1. Introduction
Rural left-behind children are defined as individuals under the age of 17 whose household registration is in rural areas and whose parents both migrate away across townships or districts for six months or more, resulting in their living separately from both parents [1]. With ongoing socioeconomic development, shifts in the urban–rural economic structure, and increasing labor migration, the number of rural left-behind children has remained high over time. Early left-behind experiences may lead to neglect, thereby increasing the risk of developing psychological problems in children [2]. Psychological resilience is considered a core mechanism for resisting the onset of mental disorders and maintaining psychological adaptation [3]. In recent years, resilience has gradually become an important indicator for assessing individual mental health and capacity to cope with adversity. Due to prolonged lack of parental companionship and socio-emotional support during critical developmental stages, rural left-behind children generally exhibit lower levels of psychological resilience compared to their non-left-behind peers [4]. Resting-state functional magnetic resonance imaging (rs-fMRI), characterized by its absence of radiation exposure, high soft-tissue resolution, and high temporal resolution, is not only used for detecting intracranial diseases but is also widely employed in studies of brain function and network connectivity. A large body of rs-fMRI research has demonstrated [5-7] that the human brain typically exhibits small-world topological properties under healthy conditions [8]. This network topology combines integration and segregation, facilitating rapid and flexible functional transitions across different task states. At present, there is limited research, domestically and internationally, on the relationship between psychological resilience and small-world properties in populations with left-behind experiences. This study aims to explore the impact of childhood left-behind experiences on adult brain functional networks. Using rs-fMRI brain network topological analysis, we assess changes in small-world properties among adults with left-behind experiences and investigate the associations between these properties, psychological resilience, and sociological adaptation ability, in order to provide evidence for understanding the neural mechanisms through which social experiences influence brain function and mental health.
2. Materials and methods
2.1. Participants
Adults with childhood left-behind experiences were recruited via online advertisements and university outreach. The study sample was drawn from multiple county-level towns and peri-urban areas in Yunnan Province. Prior to the study, ethical approval was obtained from the Ethics Committee of the First Affiliated Hospital of Kunming Medical University (Approval No.: 2023L49). All volunteers provided written informed consent.
Inclusion criteria: (1) Left-behind experience of ≥2 years; (2) Age 20–40 years; (3) Residence in county-level or lower administrative units in Yunnan Province for ≥16 years; (4) Han ethnicity; (5) Right-handed; (6) No obvious intracranial organic lesions on MRI; (7) No history of neurological or psychiatric disorders; (8) No contraindications to MRI.
Exclusion criteria: (1) Insufficient duration of left-behind experience; (2) Excessive head motion during MRI (translation >2 mm or rotation >2°); (3) History of neurological or psychiatric disorders; (4) History of drug addiction.
A total of 149 volunteers were initially enrolled. Four were excluded due to poor image quality or incomplete data, resulting in 145 left-behind adult participants included in the final analysis.
2.2. Collection of clinical data and questionnaire measures
Basic demographic and clinical information was collected for all volunteers, including age, sex, and years of education. Detailed information on participants’ left-behind experiences and duration was also obtained. Psychological resilience was assessed using the Chinese version of the Connor-Davidson Resilience Scale (CD-RISC) as adapted by Xiao [9]. This scale comprises 25 items scored on a 5-point Likert scale ranging from 1 ("not true at all") to 5 ("true nearly all the time"), yielding a total score between 25 and 125. Higher scores indicate greater psychological resilience.
To facilitate effective comparison of resilience differences and stratified network analyses, the median-split method was employed to divide the sample into high- and low-resilience groups. Based on the median CD-RISC score of 62, participants with scores ≥62 were classified into the high-resilience group (n = 75), and those with scores <62 into the low-resilience group (n = 70).
