1. Case data
The patient, a 58-year-old male, height 172 cm, weight 86 kg, was admitted to the First Affiliated Hospital of Shihezi University in mid-November 2021. He was diagnosed with right-sided small cell lung cancer with mediastinal, hilar, cervical lymph node, and adrenal metastases, stage IV (cT3N3M1b). Tumor markers: carcinoembryonic antigen (CEA) 13.41 ng/mL; carbohydrate antigen (CA) 19-9 44.21 U/mL; CA 72-4 8.75 U/mL; neuron-specific enolase (NSE) 263.00 ng/mL; pro-gastrin-releasing peptide (Pro-GRP) 430.6 pg/mL. PD-L1 expression: not tested. Immunohistochemistry: Napsin A (-), CK7 (-), TTF-1 (+), CK5/6 (-), P40 (-), P63 (-), CD56 (+), AE1/3 (partially +), CgA (+), Syn (+), EBER (-), CD5 (-), Ki-67 (70%). Treatment history: From November 30, 2021, to February 28, 2022, the patient underwent five cycles of first-line chemotherapy consisting of “atezolizumab 1200 mg + carboplatin 800 mg + etoposide 590 mg.” After chemotherapy, the cervical mass showed significant reduction, and the therapeutic evaluation indicated a major partial response (PR). Subsequently, the patient received maintenance monotherapy with atezolizumab (1200 mg per cycle, every three weeks) up to the present. The most recent immunotherapy session was on March 28, 2024. Past medical history: prior hernia surgery; denies any family history of hereditary diseases, autoimmune disorders, or drug allergies.
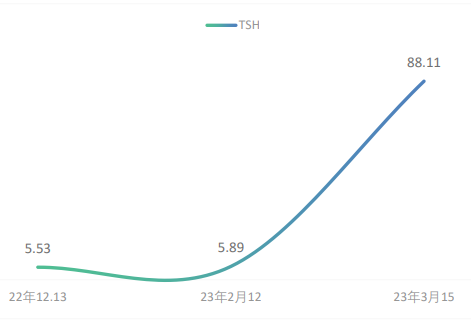
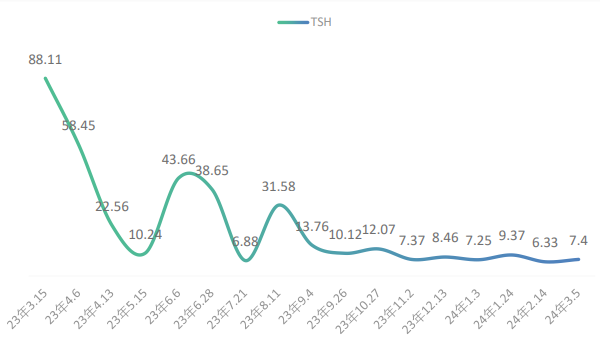
On December 13, 2022, after nine cycles of maintenance monotherapy, the patient developed thyroid dysfunction with the following thyroid function test results: thyroid-stimulating hormone (TSH) 5.53 mIU/L, free triiodothyronine (FT3) 4.23 pmol/L, and free thyroxine (FT4) 15.84 pmol/L. According to the toxicity grading and management principles outlined in the 2023 edition of the CSCO Guidelines for the Management of Toxicities Related to Immune Checkpoint Inhibitors (hereinafter referred to as the same principles), the condition was graded as G1. No special intervention was performed, and the patient proceeded to the next chemotherapy cycle. On February 12, 2023 (after the 11th cycle), repeat thyroid function tests showed TSH 5.89 mIU/L, FT3 4.78 pmol/L, and FT4 13.88 pmol/L. On March 15, 2023 (after the 12th cycle), thyroid function results were TSH 88.11 mIU/L, FT3 2.51 pmol/L, and FT4 4.38 pmol/L. The patient’s TSH level continued to rise abnormally (see Figure 1), and a clinical diagnosis of hypothyroidism was made. According to the toxicity grading principles, this was graded as G2. Immunotherapy with immune checkpoint inhibitors was suspended, and the patient was started on oral levothyroxine sodium 50 μg once daily for hypothyroidism. On April 13, 2023, the patient’s TSH level decreased to 22.56 mIU/L, and on April 23, atezolizumab monotherapy was resumed for the 13th cycle. After treatment was resumed, thyroid function tests were conducted every 4–6 weeks (see Figure 2). On June 6, 2023, TSH was found to have increased again to 43.66 mIU/L. After clinical pharmacist intervention, poor medication adherence was suspected. It was recommended that the patient’s adherence be assessed using the Morisky Medication Adherence Scale, and targeted medication education and counseling for thyroid dysfunction were provided. Subsequently, the patient returned regularly every 4–6 weeks for follow-up. His TSH levels remained stably at a relatively low abnormal range, and his hypothyroidism was brought under control.
