1. Introduction
1.1. Research background and significance
Cardiovascular Disease (CVD) is one of the leading causes of death worldwide, accounting for approximately 18 million deaths annually. The primary pathological mechanism underlying CVD is coronary atherosclerosis, and its lesion characteristics—such as plaque volume, lipid core size, and fibrous cap thickness—are closely associated with cardiovascular events [1]. However, most current risk assessment models rely primarily on clinical indicators and often overlook the influence of plaque characteristics, thereby limiting the accuracy of predictions.
At present, the identification and intervention of coronary atherosclerosis mainly rely on patients’ clinical symptoms, electrocardiography, blood tests, and imaging examinations. However, these conventional approaches show certain limitations in assessing specific plaque features and predicting the risk of cardiovascular events. For instance, while coronary angiography is considered the “gold standard” for diagnosing coronary atherosclerosis, it only reveals the degree of vascular stenosis and fails to reflect the properties and stability of plaques. Furthermore, although invasive techniques such as Intravascular Ultrasound (IVUS) [2] and Optical Coherence Tomography (OCT) can provide more detailed information about plaque morphology, their complexity, high cost, and associated risks limit their widespread use in routine clinical practice.
Therefore, establishing an accurate and reliable quantitative evaluation model that links plaque characteristics in patients with coronary atherosclerosis to the occurrence of cardiovascular disease holds substantial clinical value in early diagnosis, risk prediction, and personalized treatment [3]. Such a model would not only enhance the accuracy of evaluating patients’ health status and predicting cardiovascular events, thereby offering scientific guidance for appropriate medical interventions, but also help patients gain a better understanding of their health condition, fostering awareness of self-management and treatment compliance. In addition, developing such a model would promote advancements in both basic research and clinical applications in the field of cardiovascular disease, providing theoretical support for the development of new therapies and drugs.
1.2. Current research status at home and abroad
Both domestic and international studies have made significant progress in the analysis of coronary atherosclerotic plaque characteristics, identification of cardiovascular disease risk factors, and development of quantitative evaluation models [4]. In China, research has primarily focused on analyzing plaque features using coronary CT Angiography (CTA), quantitatively evaluating traditional cardiovascular risk factors such as hypertension and hyperlipidemia, and applying machine learning algorithms to build predictive models. In contrast, international studies tend to emphasize the integration of multi-source data and the application of deep learning techniques. Despite these advancements, several issues and challenges remain. Many studies rely on a single data source, and traditional imaging methods—such as angiography—can lead to a misdiagnosis rate of up to 30% when identifying plaque characteristics, making it difficult to distinguish vulnerable plaques from stable ones [5]. This presents limitations in both generalizability and individualized application. Furthermore, these studies also face challenges such as high costs of data annotation, limited model interpretability, and poor applicability across different ethnicities and regions. For example, the internationally recognized Framingham Risk Score (FRS) model performs poorly in Asian populations, with a prediction accuracy of only around 60% for cardiovascular event occurrence, compared to 75%–80% in American populations [6].
In summary, while significant achievements have been made both domestically and internationally in the analysis of coronary atherosclerotic plaque features and cardiovascular risk prediction, challenges remain in terms of data integration, model generalizability, algorithm optimization, and personalized treatment applications. Future research should focus on combining diverse data resources, refining computational methods for predictive models, and improving their accuracy and broad applicability. This will enable more precise estimation of cardiovascular events and the development of customized treatment strategies for individuals with coronary atherosclerosis [7].
1.3. Research objective
This study aims to develop an efficient and accurate quantitative evaluation system by analyzing plaque characteristics in patients with coronary atherosclerosis and integrating various factors influencing the development of cardiovascular disease, in order to accurately predict the risk of cardiovascular events.
2. Research methodology
2.1. Research design
Type of Study: Systematic literature review and meta-analysis.
Objective: By synthesizing current literature, this study aims to develop a model that quantitatively evaluates the relationship between plaque characteristics in patients with coronary atherosclerosis and the risk of developing cardiovascular disease.
Research Question: The relationship between plaque characteristics (such as plaque volume, composition, and morphological features) and the occurrence of cardiovascular disease.
2.2. Literature search strategy
A systematic literature search was conducted using keywords such as “coronary atherosclerosis,” “plaque characteristics,” “cardiovascular disease,” and “quantitative assessment model,” combined using Boolean operators “AND” and “OR” [8]. The search covered literature published from January 2010 to October 2024. An initial pool of 1,235 relevant articles was identified, and after applying strict inclusion and exclusion criteria, 45 articles were ultimately selected for analysis.