2.3. Rs-fmri scanning and parameters
All MRI data were acquired by certified imaging technicians at the Department of Medical Imaging, First Affiliated Hospital of Kunming Medical University. Imaging was performed using a GE Silent MR 750w 3.0 T scanner equipped with the manufacturer’s 24-channel head-and-neck combined coil. Conventional structural brain imaging was first performed to rule out substantial intracranial pathology. Functional imaging employed a Blood Oxygen Level–Dependent (BOLD)–sensitive sequence using Echo-Planar Imaging (EPI) with the following parameters: Repetition Time (TR) = 2000 ms; Echo Time (TE) = 30 ms; flip angle = 90°; Field of View (FOV) = 224 mm × 224 mm; slice thickness = 3 mm; inter-slice gap = 1 mm; 36 slices; 160 time points. During scanning, participants were instructed to keep their eyes closed while remaining awake and to avoid deliberately focusing on any specific thoughts, in order to capture spontaneous brain activity in a natural resting state.
2.4. Image processing and brain network metric extraction
Preprocessing of resting-state fMRI data was performed using the Graph Theoretical Network Analysis Toolbox (GRETNA) [10] on the MATLAB 2021a platform. Steps included DICOM format conversion; removal of the first 10 time points; slice timing correction; head motion correction; spatial normalization; spatial smoothing; linear trend removal; nuisance covariate regression; and band-pass filtering.
For network construction, the Automated Anatomical Labeling (AAL) atlas was used to parcellate the whole brain into 90 cortical and subcortical regions, which served as the nodes of the network graph. The sparsity range was set from 0.05 to 0.40 with a step size of 0.01, resulting in 36 threshold points in total. At each sparsity level, GRETNA automatically thresholded the Pearson correlation matrix to generate an undirected binary network graph. The software calculated both global and nodal properties of the network. Global properties, reflecting overall network integration, local clustering, and information transfer efficiency, included normalized clustering coefficient (γ), normalized characteristic path length (λ), clustering coefficient (Cp), average path length (Lp), global efficiency (Eg), local efficiency (Eloc), and small-world coefficient (σ). The small-world property is defined as σ = γ/λ, with a network exhibiting small-world characteristics when γ > 1 and λ ≈ 1 [8]. Nodal properties mainly included Degree Centrality (DC) and Nodal Efficiency (NE) for each brain region across all sparsity levels. For each metric, the Area Under the Curve (AUC) [11] was calculated as a summary measure for each participant, reducing subjective bias from sparsity selection and enhancing stability in inter-individual comparisons.
2.5. Statistical analysis
Data were analyzed using SPSS version 27.0. Basic demographic characteristics (age, sex, years of education) were summarized and compared between groups. Continuous variables were expressed as mean ± standard deviation (x̄ ± s) and compared using independent-samples t-tests; categorical variables were presented as counts and compared using chi-square tests (χ²). Global and nodal brain network properties between the high- and low-resilience groups were compared using independent-samples t-tests, with P < 0.05 indicating statistical significance. False Discovery Rate (FDR) correction was applied with age, sex, and years of education as covariates; differences were considered statistically significant at P < 0.05 after correction. For brain regions showing significant group differences, the correlations between network properties and psychological resilience scores among left-behind participants were examined using Pearson or Spearman correlation analyses. Visualization of the results was performed using the BrainNet Viewer toolbox [12] on the MATLAB 2021a platform.
3. Results
3.1. Clinical data descriptive statistics
Age and years of education in both the high-resilience and low-resilience groups were normally distributed and are expressed as mean ± standard deviation. Independent-samples t-tests showed no statistically significant differences between groups (P > 0.05). Sex distribution was also compared using Pearson’s chi-square test, with no significant difference (P > 0.05) (Table 1). These results indicate that sex and age were evenly distributed between the groups, and that the groups were highly homogeneous in terms of educational background.
|
High-Resilience Group (n=75) |
Low-Resilience Group (n=70) |
Test Type |
p Value |
|
|
Age (years) |
24.72 ± 3.27 |
24.43 ± 3.39 |
t test |
0.38 |
|
Sex (male/female) |
33/ 42 |
30 / 40 |
χ2 test |
0.890 |
|
Years of education |
16.68 ± 1.80 |
16.94 ± 1.79 |
t test |
0.967 |
3.2. Comparison of brain network small-world properties
Both high- and low-resilience groups exhibited small-world network characteristics across all sparsity levels (γ > 1, λ ≈ 1, σ > 1). There were no statistically significant differences between groups in the global properties of Eg, Cp, Lp, or λ (P > 0.05, FDR-corrected for multiple comparisons). However, σ, γ, and Eloc showed statistically significant differences between groups (P < 0.05, FDR-corrected). See Table 2.
