1. Introduction
Esophageal Cancer (EC) is the ninth most common cancer globally and the sixth leading cause of cancer-related mortality [1]. It is a severe malignancy in terms of both mortality and prognosis, with approximately 500,000 new cases and 400,000 deaths reported annually worldwide. The five-year survival rate for EC is less than 20% [2]. As a growing public health concern, its incidence is projected to increase over the next decade. Squamous cell carcinoma is the most common histological type globally, with particularly high incidence in developing countries. Conversely, in developed countries, the prevalence of Gastroesophageal Reflux Disease (GERD) and obesity has led to a sharp increase in esophageal adenocarcinoma over the past 40 years [3]. In Asia, squamous cell carcinoma remains dominant, whereas adenocarcinoma is becoming more common in Western countries [4]. Known risk factors include smoking, alcohol consumption, nutritional deficiencies (such as vitamins and minerals), chronic esophagitis, obesity, and GERD. Genetic, environmental, and lifestyle factors also contribute to EC risk [5]. Primary prevention strategies, such as dietary and lifestyle modifications, can help reduce the incidence of EC [6].
Tea is widely consumed around the world. Based on the degree of fermentation, tea is typically categorized into six types: white, green, yellow, oolong, black, and dark tea. It contains various phytochemicals, including polyphenols, pigments, polysaccharides, alkaloids, free amino acids, and saponins. Numerous studies have shown that tea has multiple health benefits, including antioxidant, anti-inflammatory, immunomodulatory, anticancer, cardioprotective, antidiabetic, anti-obesity, and hepatoprotective effects [7]. Existing research on the causal link between tea consumption and esophageal cancer mainly consists of case-control studies. Some findings suggest that tea may have inhibitory effects on the development of certain cancers, particularly esophageal cancer [8]. However, there is insufficient evidence to support a protective effect of tea against lung, esophageal, or gastric cancers [9]. Although Randomized Controlled Trials (RCTs) are ideal for resolving such questions, they are resource-intensive. In recent years, Mendelian Randomization (MR) has become a widely adopted method to estimate causal effects between modifiable exposures and disease traits. MR leverages the principle of Mendel’s random assortment and independent segregation of alleles, reducing confounding and reverse causality that often affect observational studies. It is considered a viable alternative to RCTs. Given the lack of previous MR studies in this area, the present study aims to rigorously evaluate the causal relationship between tea consumption and esophageal cancer through Mendelian Randomization analysis.
2. Materials and methods
2.1. Study design
Mendelian Randomization (MR) is an analytical method that uses genetic variants as instrumental variables (IVs) to assess causality. It relies on three core assumptions: (1) The genetic IVs are strongly associated with the exposure. (2) The genetic IVs are not associated with any confounding factors. (3) The genetic IVs influence the outcome solely through the exposure, and not through alternative pathways (see Figure 1) [10]. This study adopted a two-sample, bidirectional MR design using two large GWAS datasets. All informed consent and ethical approvals were obtained from the original studies.
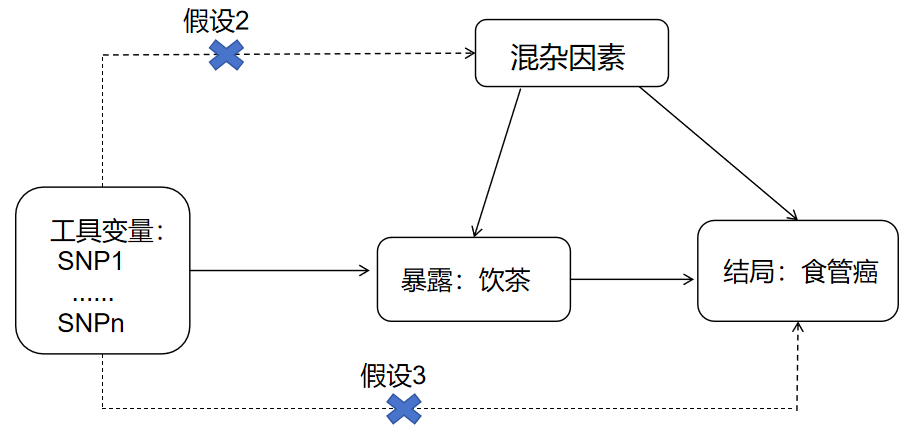
Figure 1. Schematic diagram of Mendelian randomization study design
2.2. Data sources
The GWAS summary statistics for tea consumption were obtained from the UK Biobank (Phenotype Code: 1488_raw), comprising 447,485 individuals of European ancestry. Tea intake data were self-reported via questionnaire, based on the question: “How many cups of tea (including black and green tea) do you drink daily?” The GWAS adjusted for sex, genotyping array, and the top ten principal components. The average tea intake was 3.51 ± 2.85 cups/day. Detailed data are available at https://gwas.mrcieu.ac.uk/ (GWAS ID: ukb-b-6066).
