. Front Public Health, 10, 989566.
[8]. Zhanghuang, C., Wang, J., Yao, Z. (2022). Development and Validation of a Nomogram to Predict Cancer-Specific Survival in Elderly Patients With Papillary Renal Cell Carcinoma. Front Public Health, 10, 874427.
[9]. Haddad, M. M., Schmit, G. D., Kurup, A. N. (2018). Percutaneous Cryoablation of Solitary, Sporadic Renal Cell Carcinoma: Outcome Analysis Based on Clear-Cell versus Papillary Subtypes. J Vasc Interv Radiol, 29(8), 1122-1126.
[10]. Nabavizadeh, R., Nooralia, A. A., Makhani, S. S. (2020). Transplant Radical Nephrectomy and Transplant Radical Nephroureterectomy for Renal Cancer: Postoperative and Survival Outcomes. Ann Transplant, 25, e925865.
[11]. Zeng, Q., Luo, S., Chen, L. (2023). Bladder metastasis from type 2 papillary renal cell carcinoma: A case report. Oncol Lett, 25(6), 270.
[12]. Maughan, B. L., & Sirohi, D. (2023). Papillary Renal Cell Carcinoma: A Review of Prospective Clinical Trials. Curr Treat Options Oncol, 24(9), 1199-1212.
Cite this article
Tang,Q.;Wan,Y. (2025). Constructing and validating prognostic models for papillary renal cell carcinoma after different surgical procedures based on the SEER database. Journal of Clinical Technology and Theory,3(1),45-50.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Clinical Technology and Theory
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bahadoram, S., Davoodi, M., Hassanzadeh, S. (2022). Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G Ital Nefrol, 39(3).
[2]. Angori, S., Lobo, J., & Moch, H. (2022). Papillary renal cell carcinoma: current and controversial issues. Curr Opin Urol, 32(4), 344-351.
[3]. Doll, K. M., Rademaker, A., & Sosa, J. A. (2018). Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg, 153(6), 588-589.
[4]. Caraballo, B., Abdulla, M., Sham, S. (2022). Papillary Renal Cell Carcinomas Demonstrating Micropapillary Features: An Investigation Into the Diagnostic and Prognostic Implications. Cureus, 14(5), e24944.
[5]. Qian, X., Wan, J., Qian, C. (2021). Clinicopathological Features and Prognostic Outcomes of Papillary Renal Cell Carcinoma. Int J Gen Med, 14, 7523-7531.
[6]. Yan, H., Wei, X., Wu, A. (2020). Nomograms for predicting overall and cancer-specific survival in patients with papillary renal cell carcinoma: a population-based study using SEER database. Transl Androl Urol, 9(3), 1146-1158.
[7]. Hu, Y., Xu, S., Qi, Q. (2022). A novel nomogram and risk classification system predicting the overall survival of patients with papillary renal cell carcinoma after nephrectomy: A population-based study. Front Public Health, 10, 989566.
[8]. Zhanghuang, C., Wang, J., Yao, Z. (2022). Development and Validation of a Nomogram to Predict Cancer-Specific Survival in Elderly Patients With Papillary Renal Cell Carcinoma. Front Public Health, 10, 874427.
[9]. Haddad, M. M., Schmit, G. D., Kurup, A. N. (2018). Percutaneous Cryoablation of Solitary, Sporadic Renal Cell Carcinoma: Outcome Analysis Based on Clear-Cell versus Papillary Subtypes. J Vasc Interv Radiol, 29(8), 1122-1126.
[10]. Nabavizadeh, R., Nooralia, A. A., Makhani, S. S. (2020). Transplant Radical Nephrectomy and Transplant Radical Nephroureterectomy for Renal Cancer: Postoperative and Survival Outcomes. Ann Transplant, 25, e925865.
[11]. Zeng, Q., Luo, S., Chen, L. (2023). Bladder metastasis from type 2 papillary renal cell carcinoma: A case report. Oncol Lett, 25(6), 270.
[12]. Maughan, B. L., & Sirohi, D. (2023). Papillary Renal Cell Carcinoma: A Review of Prospective Clinical Trials. Curr Treat Options Oncol, 24(9), 1199-1212.