|
Metric |
High-Resilience Group |
Low-Resilience Group |
t Value |
p Value |
|
Eloc |
0.732 ± 0.016 |
0.721 ± 0.026 |
2.985 |
0.003 |
|
Eg |
0.525 ± 0.019 |
0.517 ± 0.028 |
2.078 |
0.040 |
|
Lp |
2.09 ± 0.131 |
2.15 ± 0.211 |
-1.899 |
0.060 |
|
Cp |
0.570 ± 0.023 |
0.566 ± 0.023 |
0.936 |
0.351 |
|
σ |
1.807 ± 0.268 |
1.623 ± 0.264 |
4.478 |
<0.001 |
|
λ |
1.113 ± 0.035 |
1.121 ± 0.047 |
-1.209 |
0.229 |
|
γ |
2.058 ± 0.311 |
1.846 ±- 0.273 |
4.644 |
<0.001 |
3.3. Node property analysis
Analysis of local brain network properties at the nodal level revealed that the high-resilience group showed significantly higher degree centrality (DC) and nodal efficiency (NE) in the right medial orbitofrontal superior frontal gyrus, left insula, and left anterior cingulate gyrus compared to the low-resilience group. Conversely, DC and NE in the left middle temporal gyrus were lower in the high-resilience group than in the low-resilience group. See Table 3 and Figure 1.
|
Brain Region |
DC |
NE |
|
|
High-Resilience Group VS Low-Resilience Group |
ORBsupmed.R |
t = 2.100 p = 0.038 |
t = 2.353 p = 0.020 |
|
INS.L |
t = 1.195 p = 0.029 |
t = 2.336 p = 0.021 |
|
|
ACG.L |
t = 2.870 p = 0.005 |
t = 3.288 p = 0.001 |
|
|
MTG.L |
t = -2.736 p = 0.007 |
t = -2.410 p = 0.017 |
Note: ORBsupmed.R = right medial orbitofrontal superior frontal gyrus; INS.L = left insula; ACG.L = left anterior cingulate gyrus; MTG.L = left middle temporal gyrus.
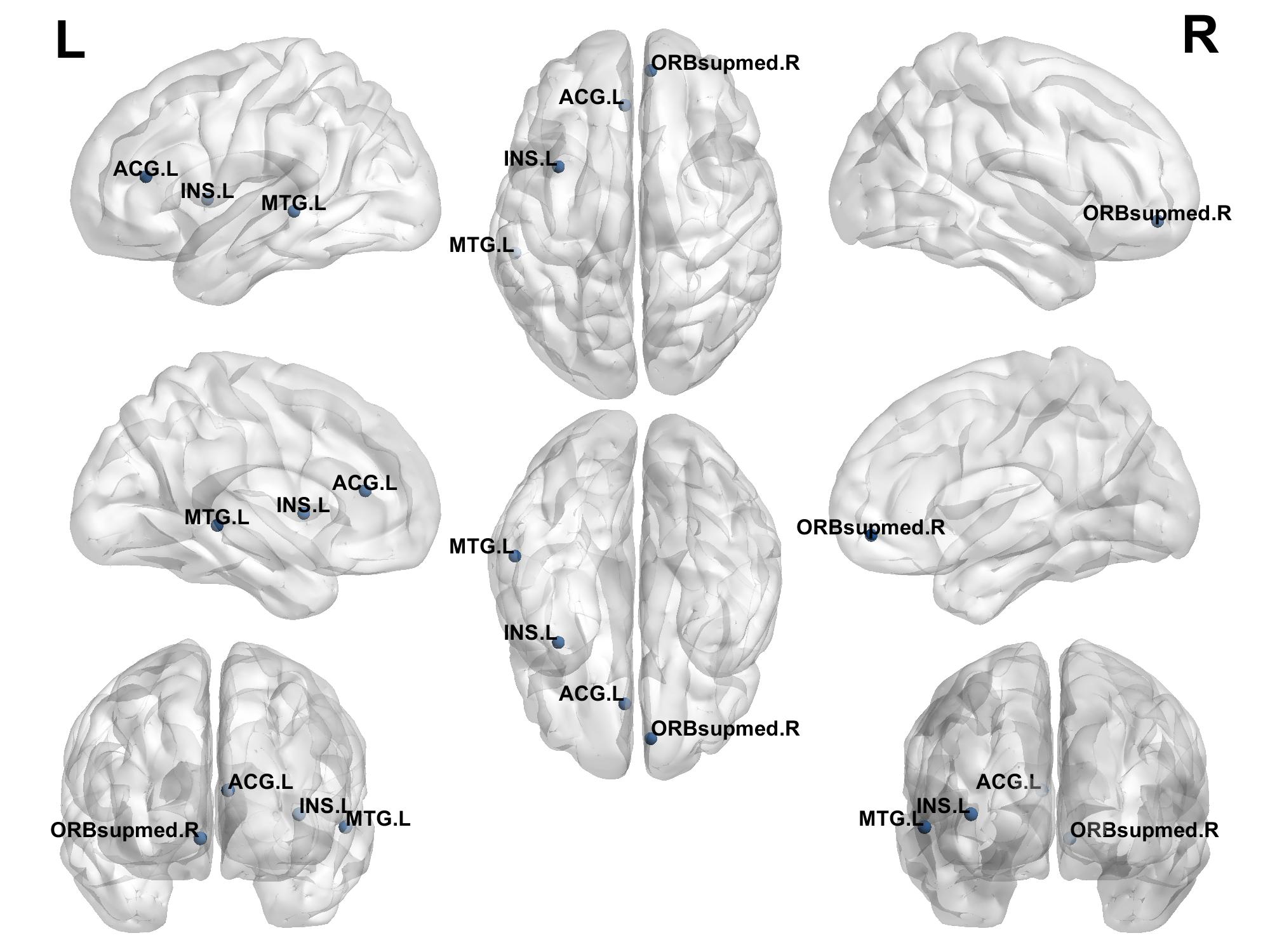
3.4. Correlation analysis
The small-world properties σ, γ, Eg, and Eloc were positively correlated with psychological resilience scores (P < 0.05). Among brain regions, DC and NE values in the right medial orbitofrontal superior frontal gyrus, left insula, and left anterior cingulate gyrus were positively correlated with psychological resilience scores (P < 0.05), while DC and NE values in the left middle temporal gyrus were negatively correlated with resilience scores (P < 0.05). See Table 4.
|
Small-World Properties |
Brain Region |
r Value |
p Value |
|
σ |
- |
0.334 |
<0.001 |
|
γ |
- |
0.329 |
<0.001 |
|
Eg |
- |
0.237 |
0.004 |
|
Eloc |
- |
0.258 |
0.002 |
|
DC |
ORBsupmed.R |
0.203 |
0.014 |
|
INS.L |
0.192 |
0.021 |
|
|
ACG.L |
0.191 |
0.021 |
|
|
MTG.L |
-0.211 |
0.011 |
|
|
NE |
ORBsupmed.R |
0.266 |
0.001 |
|
INS.L |
0.245 |
0.003 |
|
|
ACG.L |
0.258 |
0.002 |
|
|
MTG.L |
-0.167 |
0.045 |
4. Conclusion and discussion
4.1. Alterations in brain network small-world properties in adults with left-behind experiences