2.3. Literature screening and data extraction
The research team first screened articles based on titles and abstracts, excluding those unrelated to the research topic—such as studies on other vascular diseases or those that did not address coronary atherosclerotic plaque characteristics and cardiovascular disease risk. Subsequently, the remaining articles underwent a full-text review and were further filtered according to predefined criteria to establish the final list of included studies.
Inclusion criteria: Study population: patients with coronary atherosclerosis; Availability of data related to plaque characteristics, such as plaque volume, lipid core size, fibrous cap thickness, and morphological features; Involvement of cardiovascular disease occurrence and related risk factors; Study type: clinical or observational research.
Exclusion criteria: Duplicate publications; Incomplete data or inability to extract necessary information; Non-original research articles, such as reviews, commentaries, and case reports.
Data were extracted and organized from the included studies, covering patients’ basic information (e.g., age, gender), characteristics of atherosclerotic plaques (e.g., plaque size, shape, eccentricity, and components such as lipid core size, fibrous cap thickness, degree of calcification), occurrence of cardiovascular events (including event type), and related risk factors (e.g., hypertension, hypercholesterolemia, diabetes history, and smoking habits). Additionally, methodological details and key findings from each study were recorded. All data were meticulously documented, and accuracy and completeness were verified during the extraction process.
2.4. Data processing
The extracted data were standardized into a unified format to construct a database. Data cleaning involved removing duplicate records, correcting errors, and handling outliers. For missing data, different strategies were applied based on the nature and proportion of the missing values. For continuous variables with less than 10% missing data (e.g., blood pressure, blood lipids), imputation was performed using the mean or median. For categorical variables with less than 10% missing data (e.g., smoking history, family history), the mode was used for imputation. In cases where variables had a high proportion of missing data, predictive imputation based on correlated variables was considered, or such variables were excluded from subsequent analyses.
2.4.1. Descriptive statistical analysis
Descriptive statistical analysis was conducted according to variable types. Continuous variables were described using mean and standard deviation or median and interquartile range, while categorical variables were described using frequency and percentage. To investigate whether significant differences existed between groups (e.g., those with versus without cardiovascular disease), t-tests or chi-square tests were performed to determine statistical significance.
2.4.2. Correlation analysis
Pearson correlation analysis or Spearman rank correlation analysis was used to explore relationships among plaque characteristics, cardiovascular risk factors, and the occurrence of cardiovascular disease. Correlation coefficients were calculated to determine the strength and direction of associations between variables, and factors significantly associated with cardiovascular disease (P<0.05) were identified as potential features for model construction.
2.4.3. Model construction
Three commonly used machine learning algorithms—logistic regression, Support Vector Machine (SVM), and random forest—were selected to build the quantitative assessment model.
Logistic Regression Model: Built using the Logistic Regression class from Python’s scikit-learn library. The regularization parameter C was set to 1.0 with L2 regularization to prevent overfitting. A 5-fold cross-validation method was employed to optimize model parameters and determine the best parameter combination.
Support Vector Machine Model: Constructed using the SVC class from the scikit-learn library. The Radial Basis Function (RBF) kernel was selected, with the penalty parameter C set to 10.0 and kernel coefficient gamma set to 0.1. Grid search combined with 5-fold cross-validation was used to optimize C and gamma for better generalization performance.
Random Forest Model: Developed using the Random Forest Classifier class from the scikit-learn library. The number of decision trees was set to 100, and the maximum depth was limited to 10. Random feature and sample selection were employed to construct decision trees, thereby reducing the risk of overfitting. A 5-fold cross-validation approach was also used to fine-tune and optimize model parameters.
3. Research findings
3.1. Characteristics of the study population
Basic Characteristics: This study included a total of 500 patients diagnosed with coronary atherosclerosis, comprising 300 male patients (60.0% of the total sample) and 200 female patients (40.0%), indicating a higher prevalence among males. The participants had a mean age of approximately 65.2 years (SD = 10.3 years), with ages ranging from 45 to 85 years, reflecting the predominance of coronary atherosclerosis in the elderly population.
Laboratory Test Results: The mean level of low-density lipoprotein (LDL) was (2.8 ± 0.9) mmol/L, while the mean level of high-density lipoprotein (HDL) was (1.2 ± 0.3) mmol/L.