2. Discussion
2.1. Evaluation of the association between atezolizumab and hypothyroidism
Based on the case, the association between atezolizumab and hypothyroidism in this patient was evaluated as follows: (1) Data collection: According to the provided information, the patient developed symptoms of hypothyroidism after the initiation of atezolizumab, with elevated TSH and decreased FT levels observed. (2) Temporal relationship: Hypothyroidism symptoms appeared after the administration of atezolizumab, and the rise in TSH coincided with the onset of symptoms (progressive elevation in TSH from the 9th cycle, reaching 88.11 mIU/L after the 10th cycle, with FT3 2.51 pmol/L and FT4 4.38 pmol/L), indicating a temporal correlation. (3) Drug labeling: According to the prescribing information for atezolizumab, immune-related hypothyroidism is explicitly listed as a possible adverse reaction. (4) Exclusion of other factors: No other concomitant medications were used. After atezolizumab administration, TSH increased and FT levels decreased. Following clinical physician and pharmacist intervention, treatment with levothyroxine sodium normalized FT levels, and TSH gradually improved.
According to the causality assessment criteria for adverse drug reactions [1], the association between atezolizumab and hypothyroidism in this case can be preliminarily assessed as “very likely.”
2.2. Immune checkpoint inhibitors causing immune-related hypothyroidism
Some immune checkpoint inhibitors (ICIs) may be associated with immune-related hypothyroidism, a condition known as immune-related thyroid disease. Certain ICIs can induce the immune system to attack thyroid tissue, leading to hypothyroidism [2].
A real-world study indicated that the incidence of thyroid-related adverse events (T-rAEs) varied across studies, ranging from approximately 13.4% to 42.0% [3]. In this study, thyroid immune-related adverse events (T-irAEs) caused by PD-1 inhibitors were found to be the most common, with an incidence ranging between 0% and 4%, which was relatively low. By comparison, the incidence of T-irAEs induced by PD-L1 inhibitors and CTLA-4 inhibitors was 0%–10% and 0%–7%, respectively [4], still indicating a certain degree of risk.
Yuan, X. [5] and colleagues conducted a literature search of databases and included 68 randomized controlled trials (RCTs). They analyzed the characteristics and patterns of thyroid-related adverse events induced by ICIs in China and found that, compared with their respective control groups, the risk of hypothyroidism in tumor patients treated with PD-1 inhibitors [RR: 7.13, 95% CI (5.79–8.79)], PD-L1 inhibitors [RR: 7.00, 95% CI (4.81–10.18)], and CTLA-4 inhibitors [RR: 5.21, 95% CI (2.69–10.11)] was increased to varying degrees.
2.3. Possible pathogenic mechanisms of immune-related hypothyroidism induced by immune checkpoint inhibitors
The exact mechanism underlying immune-related hypothyroidism (IRH) remains hypothetical and has not been fully established. However, several studies and hypotheses have been proposed to explain the relationship between immune checkpoint inhibitors (ICIs) and hypothyroidism. ICIs are a class of immunotherapeutic agents that enhance the activity of the host immune system by inhibiting tumor-mediated suppression of immune responses. These drugs can activate the immune system to specifically attack tumor cells. However, this enhanced immune activity may lead to excessive autoimmune responses. At the hypothesis stage, some researchers propose that ICIs may overactivate the host immune system, thereby triggering autoimmune reactions directed against the thyroid.