The “small-world” property of brain networks is an important theoretical model for understanding the mechanisms of information processing and the organization of cognitive functions in the brain. The concept was first proposed by Watts and Strogatz in 1998 [8] to describe a network structure that combines high local clustering with short path length efficiency. This model has been widely used to explain how brain regions can achieve efficient information transfer and cognitive resource integration through limited structural or functional connections [13]. The relationship between brain functional networks and psychological resilience has become a major focus of interdisciplinary research in neuroscience and psychology in recent years. From a developmental neuropsychology perspective, social experiences during childhood and adolescence profoundly influence the formation of brain structure and functional connectivity patterns. In this study, both the high-resilience and low-resilience groups of adults with left-behind experiences exhibited small-world network properties. However, the high-resilience group showed higher σ, γ, and Eloc values compared to the low-resilience group, suggesting that individuals with higher resilience possess more efficient information transmission and better structural integration in their brain functional connectivity. In contrast, the reductions in σ, γ, and Eloc observed in the low-resilience group reflect a shift toward more random network organization, indicating reduced integrative capacity and decreased efficiency of information processing. These topological changes may underlie the neurobiological basis for characteristics typically associated with low resilience, such as emotional instability, slower responses to stress, and cognitive decline. Previous studies [13-15] have suggested that individuals with high psychological resilience generally possess stronger cognitive control and emotion regulation abilities when facing stress or negative emotions, with neural mechanisms likely involving efficient functional integration between prefrontal and limbic systems. The present study provides further evidence at the whole-brain network topology level, showing that higher σ, γ, and Eloc values in the high-resilience group support a highly efficient information processing mode for integrated brain functions. This configuration meets the dual demands of cognitive and emotional regulation in complex environments, reflecting the adaptive capacity of the neural system.