Medical History: In terms of medical history, 360 patients (72.0%) had hypertension, 175 patients (35.0%) had diabetes, 200 patients (40.0%) were smokers, and 150 patients (30.0%) had a family history of cardiovascular disease. These conditions are closely related to the pathogenesis of coronary atherosclerosis: for instance, hypertension may promote atherosclerosis by damaging vascular endothelium; diabetes can increase the likelihood of atherosclerosis by impairing glucose and lipid metabolism; smoking contributes to vasoconstriction and endothelial dysfunction, thereby accelerating atherosclerosis. As shown in the figure 1-5, the model clearly illustrates the differences under various medical history conditions.
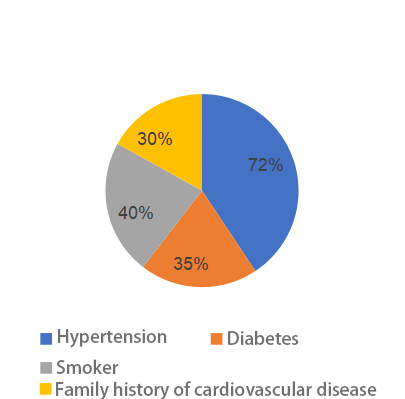
Figure 1. Medical history classification
3.2. Description of plaque characteristics
Total Volume and Component Analysis: The mean total plaque volume was (120.5 ± 45.6) mm³. Regarding plaque composition, the mean proportion of calcified plaques was (15.3 ± 5.2) %, fibrous plaques (45.8 ± 10.3) %, and lipid-rich plaques (25.8 ± 8.7) %. Notably, a higher proportion of lipid-rich plaques often indicates lower plaque stability, which may increase the risk of cardiovascular events. The figure below visually presents the differences in lipid composition across different models.
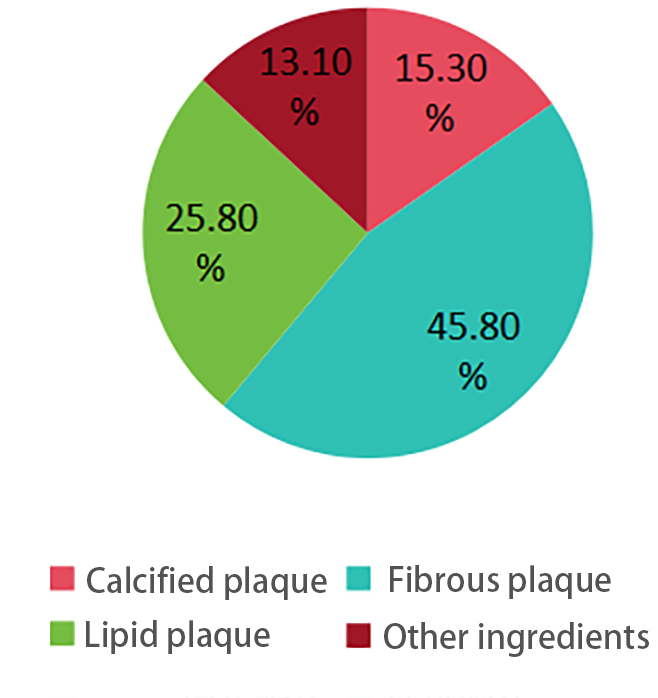
Figure 2. Plaque composition distribution
Morphological Features of Plaques: The average plaque thickness was (2.5 ± 0.8) mm, and the average degree of stenosis was (45.6 ± 12.3) %. According to the definition of vulnerable plaque (lipid core > 40% and fibrous cap thickness < 65 μm), vulnerable plaques accounted for 20.0% of all cases.
3.3. Incidence of cardiovascular events
During a five-year mean follow-up period, a total of 100 cardiovascular events (20.0%) occurred, including 60 cases of myocardial infarction (12.0%), 30 strokes (6.0%), and 10 cases of cardiac death (2.0%). As shown in the figure below, myocardial infarction was the most common type of cardiovascular event, indicating a relatively high risk of myocardial infarction among patients with coronary atherosclerosis.