GWAS data for esophageal cancer were also derived from UK Biobank, comprising 998 EC cases and 475,308 controls, all of European ancestry. As the datasets are publicly available, raw data lists are not included.
2.3. Instrumental variable selection and harmonization
From the UK Biobank GWAS (ID: ukb-b-6066), 41 SNPs significantly associated with tea consumption at the genome-wide level (P < 5×10⁻⁸) and meeting linkage disequilibrium criteria (r² < 0.001 within a 10,000 kb window) were initially selected. These SNPs were then extracted from the esophageal cancer GWAS dataset for subsequent analyses. After aligning SNPs by chromosome and position, eight palindromic SNPs with intermediate allele frequencies (rs11164870, rs132904, rs1453548, rs2273447, rs2783129, rs56348300, rs713598, rs9302428) were excluded due to ambiguity. Ultimately, 33 SNPs were retained. To assess instrument strength, the F-statistic was calculated for each SNP, with all exceeding F > 10, indicating no weak instrument bias. Details are presented in Table 1.
Table 1. Information on instrumental variables related to tea consumption
SNP | Position | EAF | EA | OA | BETA | Sx̅ | P | N | R2 | F |
rs34619 | 5:60465365 | 0.431 | A | G | 0.012 | 0.002 | 4.30E-08 | 447485 | 6.73E-05 | 30.105 |
rs17576658 | 13:100272019 | 0.247 | A | G | -0.013 | 0.002 | 4.10E-08 | 447485 | 6.76E-05 | 30.261 |
rs2279844 | 17:40819809 | 0.379 | A | G | -0.012 | 0.002 | 4.00E-08 | 447485 | 6.77E-05 | 30.283 |
rs2645929 | 13:56444529 | 0.813 | G | A | -0.015 | 0.003 | 3.50E-08 | 447485 | 6.83E-05 | 30.543 |
rs6829 | 13:111531264 | 0.596 | T | C | -0.012 | 0.002 | 3.70E-08 | 447485 | 6.84E-05 | 30.598 |
rs7757102 | 6:137222671 | 0.555 | G | A | -0.012 | 0.002 | 3.10E-08 | 447485 | 6.88E-05 | 30.793 |
rs17245213 | 11:1679769 | 0.208 | A | G | -0.015 | 0.003 | 2.00E-08 | 447485 | 7.07E-05 | 31.642 |
rs10764990 | 10:129152608 | 0.607 | A | G | -0.012 | 0.002 | 1.90E-08 | 447485 | 7.09E-05 | 31.725 |
rs57462170 | 3:50239803 | 0.109 | A | G | 0.019 | 0.003 | 1.90E-08 | 447485 | 7.11E-05 | 31.821 |
rs57631352 | 19:4338173 | 0.297 | G | A | -0.013 | 0.002 | 1.70E-08 | 447485 | 7.17E-05 | 32.078 |
rs2351187 | 10:86850616 | 0.319 | A | G | 0.013 | 0.002 | 1.60E-08 | 447485 | 7.23E-05 | 32.364 |
rs9648476 | 7:39293033 | 0.623 | A | G | 0.013 | 0.002 | 1.10E-08 | 447485 | 7.34E-05 | 32.855 |
rs13282783 | 8:22088975 | 0.286 | T | C | -0.014 | 0.002 | 7.90E-09 | 447485 | 7.53E-05 | 33.717 |
rs1156588 | 2:58515375 | 0.210 | G | A | -0.015 | 0.003 | 2.90E-09 | 447485 | 7.93E-05 | 35.471 |
rs2117137 | 3:89525505 | 0.405 | G | A | 0.013 | 0.002 | 1.70E-09 | 447485 | 8.14E-05 | 36.425 |
rs10752269 | 10:12692902 | 0.506 | A | G | -0.013 | 0.002 | 1.30E-09 | 447485 | 8.28E-05 | 37.073 |
rs141071726 | 7:17558580 | 0.027 | A | G | 0.041 | 0.007 | 2.20E-09 | 447485 | 8.63E-05 | 38.608 |
rs149805207 | 6:137095269 | 0.009 | G | A | -0.072 | 0.013 | 1.10E-08 | 447485 | 8.76E-05 | 39.205 |
rs12591786 | 15:60902512 | 0.159 | T | C | -0.018 | 0.003 | 3.70E-10 | 447485 | 9.08E-05 | 40.656 |
rs11587444 | 1:150722844 | 0.393 | G | A | 0.014 | 0.002 | 1.00E-10 | 447485 | 9.40E-05 | 42.063 |
rs9937354 | 16:53799847 | 0.424 | A | G | -0.014 | 0.002 | 4.90E-11 | 447485 | 9.70E-05 | 43.413 |
rs4808193 | 19:19410622 | 0.335 | C | T | 0.015 | 0.002 | 1.70E-11 | 447485 | 0.00010 | 45.576 |