Degree Centrality (DC) measures the number of connections a brain region has with other regions across the whole brain; higher values indicate that the node functions as a central hub in information transfer. Nodal Efficiency (NE) reflects the efficiency with which a node exchanges information with other nodes via the shortest paths; higher values indicate greater overall efficiency of information transmission. The right medial orbitofrontal superior frontal gyrus is a key prefrontal-limbic region responsible for emotion regulation [16]. The insula and anterior cingulate cortex, along with the amygdala and other regions, form the salience network, which is involved in integrating information processing and is associated with cognitive functions [17]. Our results showed that the high-resilience group had significantly higher DC and NE in the right medial orbitofrontal superior frontal gyrus, left insula, and left anterior cingulate gyrus. This suggests these regions have broader connectivity and more efficient information transfer within the functional network, supporting stronger integration of emotional regulation and cognitive functions. Such features may enable high-resilience individuals to better manage stress, control impulses, and remain calm. The left middle temporal gyrus is involved in emotional regulation and language processing (especially semantic aspects) [18], and is an important node within the Default Mode Network (DMN) [19]. In this study, reduced DC and NE in the left middle temporal gyrus in the high-resilience group suggest decreased neural network connectivity in this region, possibly indicating a reduced role for this semantic-processing region during stress and emotion regulation. Instead, high-resilience individuals may rely more on regions directly involved in emotion and cognitive control, such as the prefrontal cortex, insula, and cingulate cortex. This interpretation aligns with findings by Miyagi et al. [20], who observed that psychological resilience may be related to the stability of the DMN, with high-resilience individuals showing more stable DMN connectivity changes during complex tasks. Meike et al. [21] also reported that improved functional network stability enhances the efficiency of emotion-regulation pathways, reducing the reliance on complex cognitive regions.
4.2. Correlation between small-world properties and psychological resilience scores
This study found that the small-world properties σ, γ, Eg, and Eloc in the brain networks of adults with left-behind experiences were positively correlated with psychological resilience scores. This suggests that improvements in global network properties—particularly increases in σ and γ, which are related to information integration and transmission—may contribute to the development of higher psychological resilience. At the nodal level, DC and NE values in the right medial orbitofrontal superior frontal gyrus, left insula, and left anterior cingulate gyrus were positively correlated with resilience scores, while those in the left middle temporal gyrus were negatively correlated. From a neurofunctional perspective, enhancements in brain network properties in regions involved in emotional regulation and cognitive control may strengthen an individual’s ability to cope with adversity, reduce negative emotions, and modulate their own psychological resilience.
4.3. Limitations
This study has several limitations. First, it primarily relies on rs-fMRI-derived brain network metrics to infer the neural mechanisms underlying the association between psychological resilience and brain function. This inferential approach is inherently indirect in theoretical logic. Second, there is a relative scarcity of prior research specifically examining the relationship between psychological resilience and small-world properties of brain networks, which may render some of our interpretations more subjective. Third, this study focused mainly on a young adult population, which limits the generalizability of the results to other stages of the lifespan.