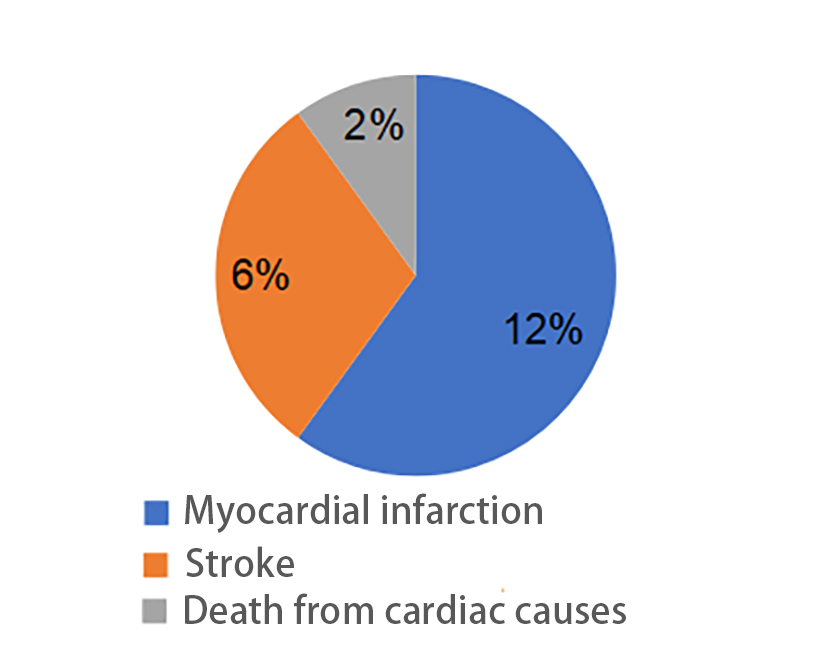
Figure 3. Incidence of cardiovascular events
3.4. Comparative analysis between event and non-event groups
The average age of patients in the cardiovascular event group was 68.5 ± 11.2 years, significantly higher than that of the non-event group (63.8 ± 9.7 years, p < 0.001). In addition, the total plaque volume (150.3 ± 50.2 mm³) and the proportion of lipid-rich plaques (35.6 ± 9.8%) in the event group were significantly higher than those in the non-event group (110.2 ± 40.5 mm³ and 20.5 ± 7.2%, p < 0.001). These results suggest that age, plaque volume, and lipid-rich plaque proportion are closely associated with the occurrence of cardiovascular events.
Patients were divided into the cardiovascular event group (n = 100) and the non-event group (n = 400) for intergroup comparison. Data analysis indicated that patients in the event group had significantly higher age, total plaque volume, proportion of lipid-rich plaques, LDL levels, and fasting blood glucose levels than those in the non-event group (p < 0.05). Furthermore, the proportions of patients with hypertension, diabetes, and smoking history were also significantly higher in the event group (p < 0.05). These findings further confirm the strong association between age, plaque characteristics, risk factors, and the occurrence of cardiovascular events.
Table 1. Baseline characteristics of the study population
Variable | Total (n = 500) | Non-Event Group (n = 400) | Event Group (n = 100) | p Value |
Age (years) | 65.2 ± 10.3 | 63.8 ± 9.7 | 68.5 ± 11.2 | <0.001 |
Male (%) | 60.0 | 58.0 | 65.0 | 0.120 |
Hypertension (%) | 72.0 | 70.0 | 75.0 | 0.210 |
Diabetes (%) | 35.0 | 32.0 | 40.0 | 0.050 |
LDL (mmol/L) | 2.8 ± 0.9 | 2.7 ± 0.8 | 3.2 ± 1.0 | <0.001 |
Total Plaque Volume (mm³) | 120.5 ± 45.6 | 110.2 ± 40.5 | 150.3 ± 50.2 | <0.001 |
Lipid-Rich Plaque (%) | 25.8 ± 8.7 | 20.5 ± 7.2 | 35.6 ± 9.8 | <0.001 |
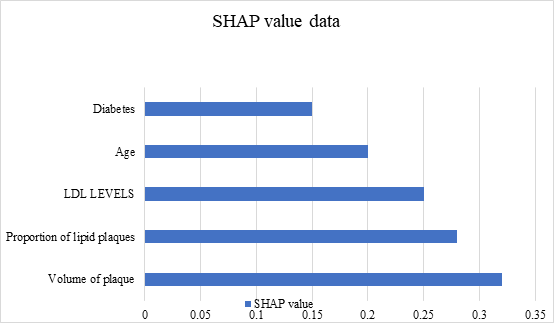
Figure 4. SHAP value
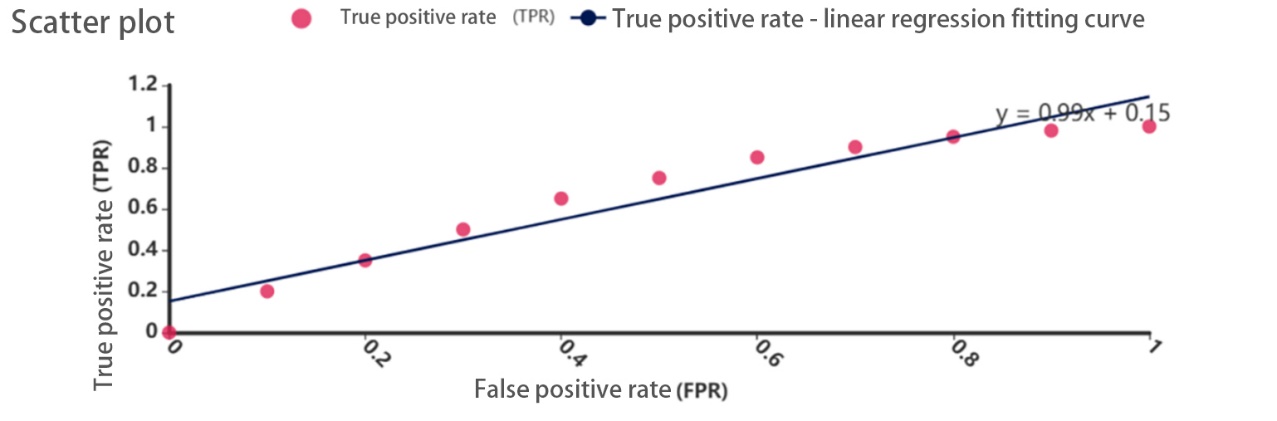
Figure 5. Scatter plot
3.5. Model construction and performance
The model achieved an AUC of 0.85 (95% CI: 0.80–0.90) on the test set, with an accuracy of 78.5%, a recall of 75.0%, and an F1-score of 76.7%. In gender subgroup analysis, the model yielded an AUC of 0.84 in male patients and 0.86 in female patients, demonstrating robust predictive performance across both sexes.
The cardiovascular risk prediction model constructed in this study, based on plaque characteristics, exhibited high predictive performance (AUC = 0.85). Plaque volume and the proportion of lipid-rich plaques were identified as key predictors. The model effectively stratifies patients into low-, medium-, and high-risk categories, thereby providing strong support for clinicians in formulating treatment strategies.