Puzanov et al. [6] support the notion that ICIs disrupt the host’s immune tolerance to self-antigens, thereby leading to immune-related adverse events (irAEs), including adverse effects on the endocrine system. Under normal circumstances, the immune system maintains tolerance to antigens derived from self-tissues to prevent excessive immune activation that could result in autoimmune disease. However, ICIs can disturb this balance of immune tolerance. The mechanism of action of ICIs involves inhibitory regulatory molecules, such as CTLA-4 and PD-1, which are key mediators of self-tolerance. By inhibiting the function of these molecules, ICIs may impair the regulation of self-antigens, causing the immune system to become overactivated against host tissues. In this context, the immune system may begin to attack normal tissues, including those of the endocrine system. This can result in immune-related endocrine adverse events, such as hypothyroidism, pituitary dysfunction, and adrenal cortical dysfunction.
Osorio et al. [7] reported that patients who are positive for thyroid autoantibodies prior to immunotherapy are more prone to developing immune-related thyroid dysfunction. A recent study from China [8] further confirmed that positive thyroglobulin antibodies (TgAb) and thyroid peroxidase antibodies (TPOAb) are considered potential contributors to immune checkpoint inhibitor (ICPi)-induced hypothyroidism. When patients receive ICI therapy, pre-existing or induced thyroid autoimmunity may trigger or exacerbate thyroid dysfunction. In patients undergoing ICI treatment, testing for TPOAb and TgAb is commonly used as a predictive marker for thyroid dysfunction, with positive results indicating a higher risk of developing hypothyroidism.
However, it should be noted that these are currently hypotheses and lack sufficient experimental validation. The precise mechanisms underlying immune-related hypothyroidism require further investigation to be confirmed and fully elucidated.
2.4. Treatment and prevention of immune-related hypothyroidism induced by immune checkpoint inhibitors
2.4.1. Treatment of immune-related hypothyroidism induced by immune checkpoint inhibitors
(1) Management principles [9]: Clinical management of thyroid injury induced by immune checkpoint inhibitors (ICIs) requires consideration of multiple factors, including the presence of clinical symptoms, the severity of symptoms, and the type of thyroid dysfunction. In addition, according to the grading of immune-related adverse events (irAEs), it is necessary to assess whether ICI therapy should be paused or discontinued and to develop an appropriate treatment plan. Endocrinology and oncology specialists should collaborate to formulate the optimal strategy to ensure the patient achieves the best therapeutic outcomes and quality of life.
(2) Recommended dosage: Patients with hypothyroidism who exhibit clinical symptoms or have TSH >10 mIU/L should receive treatment. For TSH levels between 5 and 10 mIU/L, treatment decisions should be based on clinical symptoms and TPOAb status. The recommended dose of levothyroxine sodium is 1–1.6 μg·kg⁻¹·d⁻¹, with an initial dose of 25–50 μg/day; however, adjustments should be made according to age, comorbidities, and the patient’s prognosis (for elderly patients or those with cardiac disease, an initial dose of 12.5 μg/day is recommended).
(3) Timing of treatment and control targets:① Timing of treatment: When the patient is asymptomatic, only clinical diagnostic observation is required, and no treatment is necessary; when clinical symptoms affecting daily activities appear, appropriate pharmacotherapy should be administered according to functional changes.② Control targets: Relief of clinical symptoms and reduction of disease grading to grade 1 or below.
(4) Monitoring indicators and control range:① Monitoring indicators: For patients with ICI-related thyroid dysfunction, TSH and FT4 levels should be monitored, with follow-up every 4–6 weeks. For patients with thyrotoxicosis who may develop hypothyroidism, monitoring should be performed every 2–3 weeks.② Control range: No clear target range has been established; individualized treatment plans should be provided based on the patient’s condition.