rs10741694 | 11:16286183 | 0.628 | C | T | 0.015 | 0.002 | 7.90E-12 | 447485 | 0.00011 | 47.075 |
rs4817505 | 21:34343828 | 0.390 | C | T | 0.015 | 0.002 | 4.20E-12 | 447485 | 0.00011 | 48.345 |
rs72797284 | 5:152031650 | 0.271 | G | A | -0.017 | 0.002 | 7.00E-13 | 447485 | 0.00012 | 51.771 |
rs56188862 | 1:174189269 | 0.387 | C | T | -0.016 | 0.002 | 4.30E-13 | 447485 | 0.00012 | 52.742 |
rs977474 | 12:11284772 | 0.834 | T | C | 0.022 | 0.003 | 2.40E-14 | 447485 | 0.00013 | 58.862 |
rs1481012 | 4:89039082 | 0.112 | G | A | -0.026 | 0.003 | 5.30E-15 | 447485 | 0.00014 | 61.411 |
rs2478875 | 6:51283110 | 0.209 | G | A | 0.022 | 0.003 | 5.10E-17 | 447485 | 0.00016 | 70.874 |
rs17685 | 7:75616105 | 0.278 | A | G | 0.023 | 0.002 | 1.60E-22 | 447485 | 0.00021 | 95.485 |
rs9624470 | 22:24820268 | 0.580 | A | G | 0.025 | 0.002 | 1.30E-31 | 447485 | 0.00031 | 138.563 |
rs4410790 | 7:17284577 | 0.631 | C | T | 0.041 | 0.002 | 3.40E-76 | 447485 | 0.00077 | 342.831 |
rs2472297 | 15:75027880 | 0.262 | T | C | 0.053 | 0.002 | 2.30E-109 | 447485 | 0.00110 | 493.046 |
Note: SNP ID denotes single nucleotide polymorphism; EA = effect allele; OA = other (non-effect) allele; Sx̄ = standard error.
2.4. Statistical analysis
2.4.1. Assessing the causal effect of tea consumption on esophageal cancer
After harmonizing the effect alleles for tea consumption and esophageal cancer, several MR methods were applied to estimate causality, including: Inverse-Variance Weighted (IVW); Weighted median; MR-Egger; Weighted mode. Each method relies on different assumptions regarding the validity of instrumental variables. IVW assumes that all IVs are valid and combines individual SNP-specific MR estimates to produce a pooled causal effect, making it the most commonly used approach [11]. The weighted median method yields consistent estimates if at least 50% of the weight comes from valid instruments [12]. MR-Egger allows for directional pleiotropy and adjusts for its bias [13]. The weighted mode approach groups SNPs based on similarity and weights each by the inverse of its variance, enhancing robustness [14].
2.4.2. Sensitivity analyses
To examine heterogeneity and pleiotropy, multiple sensitivity analyses were performed: IVW and MR-Egger regression were used along with Cochran's Q statistic to test for heterogeneity. MR-PRESSO was employed to detect horizontal pleiotropy and potential outliers. To assess assumption violations (assumptions 2 and 3), the MR-Egger intercept was examined. If the intercept is near zero (<0.1) and P > 0.05, the results are considered reliable, suggesting no horizontal pleiotropy. The CAUSE method was applied to account for both correlated and uncorrelated pleiotropy. Funnel plots were used for visual assessment of symmetry, indicating absence or presence of pleiotropy. Leave-one-out analyses evaluated the influence of individual SNPs on overall estimates. Power calculations were conducted using the mRn tool (https://shiny.cnsgenomics.com/mRnd/).
2.4.3. Reverse Mendelian randomization analysis
The reverse MR analysis treated esophageal cancer as the exposure and tea consumption as the outcome, using the same procedures described above. All analyses were performed using R version 4.4.1, with a significance threshold of α = 0.05.