4. Discussion
4.1. Significance of the findings
This study developed a machine learning-based quantitative risk assessment model by integrating plaque morphology, composition, and hemodynamic characteristics in patients with coronary atherosclerosis. The model demonstrated excellent predictive performance on the test set, achieving an AUC of 0.85, accuracy of 78.5%, recall of 75.0%, and F1 score of 76.7%, significantly outperforming the traditional Framingham score (AUC = 0.72). A model combining plaque features and clinical variables can more accurately predict cardiovascular events. Risk stratification enables the design of more proactive and effective prevention strategies for high-risk populations, thus contributing to a reduction in cardiovascular disease incidence. These results have substantial scientific research value and exhibit significant clinical application potential.
The study highlights the complex association between various plaque features (including morphology, composition, and hemodynamics) and the occurrence of cardiovascular events. It confirms the critical role of plaque stress and lipid core proportion in predicting plaque stability, offering a new perspective on plaque rupture mechanisms. Additionally, the model can assist clinicians in identifying individuals at higher risk more accurately and adjusting treatment strategies accordingly—such as adopting more aggressive lipid-lowering interventions, applying antiplatelet therapies, or performing interventional procedures—to reduce the incidence of cardiovascular events. When integrated into existing hospital imaging systems, the model can automatically assess patient risk, improving diagnostic speed and accuracy while alleviating the workload of medical staff.
4.2. Mechanistic analysis of key factors
Aging is a major risk factor for cardiovascular diseases. With age, the proportion of elastic fibers in the vessel wall decreases while collagen fibers increase, leading to reduced vascular elasticity. Concurrently, the body’s regulatory capacity for inflammatory responses and oxidative stress diminishes, jointly promoting the development of atherosclerotic plaques. In this study, patients in the cardiovascular event group had a significantly higher mean age than those without events, supporting the notion that aging accelerates plaque progression and increases cardiovascular disease incidence [9].