2.4.2. Prevention of immune-related hypothyroidism induced by immune checkpoint inhibitors
The precise methods for preventing immune-related hypothyroidism are still under investigation and may vary among individuals. However, the following measures may help reduce the risk of developing immune-related hypothyroidism:
(1) Close monitoring of thyroid function: For patients receiving immune checkpoint inhibitor therapy, regular thyroid function tests should be conducted, including measurement of thyroid hormone levels such as TSH, T4, and T3. This allows for early detection of hypothyroidism and timely initiation of appropriate treatment.
(2) Patient education: Provide comprehensive education and information to ensure that patients understand the potential adverse effects and complications of immune checkpoint inhibitors, including immune-related hypothyroidism. Patients should be informed about the early symptoms of hypothyroidism and be instructed on how to maintain communication with their healthcare providers.
(3) Multidisciplinary team management: Establish a multidisciplinary team, including oncologists, internists, and endocrinologists, to ensure comprehensive monitoring and management of patients during immune checkpoint inhibitor therapy.
(4) Personalized treatment plans: Develop individualized treatment plans based on the patient’s condition and response to immune checkpoint inhibitor therapy. This may include dose adjustments, combination therapy, or extended treatment intervals to reduce the risk of immune-related hypothyroidism.
2.5. Discharge education provided by clinical pharmacists
(1) Medication timing: Instruct the patient to take the medication on an empty stomach in the morning (at least 30 minutes before breakfast), with a moderate amount of liquid (e.g., half a glass of water). The patient must not adjust or discontinue the dose on their own.
(2) Grapefruit juice: Grapefruit juice may delay the absorption of levothyroxine and reduce its efficacy. Avoid consuming grapefruit or grapefruit juice during treatment.
(3) Dietary considerations: Soy products (such as soy milk), high-fiber foods, dairy products, eggs, and walnuts may reduce the absorption of levothyroxine. These foods should be avoided during treatment, or if co-ingested, a minimum interval of 2 hours should be maintained.
(4) Calcium salts: Calcium salts (e.g., calcium carbonate, calcium citrate) can diminish the pharmacological effect of levothyroxine. If co-administered, a minimum interval of 4 hours should be observed.
(5) Overdose or rapid dose increase: Excessive dosing or rapid dose escalation may cause symptoms of hyperthyroidism, including arrhythmias (such as tachycardia and palpitations), headache, flushing, fever, vomiting, insomnia, excessive sweating, weight loss, and diarrhea. Patients experiencing these symptoms should seek medical attention promptly.
3. Conclusion
In this case, the patient developed immune-related hypothyroidism. The clinical pharmacist provided corresponding medication recommendations and conducted pharmacotherapy monitoring throughout the treatment process, and informed the oncology physician that the use of atezolizumab may increase the risk of immune-related hypothyroidism. Subsequently, the clinician, after considering the patient’s primary disease and medication regimen, determined whether to discontinue atezolizumab and prescribed levothyroxine sodium to manage hypothyroidism, thereby selecting a more appropriate therapeutic strategy for the patient. This case highlights that both clinicians and clinical pharmacists should consider the risk of immune-related hypothyroidism when using atezolizumab for the treatment of lung cancer, in order to ensure medication safety.
References
[1]. Li, B., Gao, R., Li, R., et al. (2014). Research on methods for determining the causality of adverse reactions/adverse events in drug clinical trials.Chinese Journal of New Drugs, 23(12), 1465–1470.
[2]. Kennedy, L. B., & Salama, A. K. S. (2020). A review of cancer immunotherapy toxicity.CA: A Cancer Journal for Clinicians, 70(2), 86–104. https: //doi.org/10.3322/caac.21596
[3]. Spagnolo, C. C., Giuffrida, G., Cannavò, S., et al. (2022). Management of endocrine and metabolic toxicities of immune-checkpoint inhibitors: From clinical studies to a real-life scenario.Cancers (Basel),15(1), 246. https: //doi.org/10.3390/cancers15010246
[4]. Kwon, H., Roh, E., Ahn, C. H., et al. (2022). Immune checkpoint inhibitors and endocrine disorders: A position statement from the Korean Endocrine Society.Journal of Endocrinology and Metabolism, 37(6), 839–850.