3. Results
3.1. Causal effect of tea consumption on esophageal cancer
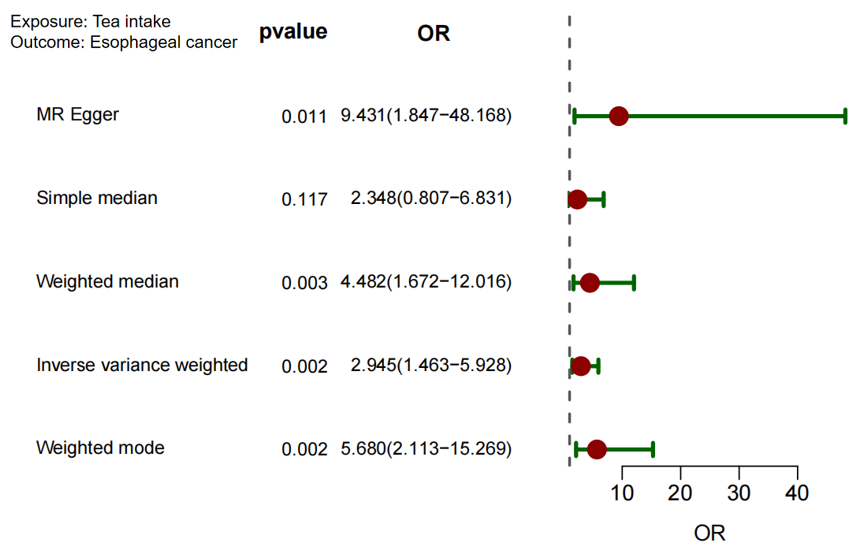
Using 33 SNPs associated with tea consumption, MR analyses were conducted to evaluate its causal effect on esophageal cancer. The results showed statistically significant associations across all methods: Inverse-Variance Weighted (IVW): OR = 2.945, 95% CI: 1.551–5.592. Weighted Mode: OR = 5.590, 95% CI: 2.056–15.201. Weighted Median: OR = 4.446, 95% CI: 1.238–8.621. These findings suggest that increased tea consumption is causally associated with a higher risk of esophageal cancer (see Figure 2 and Table 2). However, heterogeneity was detected through IVW and Cochran's Q tests.
3.2. Sensitivity analyses
In the MR analysis assessing the causal relationship between tea intake and esophageal cancer, the MR-Egger intercept had a P-value greater than 0.05 (P = 0.273), indicating no evidence of horizontal pleiotropy. Additionally, the CAUSE results showed: Variance explained in exposure: 0.0056; Variance explained in outcome: 0.00012; Directionality p-value: 9.53e–207. These results support a positive causal relationship between tea consumption and increased esophageal cancer risk, even after correcting for correlated and uncorrelated pleiotropy. The scatter plot (Figure 2) illustrates the effect size of each SNP on tea consumption and esophageal cancer risk. The funnel plot (Figure 3) shows symmetrical variation in effect sizes around the point estimates, further indicating no substantial pleiotropy. Finally, a leave-one-out sensitivity analysis (Figure 4) confirmed that no single SNP disproportionately influenced the overall association. Taken together, these findings strongly suggest that increased tea consumption is associated with an elevated risk of developing esophageal cancer.
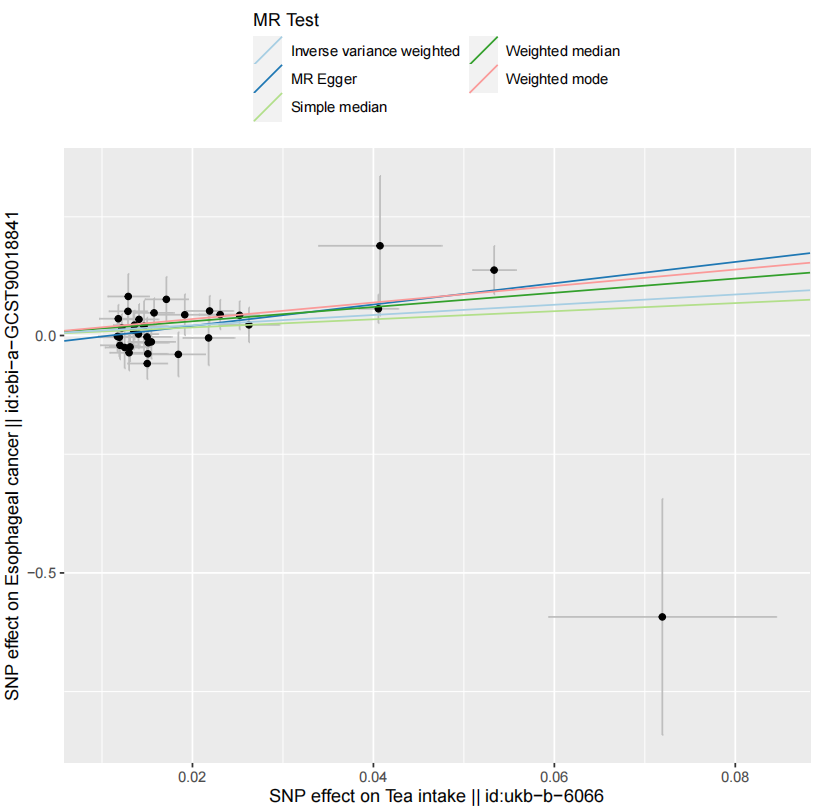
Figure 2. Scatter plot of Mendelian randomization analysis