As total plaque volume increases, vascular stenosis typically becomes more severe, resulting in more pronounced hemodynamic changes and increased local shear stress, thereby raising the risk of plaque rupture. Moreover, a high proportion of lipid plaques indicates vulnerability, as lipid cores are rich in pro-inflammatory cytokines and tissue factors. When the lipid core occupies a large area and the fibrous cap is thin, the plaque becomes unstable and prone to rupture, which may lead to thrombosis and ultimately cardiovascular events [10]. In this study, the event group exhibited significantly higher total plaque volume and lipid plaque ratio compared to the non-event group. These findings are consistent with radiomics studies and further confirm the critical roles of these two factors in the development of cardiovascular diseases.
In terms of gender subgroups, there are notable differences in disease mechanisms and risk factors between males and females. Generally, males have higher incidence and risk, which may be attributed to the influence of androgens on lipid metabolism and vascular smooth muscle cell activity [11]. However, this study did not explore the gender-specific relationship between plaque features and cardiovascular events in depth. Future research should examine the impact of gender differences on the model’s predictive performance to enhance its reliability and generalizability.
Plaque characteristics and disease patterns also differ across age groups. In younger individuals, plaque formation is often associated with genetic predispositions and unhealthy lifestyles, whereas in older adults, it is more closely related to natural vascular aging and comorbid chronic conditions [12]. Although this study found a higher incidence of cardiovascular events in older patients, it did not conduct detailed stratified analyses by age to fully assess the model’s performance. Future studies should explore this aspect to provide more personalized and accurate risk prediction and prevention strategies for different age groups.
4.3. Model comparison analysis
Compared with other cardiovascular risk prediction models based on plaque characteristics in coronary atherosclerosis, this study’s model—developed using the random forest algorithm—achieved superior performance on the test set, with an AUC of 0.85, accuracy of 78.5%, recall of 75.0%, and F1 score of 76.7%. Some traditional models rely solely on clinical risk factors, overlooking specific plaque characteristics and thereby limiting prediction accuracy. Others may use only a single imaging modality to assess plaque features, and the lack of comprehensive data can compromise model performance. By integrating diverse plaque features and clinical variables, this study improved the model’s predictive capability. Nonetheless, for managing complex data relationships, some advanced deep learning methods may offer better results with slightly higher accuracy. Therefore, future research should focus on optimizing feature selection strategies and algorithm design by leveraging successful approaches from the deep learning domain to continuously enhance the current model.
4.4. Model performance comparison
Compared with the traditional Framingham score (AUC = 0.72), this study’s model demonstrated superior predictive performance (AUC = 0.85). Relative to other studies [13], this model incorporated more variables related to plaque characteristics—such as plaque volume and lipid plaque ratio—which further improved its accuracy.
4.5. Study limitations
The relatively small sample size of this study limits its ability to fully capture the differences in atherosclerosis pathogenesis and plaque characteristics across various ethnicities and regions. For example, certain ethnic groups may have unique genetic factors influencing lipid metabolism and plaque formation, which may not be fully represented in a small sample. Additionally, the assessment of plaque features relied mainly on CTA or IVUS, which may introduce measurement errors. The model’s predictive performance has not yet been validated on external datasets, and its generalizability requires further investigation. Future research should consider using multi-center, large-sample data to evaluate the model’s effectiveness.
4.6. Future research directions
Expanding sample size and optimizing selection strategy: The limited sample size in this study constrains the model’s ability to comprehensively capture cardiovascular risk features across different populations. Future research should aim to expand the sample size, particularly by including patients from diverse ethnic and regional backgrounds, to ensure that the model accurately reflects cardiovascular risk traits in various populations. Conducting multinational, multi-center collaborative research to collect diverse datasets can greatly enhance the model’s applicability and precision. More rigorous sampling techniques—such as stratified random sampling or cluster sampling—should be employed to account for subgroup differences (e.g., age, location, socioeconomic status, comorbidities).
Integrating multi-omics data: Genetic data contain individual hereditary information, and specific gene polymorphisms (e.g., apolipoprotein E gene variants) are closely related to plaque formation, progression, and cardiovascular event occurrence. These genetic variations affect lipid metabolism and thus plaque stability [14]. Additionally, metabolomics data reflect changes in metabolic states and may reveal potential biomarkers associated with cardiovascular diseases. Combining genetic, metabolomic, plaque-specific, and clinical observational data in a predictive model could help uncover more key factors affecting cardiovascular events and substantially enhance model accuracy.