In summary, the study found that psychological resilience levels are associated with resting-state brain network properties, showing systematic differences especially in small-world attributes and key nodal functional connectivity patterns. Childhood experiences of being left behind represent an important environmental factor potentially affecting the development of psychological resilience, characterized by emotional deprivation, disrupted caregiving, and neglect—amounting to prolonged social deprivation. Such experiences occur during critical periods of neurodevelopment, with chronic stress exposure and emotional deprivation having a profound impact on the formation of neural network pathways [22]. While adults with left-behind experiences all showed small-world properties in their brain networks, those with higher resilience demonstrated greater information transmission efficiency and network integration. These features may provide a topological neural basis supporting their abilities in emotional regulation and cognitive control. This finding offers a novel network-level perspective for understanding the neural encoding of psychological traits in this population and provides a theoretical foundation based on network structural features for future psychological interventions in individuals with left-behind experiences.
Funding project
National Natural Science Foundation of China (82160275)
References
[1]. National Bureau of Statistics of China, United Nations Children’s Fund, & United Nations Population Fund. (2020). The status of China’s child population in 2020: Facts and data. Retrieved from National Bureau of Statistics of China website: https: //www.stats.gov.cn/zs/tjwh/tjkw/tjzl/202304/P020230419425666818737.pdf
[2]. J. R. Reinwald, R. Becker, A. S. Mallien, et al.Neural Mechanisms of Early-Life Social Stress as a Developmental Risk Factor for Severe Psychiatric Disorders [J].Biol Psychiatry, 2018, 84(2): 116-128.
[3]. Liu, H., Zhang, C., & Yang, L. (2019). Psychological resilience and its neural mechanisms: Evidence from non-human animal models.Advances in Psychological Science, 27(2), 312–321.
[4]. Pan, G., Li, B., Wang, J., et al. (2019). Childhood maltreatment and mental health status among college students with left-behind experience.Chinese Journal of Disease Control & Prevention, 23(7), 840–844.
[5]. Qi J, Li B, Zhang Y, et al. (2021) Disrupted small-world networks are associated with decreased vigilant attention after total sleep deprivation [J].Neuroscience, 471: 51-60.
[6]. Achard S, Bullmore E. (2007) Efficiency and cost of economical brain functional networks.PLoS Comput Biol.3(2): e17.
[7]. Chen, J. (2024). Analysis and classification of adolescent depression brain networks based on fMRI images (Master’s thesis). China University of Mining and Technology. https: //doi.org/10.27623/d.cnki.gzkyu.2024.001043.
[8]. Watts D J, Strogatz S H. (1998) Collective dynamics of 'small-world’networks [J].Nature, 393(6684): 440-442.
[9]. Yu X,Zhang J. Factor analysis and psychometric evaluation of the Connor-Davidson Resilience Scale (CD-RISC) with Chinese people [J].SOCIAL BEHAVIOR AND PERSONALITY,2017,35(1): 19-30.
[10]. Wang J, Wang X, Xia M, Liao X, Evans A, He Y. (2015) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics.Front Hum Neurosci. 9: 386.
[11]. Suo X, Lei D, Li K, et al. (2015) Disrupted brain network topology in pediatric posttraumatic stress disorder: A resting-state f MRI study [J].Human Brain Mapping, 36(9): 3677-3686.
[12]. Xia M, Wang J, He Y (2013) BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics.PLoS ONE8: e68910.
[13]. Han, Y., Li, Y., Zhang, H., et al. (2024). Research progress on large-scale brain networks in Alzheimer’s disease based on MRI analysis.Journal of Practical Medicine, 40(4), 575–579.
[14]. J. R. Reinwald, R. Becker, A. S. Mallien, et al. (2018) Neural Mechanisms of Early-Life Social Stress as a Developmental Risk Factor for Severe Psychiatric Disorders [J].Biol Psychiatry, 84(2): 116-128.
[15]. Gupta A, Love A, Kilpatrick LA, Labus JS, Bhatt R, Chang L, Tillisch K, Naliboff B, Mayer EA. (2017) Morphological brain measures of cortico-limbic inhibition related to resilience.J Neurosci Res, 95(9): 1760-1775. doi: 10.1002/jnr.24007IF: 2.9 Q2 . Epub 2016 Dec 28. PMID: 28029706; PMCID: PMC5512424.
[16]. Feng, Z., & Liao, C. (2023). Advances in research on the neural model of negative emotion processing and cognitive control in depression.Journal of Army Medical University,45(23), 2395–2402.