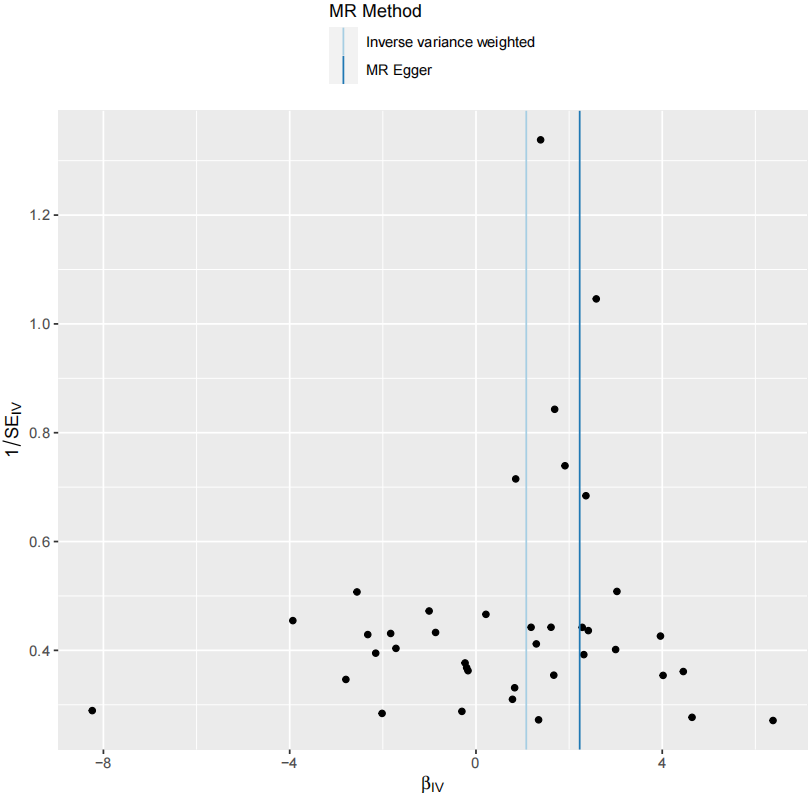
Figure 3. Funnel plot of Mendelian randomization analysis
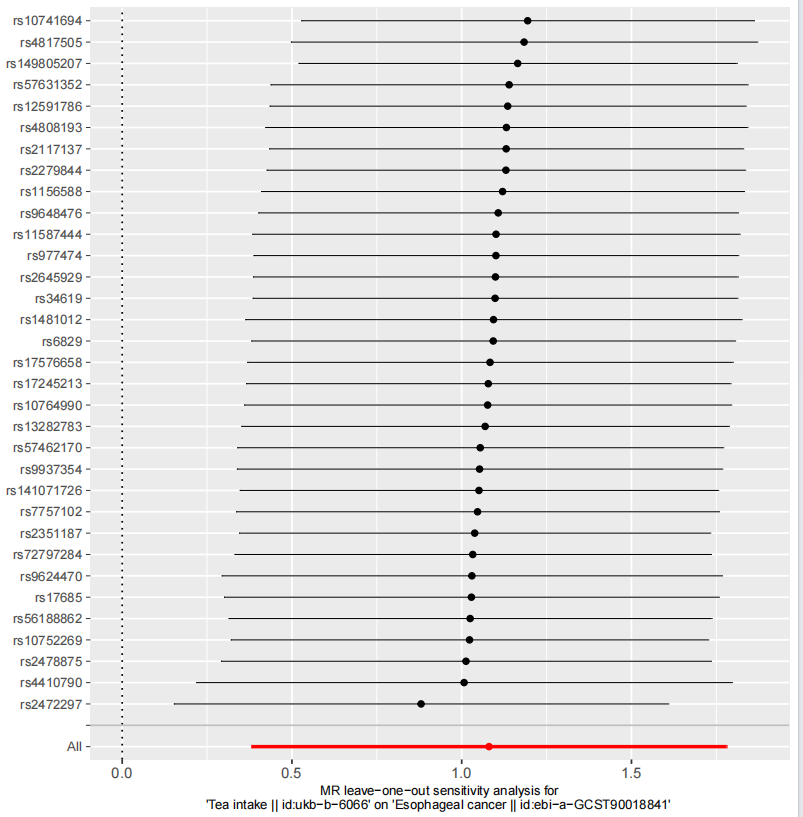
Figure 4. Leave-one-out sensitivity analysis
3.3. Reverse Mendelian randomization analysis
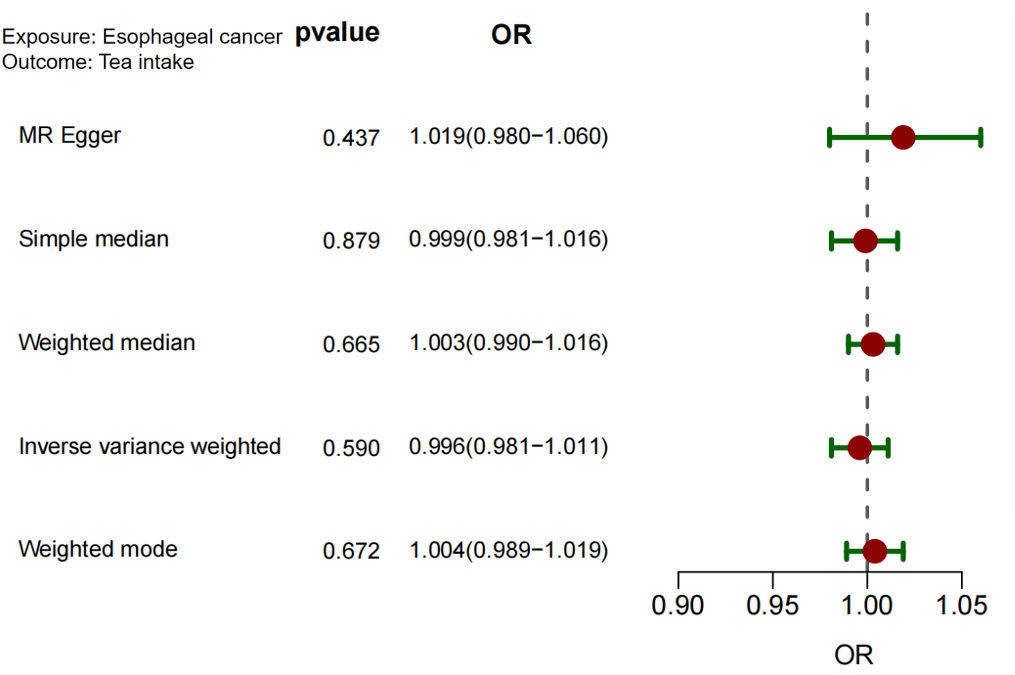
To test for reverse causality, esophageal cancer was treated as the exposure and tea consumption as the outcome. Four SNPs were used in this analysis. The IVW method yielded a non-significant result: P = 0.590. This indicates no evidence of a reverse causal relationship from esophageal cancer to tea consumption (see Figure 5 and Table 2).
|
(a) |
|
(b) |
Figure 5. Forest map
Table 2. Results of Mendelian randomization analysis
Exposure/outcome | IVW | Weighted median | MR Egger | Weighted mode | ||||
OR(95%CI) P | OR(95%CI) P | OR(95%CI) P | OR(95%CI) P | |||||
Tea intake/esophageal cancer | 2.945(1.551~5.592) | 0.001 | 4.446(1.238~8.621) | 0.004 | 9.272(1.992~43.163) | 0.005 | 5.590(2.056~15.201) | 0.001 |
Esophageal cancer/tea intake | 0.996(0.981~1.011) | 0.590 | 1.003(0.990~1.016) | 0.652 | 1.019(0.980~1.060) | 0.335 | 1.004(0.989~1.019) | 0.634 |
4. Discussion
This study employed a two-sample, bidirectional Mendelian Randomization (MR) approach to evaluate the causal relationship between tea consumption and the risk of esophageal cancer. The results indicate that increased tea intake causally raises the risk of developing esophageal cancer, while no evidence supports the existence of reverse causality. Numerous observational studies have examined the relationship between tea consumption and esophageal cancer risk, but their findings have been inconsistent. The relationship between tea and cancer risk—particularly esophageal cancer—is complex and multifaceted, as evidenced by the contradictory findings across studies. This paper underscores the value of MR as a method for exploring potential causal links, especially when conventional