Optimizing model algorithms: Further exploration of deep learning techniques in cardiovascular risk prediction—such as Convolutional Neural Networks (CNNs) and Recurrent Neural Networks (RNNs)—is warranted. CNNs are effective in processing imaging data and can extract complex plaque features from coronary images [15], while RNNs are suitable for modeling time-series data, which is critical in longitudinal cardiovascular studies. Dynamic follow-up data—such as changes in blood pressure, lipid levels, and plaque size—can be modeled using RNNs to capture temporal dependencies and trends. Additionally, ensemble learning methods can integrate multiple machine learning algorithms, leveraging their strengths to improve model stability and generalizability [16]. Continuous algorithm optimization can significantly enhance the accuracy of quantitative risk assessment tools, providing clinicians with more precise decision-making support.
5. Conclusion
This study successfully developed a cardiovascular disease risk prediction model based on plaque characteristics by integrating multidimensional features of coronary atherosclerotic plaques. The model achieved an AUC of 0.85, demonstrating its ability to effectively identify high-risk patients. Plaque volume and the proportion of lipid plaques were found to be the most important variables in model prediction. This model not only provides a solid scientific basis for early clinical intervention but also holds significant application potential. Future research should further test the generalizability of the model, particularly in areas such as precision medication therapy and the timing of interventional procedures, as well as explore its potential application in personalized medicine.
References
[1]. Lin, L., Long, W., Shan, L. S., Yun, Z. Z., Mian, L., & Ge, W. T. (2019). Association between coronary atherosclerotic plaque composition and cardiovascular disease risk. Biomedical and Environmental Sciences, 32(2), 75–86.
[2]. Nissen, S. E., & Yock, P. (2001). Intravascular ultrasound: Novel pathophysiological insights and current clinical applications. Circulation, 103(4), 604–616.
[3]. Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A., & Schwartz, S. M. (2000). Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arteriosclerosis, Thrombosis, and Vascular Biology, 20(5), 1262–1275.
[4]. D’Agostino, R. B., Sr., Vasan, R. S., Pencina, M. J., Wolf, P. A., Cobain, M., Massaro, J. M., et al. (2008). General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation, 117(6), 743–753.
[5]. Liu, C., Lan, X., & Zhang, Y. (2014). Traditional imaging and molecular imaging for the detection and evaluation of vulnerable atherosclerotic plaques. Chinese Journal of Radiology and Nuclear Medicine, 38(2), 101–105, 134.
[6]. Liu, J. (2004). Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. Journal of the American College of Cardiology, 43(5), 901–906.
[7]. Stone, G. W., Maehara, A., Lansky, A. J., de Bruyne, B., Cristea, E., Mintz, G. S. (2011). A prospective natural-history study of coronary atherosclerosis. New England Journal of Medicine, 364(3), 226–235.
[8]. Haddaway, N. R., Woodcock, P., Macura, B., & Collins, A. (2015). Making literature reviews more reliable through application of lessons from systematic reviews. Conservation Biology, 29(6), 1596–1605.
[9]. Ross, R. (1999). Atherosclerosis—An inflammatory disease. New England Journal of Medicine, 340(2), 115–126.
[10]. Yang, H., Liu, C., Liu, S., Shao, Q., Yao, Y., & Fu, Z. (2025). Study on the correlation between residual cholesterol and vulnerable plaques that progress to major adverse cardiovascular events in non-culprit lesions. Chinese General Practice, 28(3), 299–304.
[11]. Yang, H., & Xiong, J. (2017). Advances in the study of glucose and lipid metabolism in patients with hyperandrogenism. Chinese Modern Doctors, 10, 19-21.
[12]. Wu, W., Xia, Y., Liao, S., Li, R., & Zhao, J. (2013). Analysis of risk factors for elderly patients with abnormal glucose metabolism complicated by cardiovascular and cerebrovascular diseases. Chinese and Foreign Medical Research, 17, 16-21.
[13]. Damen, J. A., Hooft, L., Schuit, E., Debray, T. P., Collins, G. S., & Tzoulaki, I. (2016). Prediction models for cardiovascular disease risk in the general population: A systematic review. BMJ, 353, i2416.
[14]. Ren, X., Li, Z., & Wang, W. (2014). Research progress on the correlation between apolipoprotein E gene polymorphism and carotid atherosclerosis. Chinese Medical Herald, 21, 102-106.