[17]. Joseph D Viviano, Robert W Buchanan, Navona Calarco, et al.(2018) Resting-State Connectivity Biomarkers of Cognitive Performance and Social Function in Individuals With Schizophrenia Spectrum Disorder and Healthy Control Subjects [J].Biological psychiatry,84(9): 665-674.
[18]. Vinod Menon, Lucina Q Uddin. (2010) Saliency, switching, attention and control: a network model of insula function [J].Brain structure & function,214(5-6): 655-67. DOI: 10.1007/s00429-010-0262-0.
[19]. Zhu T, Wang Z, Wu W, Ling Y, Wang Z, Zhou C, Fang X, Huang C, Xie C, Chen J, Zhang X. (2023) Altered brain functional networks in schizophrenia with persistent negative symptoms: an activation likelihood estimation meta-analysis.Front Hum Neurosci,17: 1204632. doi: 10.3389/fnhum.2023.1204632IF: 2.7 Q2 . PMID: 37954938; PMCID: PMC10637389.
[20]. Zweerings J, Hummel B, Keller M, Zvyagintsev M, Schneider F, Klasen M, Mathiak K. (2019) Neurofeedback of core language network nodes modulates connectivity with the default-mode network: A double-blind fMRI neurofeedback study on auditory verbal hallucinations.Neuroimage,189: 533-542. doi: 10.1016/j.neuroimage.2019.01.058. Epub 2019 Jan 28. PMID: 30703519.
[21]. Miyagi T, Oishi N, Kobayashi K, Ueno T, Yoshimura S, Murai T, Fujiwara H. (2020) Psychological resilience is correlated with dynamic changes in functional connectivity within the default mode network during a cognitive task.Sci Rep,10(1): 17760.
[22]. Meike D Hettwer, Lena Dorfschmidt, Lara M C Puhlmann, et al. (2024) Longitudinal variation in resilient psychosocial functioning is associated with ongoing cortical myelination and functional reorganization during adolescence [J].Nature communications, 15(1): 6283.
[23]. Martin H Teicher, Jacqueline A Samson, Carl M Anderson, et al. (2016) The effects of childhood maltreatment on brain structure, function and connectivity [J].Nature reviews Neuroscience, 17(10): 652-66.
Cite this article
Li,L.;Mo,Y. (2025). A study on the correlation between small-world properties of brain networks and psychological resilience phenotypes in adults with childhood left-behind experiences. Journal of Clinical Technology and Theory,3(2),101-107.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Clinical Technology and Theory
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. National Bureau of Statistics of China, United Nations Children’s Fund, & United Nations Population Fund. (2020). The status of China’s child population in 2020: Facts and data. Retrieved from National Bureau of Statistics of China website: https: //www.stats.gov.cn/zs/tjwh/tjkw/tjzl/202304/P020230419425666818737.pdf
[2]. J. R. Reinwald, R. Becker, A. S. Mallien, et al.Neural Mechanisms of Early-Life Social Stress as a Developmental Risk Factor for Severe Psychiatric Disorders [J].Biol Psychiatry, 2018, 84(2): 116-128.
[3]. Liu, H., Zhang, C., & Yang, L. (2019). Psychological resilience and its neural mechanisms: Evidence from non-human animal models.Advances in Psychological Science, 27(2), 312–321.
[4]. Pan, G., Li, B., Wang, J., et al. (2019). Childhood maltreatment and mental health status among college students with left-behind experience.Chinese Journal of Disease Control & Prevention, 23(7), 840–844.
[5]. Qi J, Li B, Zhang Y, et al. (2021) Disrupted small-world networks are associated with decreased vigilant attention after total sleep deprivation [J].Neuroscience, 471: 51-60.
[6]. Achard S, Bullmore E. (2007) Efficiency and cost of economical brain functional networks.PLoS Comput Biol.3(2): e17.
[7]. Chen, J. (2024). Analysis and classification of adolescent depression brain networks based on fMRI images (Master’s thesis). China University of Mining and Technology. https: //doi.org/10.27623/d.cnki.gzkyu.2024.001043.
[8]. Watts D J, Strogatz S H. (1998) Collective dynamics of 'small-world’networks [J].Nature, 393(6684): 440-442.