[5]. Yuan, X., Wang, Y. Y., & Chen, W. (2024). Literature analysis of thyroid-related adverse reactions induced by immune checkpoint inhibitors in China.Chinese Journal of Hospital Pharmacy,44(09), 1062–1068. https: //doi.org/10.13286/j.1001-5213.2024.09.09
[6]. Puzanov, I., Diab, A., Abdallah, K., et al. (2017). Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group.Journal of Immunotherapy of Cancer, 5(1), 95. https: //doi.org/10.1186/s40425-017-0300-z
[7]. Osorio, J. C., Ni, A., Chaft, J. E., et al. (2017). Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer.Annals of Oncology,28(3), 583–589. https: //doi.org/10.1093/annonc/mdw640
[8]. Cui, W. J., Hu, X., Wei, X., et al. (2021). Research progress on thyroid dysfunction induced by immune checkpoint inhibitors.InternationalJournal of Endocrinology and Metabolism,41(1), 33–37.
[9]. Expert consensus on endocrine immune-related adverse reactions induced by immune checkpoint inhibitors (2020). Endocrine Immunology Group, Chinese Medical Association, Endocrinology Branch.Chinese Journal of Endocrinology and Metabolism,2021(01).
Cite this article
Li,H.;Li,J. (2025). A case analysis of thyroid dysfunction induced by immunotherapy in a patient with lung cancer. Journal of Clinical Technology and Theory,3(3),5-9.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Clinical Technology and Theory
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Li, B., Gao, R., Li, R., et al. (2014). Research on methods for determining the causality of adverse reactions/adverse events in drug clinical trials.Chinese Journal of New Drugs, 23(12), 1465–1470.
[2]. Kennedy, L. B., & Salama, A. K. S. (2020). A review of cancer immunotherapy toxicity.CA: A Cancer Journal for Clinicians, 70(2), 86–104. https: //doi.org/10.3322/caac.21596
[3]. Spagnolo, C. C., Giuffrida, G., Cannavò, S., et al. (2022). Management of endocrine and metabolic toxicities of immune-checkpoint inhibitors: From clinical studies to a real-life scenario.Cancers (Basel),15(1), 246. https: //doi.org/10.3390/cancers15010246
[4]. Kwon, H., Roh, E., Ahn, C. H., et al. (2022). Immune checkpoint inhibitors and endocrine disorders: A position statement from the Korean Endocrine Society.Journal of Endocrinology and Metabolism, 37(6), 839–850.
[5]. Yuan, X., Wang, Y. Y., & Chen, W. (2024). Literature analysis of thyroid-related adverse reactions induced by immune checkpoint inhibitors in China.Chinese Journal of Hospital Pharmacy,44(09), 1062–1068. https: //doi.org/10.13286/j.1001-5213.2024.09.09
[6]. Puzanov, I., Diab, A., Abdallah, K., et al. (2017). Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group.Journal of Immunotherapy of Cancer, 5(1), 95. https: //doi.org/10.1186/s40425-017-0300-z
[7]. Osorio, J. C., Ni, A., Chaft, J. E., et al. (2017). Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer.Annals of Oncology,28(3), 583–589. https: //doi.org/10.1093/annonc/mdw640
[8]. Cui, W. J., Hu, X., Wei, X., et al. (2021). Research progress on thyroid dysfunction induced by immune checkpoint inhibitors.InternationalJournal of Endocrinology and Metabolism,41(1), 33–37.
[9]. Expert consensus on endocrine immune-related adverse reactions induced by immune checkpoint inhibitors (2020). Endocrine Immunology Group, Chinese Medical Association, Endocrinology Branch.Chinese Journal of Endocrinology and Metabolism,2021(01).