observational studies are prone to confounding. Although tea has been widely recognized for its antioxidant and anti-inflammatory properties, which are often believed to confer anticancer benefits, the findings of this MR study challenge that assumption. Instead, the results suggest that excessive tea consumption may elevate cancer risk, possibly due to specific tea constituents or the conditions under which tea is consumed. The literature presents a mixed picture of tea’s health effects. For instance, a Mendelian Randomization study by Deng et al. found no causal relationship between tea consumption and breast cancer, implying that tea may not significantly affect breast cancer risk [15]. Similarly, research into tea’s effects on bone health found no causal association with major skeletal disorders, indicating that tea’s health impacts may be context-dependent or limited [16]. Conversely, Sun et al. emphasized potential adverse effects of tea, linking it to an increased risk of gastroesophageal reflux disease (GERD), a condition known to be a risk factor for esophageal cancer. This indirect association supports the findings of the present study, which suggest that, under certain conditions, high tea consumption may be harmful [17]. On the other hand, Kim and Je offered a broader perspective by reporting that moderate tea consumption was associated with reduced all-cause, cardiovascular, and cancer mortality, indicating a protective effect at lower intake levels [18]. Taken together, the evidence suggests that while moderate tea consumption may have health benefits, excessive intake could pose risks—particularly in relation to esophageal disorders. The inconsistent findings across studies highlight the need for further research that takes into account tea type, preparation methods, and individual genetic variations, to better clarify the role of tea in cancer risk and other health outcomes.
