1. Introduction
Glioma is the most common type of intracranial malignant tumor. The World Health Organization (WHO) classifies gliomas into four grades, with malignancy increasing with grade [1], and classifies grade I and II gliomas as low-grade gliomas (LGG). LGG exhibits malignant biological characteristics such as invasive growth and resistance to radiotherapy and chemotherapy, and has a tendency to progress to high-grade gliomas [2]. Meanwhile, the therapeutic effect of LGG is quite promising; therefore, the discovery of new biomarkers and early diagnosis are particularly important.
The protein encoded by the VTCN1 gene belongs to the B7 co-stimulatory protein family. When present on the surface of antigen-presenting cells and tumor cells, it interacts with ligands on the surface of T cells, and inhibits T cell-mediated cellular immunity by suppressing the proliferation of activated T cells, the production of cytokines, and the differentiation of effector T cells [3]. When expressed on the surface of tumor-associated macrophages, it plays an important role in inhibiting tumor-associated antigen-specific T cell immunity together with regulatory T cells [4]. Studies have shown that high levels of VTCN1-encoded protein are associated with the progression of cancers such as breast cancer [5-7], pancreatic cancer [8-10], and cervical cancer [11,12]; however, relevant research on VTCN1 in LGG [13] needs further expansion.
In this study, the regulatory functions and molecular mechanisms of VTCN1 in LGG were thoroughly investigated using cancer datasets, and potential collaborative activities that may be used to promote immunotherapy were predicted, providing new directions and strategies for the clinical treatment of LGG.
2. Materials and methods
2.1. Prognostic analysis
Forest plots and Kaplan-Meier curves were used to examine the relationship between VTCN1 expression and patient prognosis in 33 types of cancers, including overall survival (OS), disease-free survival (DFS), disease-specific survival (DSS), and progression-free survival (PFS). Univariate survival analysis was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs).
2.2. Prognostic model
RNA-seq data and corresponding clinical information of LGG were obtained from The Cancer Genome Atlas (TCGA) dataset (https://portal.gdc.com). First, univariate and multivariate Cox regression analyses were performed, and forest plots were generated using the "forestplot" package to display each variable (P value, HR, and 95% CI). Based on the results of multivariate Cox proportional hazards analysis, a nomogram was constructed using the "rms" package to predict the X-year overall recurrence rate. The nomogram provides a graphical representation of these factors, and the prognostic risk of individual patients can be calculated based on the points associated with each risk factor.
2.3. Tumor Immune Estimation Resource (TIMER)
The TIMER database (https://cistrome.shinyapps.io/timer/) was used to analyze the expression profile of VTCN1 and the abundance of immune infiltration in pan-cancer. The gene expression level was represented by log₂ TPM values.
2.4. Mutation analysis
RNA-seq data and corresponding clinical information of LGG were obtained from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.com). Tumor mutational burden (TMB) was obtained from the article The Immune Landscape of Cancer published by Vesteinn Thorsson et al. in 2018; microsatellite instability (MSI) was obtained from the article Landscape of Microsatellite Instability Across 39 Cancer Types published by Russell Bonneville et al. in 2017. Statistical analysis was performed using R software v4.0.3. The rank-sum test was used to compare two groups of data, and P<0.05 was considered statistically significant.
2.5. UALCAN
The UALCAN database (http://ualcan.path.uab.edu/analysis.html) was used to detect the expression level of VTCN1 between different cancers and their corresponding adjacent normal tissues. The Student's t-test was used to determine the significance of differences, and P<0.05 was considered statistically significant.
2.6. GEPIA2
The GEPIA2 database (http://gepia2.cancer-pku.cn/#index) was used to detect the correlation between VTCN1 and related genes. The Student's t-test was used to determine the significance of differences, and P<0.05 was considered statistically significant.
2.7. Ferroptosis
RNA-seq data and corresponding clinical information of LGG were obtained from The Cancer Genome Atlas (TCGA) dataset (https://portal.gdc.com). Ferroptosis-related genes were obtained from the systematic analysis of ferroptosis abnormalities and functions in cancer by Ze-Xian Liu et al. By collecting the expression levels of 24 ferroptosis-related genes, differential analysis of ferroptosis was performed among different samples.