[9]. Yu X,Zhang J. Factor analysis and psychometric evaluation of the Connor-Davidson Resilience Scale (CD-RISC) with Chinese people [J].SOCIAL BEHAVIOR AND PERSONALITY,2017,35(1): 19-30.
[10]. Wang J, Wang X, Xia M, Liao X, Evans A, He Y. (2015) GRETNA: a graph theoretical network analysis toolbox for imaging connectomics.Front Hum Neurosci. 9: 386.
[11]. Suo X, Lei D, Li K, et al. (2015) Disrupted brain network topology in pediatric posttraumatic stress disorder: A resting-state f MRI study [J].Human Brain Mapping, 36(9): 3677-3686.
[12]. Xia M, Wang J, He Y (2013) BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics.PLoS ONE8: e68910.
[13]. Han, Y., Li, Y., Zhang, H., et al. (2024). Research progress on large-scale brain networks in Alzheimer’s disease based on MRI analysis.Journal of Practical Medicine, 40(4), 575–579.
[14]. J. R. Reinwald, R. Becker, A. S. Mallien, et al. (2018) Neural Mechanisms of Early-Life Social Stress as a Developmental Risk Factor for Severe Psychiatric Disorders [J].Biol Psychiatry, 84(2): 116-128.
[15]. Gupta A, Love A, Kilpatrick LA, Labus JS, Bhatt R, Chang L, Tillisch K, Naliboff B, Mayer EA. (2017) Morphological brain measures of cortico-limbic inhibition related to resilience.J Neurosci Res, 95(9): 1760-1775. doi: 10.1002/jnr.24007IF: 2.9 Q2 . Epub 2016 Dec 28. PMID: 28029706; PMCID: PMC5512424.
[16]. Feng, Z., & Liao, C. (2023). Advances in research on the neural model of negative emotion processing and cognitive control in depression.Journal of Army Medical University,45(23), 2395–2402.
[17]. Joseph D Viviano, Robert W Buchanan, Navona Calarco, et al.(2018) Resting-State Connectivity Biomarkers of Cognitive Performance and Social Function in Individuals With Schizophrenia Spectrum Disorder and Healthy Control Subjects [J].Biological psychiatry,84(9): 665-674.
[18]. Vinod Menon, Lucina Q Uddin. (2010) Saliency, switching, attention and control: a network model of insula function [J].Brain structure & function,214(5-6): 655-67. DOI: 10.1007/s00429-010-0262-0.
[19]. Zhu T, Wang Z, Wu W, Ling Y, Wang Z, Zhou C, Fang X, Huang C, Xie C, Chen J, Zhang X. (2023) Altered brain functional networks in schizophrenia with persistent negative symptoms: an activation likelihood estimation meta-analysis.Front Hum Neurosci,17: 1204632. doi: 10.3389/fnhum.2023.1204632IF: 2.7 Q2 . PMID: 37954938; PMCID: PMC10637389.
[20]. Zweerings J, Hummel B, Keller M, Zvyagintsev M, Schneider F, Klasen M, Mathiak K. (2019) Neurofeedback of core language network nodes modulates connectivity with the default-mode network: A double-blind fMRI neurofeedback study on auditory verbal hallucinations.Neuroimage,189: 533-542. doi: 10.1016/j.neuroimage.2019.01.058. Epub 2019 Jan 28. PMID: 30703519.
[21]. Miyagi T, Oishi N, Kobayashi K, Ueno T, Yoshimura S, Murai T, Fujiwara H. (2020) Psychological resilience is correlated with dynamic changes in functional connectivity within the default mode network during a cognitive task.Sci Rep,10(1): 17760.
[22]. Meike D Hettwer, Lena Dorfschmidt, Lara M C Puhlmann, et al. (2024) Longitudinal variation in resilient psychosocial functioning is associated with ongoing cortical myelination and functional reorganization during adolescence [J].Nature communications, 15(1): 6283.
[23]. Martin H Teicher, Jacqueline A Samson, Carl M Anderson, et al. (2016) The effects of childhood maltreatment on brain structure, function and connectivity [J].Nature reviews Neuroscience, 17(10): 652-66.