References
[1]. Sheikh, M., Roshandel, G., McCormack, V., & Malekzadeh, R. (2023). Current status and future prospects for esophageal cancer. Cancers, 15(3), 765.
[2]. Li, J., Xu, J., Zheng, Y., Gao, Y., He, S., Li, H., ... & He, J. (2021). Esophageal cancer: Epidemiology, risk factors and screening. Chinese Journal of Cancer Research, 33(5), 535.
[3]. Napier, K. J., Scheerer, M., & Misra, S. (2014). Esophageal cancer: A review of epidemiology, pathogenesis, staging workup and treatment modalities. World journal of gastrointestinal oncology, 6(5), 112.
[4]. Testa, U., Castelli, G., & Pelosi, E. (2023). The molecular characterization of genetic abnormalities in esophageal squamous cell carcinoma may foster the development of targeted therapies. Current Oncology, 30(1), 610-640.
[5]. Zhang, W., Bailey-Wilson, J. E., Li, W., Wang, X., Zhang, C., Mao, X., ... & Wu, M. (2000). Segregation analysis of esophageal cancer in a moderately high–incidence area of northern China. The American Journal of Human Genetics, 67(1), 110-119.
[6]. Sohda, M., & Kuwano, H. (2017). Current status and future prospects for esophageal cancer treatment. Annals of Thoracic and Cardiovascular Surgery, 23(1), 1-11.
[7]. Tang, G. Y., Meng, X., Gan, R. Y., Zhao, C. N., Liu, Q., Feng, Y. B., ... & Li, H. B. (2019). Health functions and related molecular mechanisms of tea components: an update review. International journal of molecular sciences, 20(24), 6196.
[8]. Gao, Y. T., McLaughlin, J. K., Blot, W. J., Ji, B. T., Dai, Q., & Fraumeni, J. F. (1994). Reduced risk of esophageal cancer associated with green tea consumption. JNCI: Journal of the National Cancer Institute, 86(11), 855-858.
[9]. Trisha, A. T., Shakil, M. H., Talukdar, S., Rovina, K., Huda, N., & Zzaman, W. (2022). Tea polyphenols and their preventive measures against cancer: Current trends and directions. Foods, 11(21), 3349.
[10]. Sekula, P., Fabiola Del Greco, M., Pattaro, C., & Köttgen, A. (2016). Mendelian randomization as an approach to assess causality using observational data. Journal of the American Society of Nephrology, 27(11), 3253-3265.
[11]. Taschler, B., Smith, S. M., & Nichols, T. E. (2022). Causal inference on neuroimaging data with Mendelian randomisation. NeuroImage, 258, 119385.
[12]. Bowden, J., & Holmes, M. V. (2019). Meta‐analysis and Mendelian randomization: a review. Research synthesis methods, 10(4), 486-496.
[13]. Bucur, I. G., Claassen, T., & Heskes, T. (2020). Inferring the direction of a causal link and estimating its effect via a Bayesian Mendelian randomization approach. Statistical Methods in Medical Research, 29(4), 1081-1111.
[14]. Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., ... & Haycock, P. C. (2018). The MR-Base platform supports systematic causal inference across the human phenome. eLife, 7, e34408.
[15]. Deng, Y., Ge, W., Xu, H., & Zhang, J. (2022). A Mendelian randomization study of the effect of tea intake on breast cancer. Frontiers in Nutrition, 9, 956969.
[16]. Chen, S., Chen, T., Chen, Y., Huang, D., Pan, Y., & Chen, S. (2022). Causal association between tea consumption and bone health: a mendelian randomization study. Frontiers in Nutrition, 9, 872451.
[17]. Sun, Q., Gao, N., Song, J., Jia, J., Dong, A., & Xia, W. (2024). The association between tea consumption and non-malignant digestive system diseases: a Mendelian randomized study. Clinical Nutrition ESPEN, 60, 327-332.