[15]. Meng, Y., Du, Z., Zhao, C., Dong, M., & Pienta, D. (2023). Automatic extraction of coronary arteries using deep learning in invasive coronary angiograms. Technology and Health Care: Official Journal of the European Society for Engineering and Medicine, 19, 36-39.
[16]. Okatani, T., Liu, X., & Suganuma, M. (2023). Improving generalization ability of deep neural networks for visual recognition tasks. Computational Color Imaging. CCIW 2019. Lecture Notes in Computer Science, vol 11418. Springer, Cham. https://doi.org/10.1007/978-3-030-13940-7_1
Cite this article
Wang,G. (2025). Quantitative evaluation model for cardiovascular disease incidence based on plaque characteristics in patients with coronary atherosclerosis. Journal of Clinical Technology and Theory,3(2),1-9.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Clinical Technology and Theory
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Lin, L., Long, W., Shan, L. S., Yun, Z. Z., Mian, L., & Ge, W. T. (2019). Association between coronary atherosclerotic plaque composition and cardiovascular disease risk. Biomedical and Environmental Sciences, 32(2), 75–86.
[2]. Nissen, S. E., & Yock, P. (2001). Intravascular ultrasound: Novel pathophysiological insights and current clinical applications. Circulation, 103(4), 604–616.
[3]. Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A., & Schwartz, S. M. (2000). Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arteriosclerosis, Thrombosis, and Vascular Biology, 20(5), 1262–1275.
[4]. D’Agostino, R. B., Sr., Vasan, R. S., Pencina, M. J., Wolf, P. A., Cobain, M., Massaro, J. M., et al. (2008). General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation, 117(6), 743–753.
[5]. Liu, C., Lan, X., & Zhang, Y. (2014). Traditional imaging and molecular imaging for the detection and evaluation of vulnerable atherosclerotic plaques. Chinese Journal of Radiology and Nuclear Medicine, 38(2), 101–105, 134.
[6]. Liu, J. (2004). Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. Journal of the American College of Cardiology, 43(5), 901–906.
[7]. Stone, G. W., Maehara, A., Lansky, A. J., de Bruyne, B., Cristea, E., Mintz, G. S. (2011). A prospective natural-history study of coronary atherosclerosis. New England Journal of Medicine, 364(3), 226–235.
[8]. Haddaway, N. R., Woodcock, P., Macura, B., & Collins, A. (2015). Making literature reviews more reliable through application of lessons from systematic reviews. Conservation Biology, 29(6), 1596–1605.
[9]. Ross, R. (1999). Atherosclerosis—An inflammatory disease. New England Journal of Medicine, 340(2), 115–126.
[10]. Yang, H., Liu, C., Liu, S., Shao, Q., Yao, Y., & Fu, Z. (2025). Study on the correlation between residual cholesterol and vulnerable plaques that progress to major adverse cardiovascular events in non-culprit lesions. Chinese General Practice, 28(3), 299–304.
[11]. Yang, H., & Xiong, J. (2017). Advances in the study of glucose and lipid metabolism in patients with hyperandrogenism. Chinese Modern Doctors, 10, 19-21.
[12]. Wu, W., Xia, Y., Liao, S., Li, R., & Zhao, J. (2013). Analysis of risk factors for elderly patients with abnormal glucose metabolism complicated by cardiovascular and cerebrovascular diseases. Chinese and Foreign Medical Research, 17, 16-21.
[13]. Damen, J. A., Hooft, L., Schuit, E., Debray, T. P., Collins, G. S., & Tzoulaki, I. (2016). Prediction models for cardiovascular disease risk in the general population: A systematic review. BMJ, 353, i2416.
[14]. Ren, X., Li, Z., & Wang, W. (2014). Research progress on the correlation between apolipoprotein E gene polymorphism and carotid atherosclerosis. Chinese Medical Herald, 21, 102-106.
[15]. Meng, Y., Du, Z., Zhao, C., Dong, M., & Pienta, D. (2023). Automatic extraction of coronary arteries using deep learning in invasive coronary angiograms. Technology and Health Care: Official Journal of the European Society for Engineering and Medicine, 19, 36-39.
[16]. Okatani, T., Liu, X., & Suganuma, M. (2023). Improving generalization ability of deep neural networks for visual recognition tasks. Computational Color Imaging. CCIW 2019. Lecture Notes in Computer Science, vol 11418. Springer, Cham. https://doi.org/10.1007/978-3-030-13940-7_1