[18]. Kim, Y., & Je, Y. (2024). Tea consumption and risk of all-cause, cardiovascular disease, and cancer mortality: a meta-analysis of thirty-eight prospective cohort data sets. Epidemiology and Health, 46, e2024056.
Cite this article
Deng,S.;Zeng,H.;Zhou,P.;Guo,Z. (2025). A Mendelian randomization study reveals a causal relationship between tea consumption and esophageal cancer. Journal of Clinical Technology and Theory,3(2),23-29.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Clinical Technology and Theory
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Sheikh, M., Roshandel, G., McCormack, V., & Malekzadeh, R. (2023). Current status and future prospects for esophageal cancer. Cancers, 15(3), 765.
[2]. Li, J., Xu, J., Zheng, Y., Gao, Y., He, S., Li, H., ... & He, J. (2021). Esophageal cancer: Epidemiology, risk factors and screening. Chinese Journal of Cancer Research, 33(5), 535.
[3]. Napier, K. J., Scheerer, M., & Misra, S. (2014). Esophageal cancer: A review of epidemiology, pathogenesis, staging workup and treatment modalities. World journal of gastrointestinal oncology, 6(5), 112.
[4]. Testa, U., Castelli, G., & Pelosi, E. (2023). The molecular characterization of genetic abnormalities in esophageal squamous cell carcinoma may foster the development of targeted therapies. Current Oncology, 30(1), 610-640.
[5]. Zhang, W., Bailey-Wilson, J. E., Li, W., Wang, X., Zhang, C., Mao, X., ... & Wu, M. (2000). Segregation analysis of esophageal cancer in a moderately high–incidence area of northern China. The American Journal of Human Genetics, 67(1), 110-119.
[6]. Sohda, M., & Kuwano, H. (2017). Current status and future prospects for esophageal cancer treatment. Annals of Thoracic and Cardiovascular Surgery, 23(1), 1-11.
[7]. Tang, G. Y., Meng, X., Gan, R. Y., Zhao, C. N., Liu, Q., Feng, Y. B., ... & Li, H. B. (2019). Health functions and related molecular mechanisms of tea components: an update review. International journal of molecular sciences, 20(24), 6196.
[8]. Gao, Y. T., McLaughlin, J. K., Blot, W. J., Ji, B. T., Dai, Q., & Fraumeni, J. F. (1994). Reduced risk of esophageal cancer associated with green tea consumption. JNCI: Journal of the National Cancer Institute, 86(11), 855-858.
[9]. Trisha, A. T., Shakil, M. H., Talukdar, S., Rovina, K., Huda, N., & Zzaman, W. (2022). Tea polyphenols and their preventive measures against cancer: Current trends and directions. Foods, 11(21), 3349.
[10]. Sekula, P., Fabiola Del Greco, M., Pattaro, C., & Köttgen, A. (2016). Mendelian randomization as an approach to assess causality using observational data. Journal of the American Society of Nephrology, 27(11), 3253-3265.
[11]. Taschler, B., Smith, S. M., & Nichols, T. E. (2022). Causal inference on neuroimaging data with Mendelian randomisation. NeuroImage, 258, 119385.
[12]. Bowden, J., & Holmes, M. V. (2019). Meta‐analysis and Mendelian randomization: a review. Research synthesis methods, 10(4), 486-496.
[13]. Bucur, I. G., Claassen, T., & Heskes, T. (2020). Inferring the direction of a causal link and estimating its effect via a Bayesian Mendelian randomization approach. Statistical Methods in Medical Research, 29(4), 1081-1111.
[14]. Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., ... & Haycock, P. C. (2018). The MR-Base platform supports systematic causal inference across the human phenome. eLife, 7, e34408.
[15]. Deng, Y., Ge, W., Xu, H., & Zhang, J. (2022). A Mendelian randomization study of the effect of tea intake on breast cancer. Frontiers in Nutrition, 9, 956969.
[16]. Chen, S., Chen, T., Chen, Y., Huang, D., Pan, Y., & Chen, S. (2022). Causal association between tea consumption and bone health: a mendelian randomization study. Frontiers in Nutrition, 9, 872451.
[17]. Sun, Q., Gao, N., Song, J., Jia, J., Dong, A., & Xia, W. (2024). The association between tea consumption and non-malignant digestive system diseases: a Mendelian randomized study. Clinical Nutrition ESPEN, 60, 327-332.
[18]. Kim, Y., & Je, Y. (2024). Tea consumption and risk of all-cause, cardiovascular disease, and cancer mortality: a meta-analysis of thirty-eight prospective cohort data sets. Epidemiology and Health, 46, e2024056.