2.8. Pathway correlation
Gene sets involved in relevant pathways were collected (DOI: 10.3390/cancers12071788). According to the ssGSEA (single-sample Gene Set Enrichment Analysis) algorithm, the enrichment score of each sample in each pathway was calculated sequentially, thereby obtaining the association between LGG samples and pathways. By calculating the correlation between gene expression and pathway scores, the relationship between VTCN1 and pathways was finally obtained by analyzing the correlation between VTCN1 and pathway scores using Spearman correlation analysis.
2.9. CCLE database
The gene expression matrix of LGG cell lines was obtained from the CCLE (Cancer Cell Line Encyclopedia) database (https://portals.broadinstitute.org/ccle/about). The expression level of VTCN1 in the same tumor was observed to obtain the expression status of this gene in different cell lines.
3. Results
3.1. Relationship between VTCN1 expression level and survival prognosis of glioma
Univariate Cox analysis of VTCN1 for OS, DFS, DSS, and PFS in multiple tumors showed that VTCN1 may act as a protective factor affecting the prognosis of brain low-grade glioma (all P<0.05; Figure 1). Kaplan-Meier survival curve analysis showed that patients were divided into high-expression and low-expression groups based on the median VTCN1 expression level. The results indicated that the median overall survival of the high VTCN1 expression group was significantly longer than that of the low-expression group (P<0.01, HR=0.552, 95%CI=0.385-0.79; Figure 2).
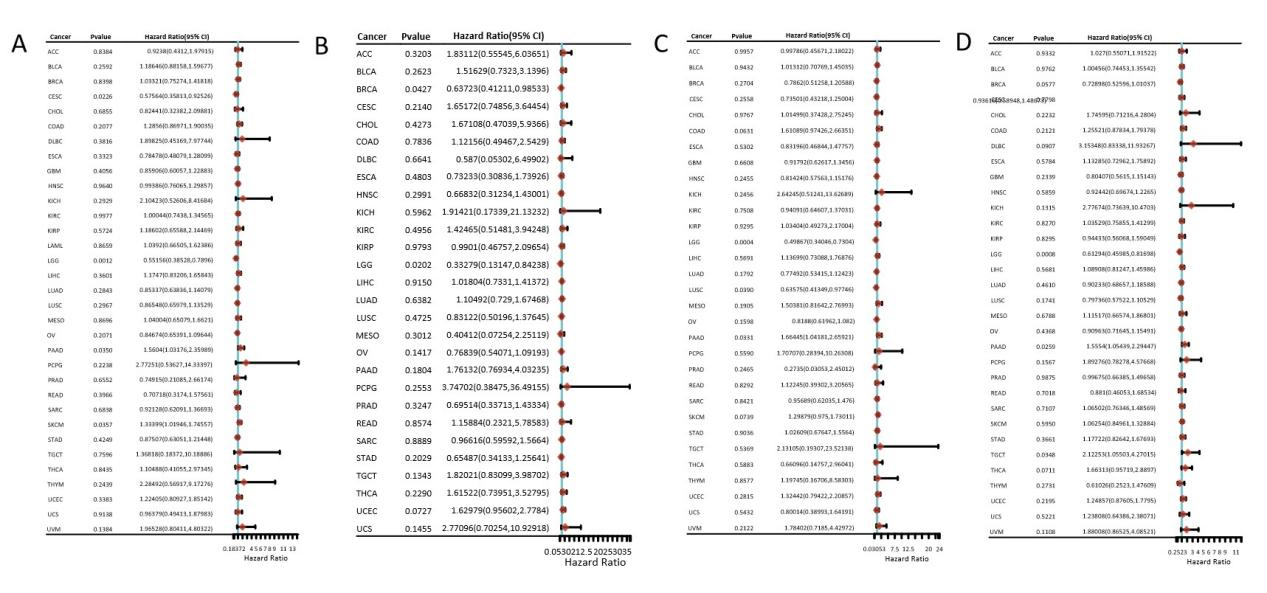
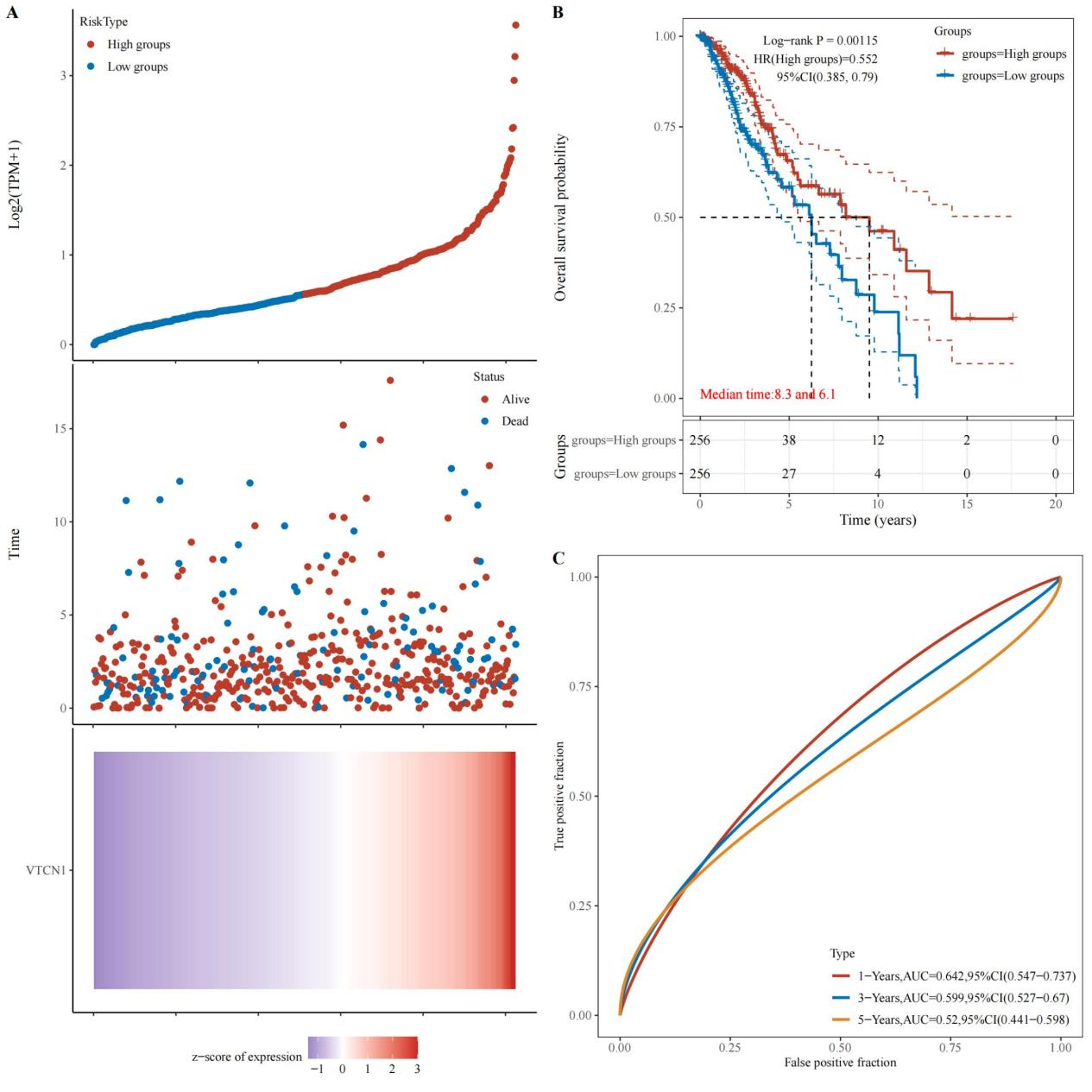
Figure 2. Kaplan-Meier survival curves of VTCN1 in LGG. A: The relationship between gene expression and survival time as well as survival status in TCGA data. B: KM survival curve of the gene in TCGA data. C: ROC curves and AUC values of the gene at different time points.
3.2. Study on VTCN1 gene correlation
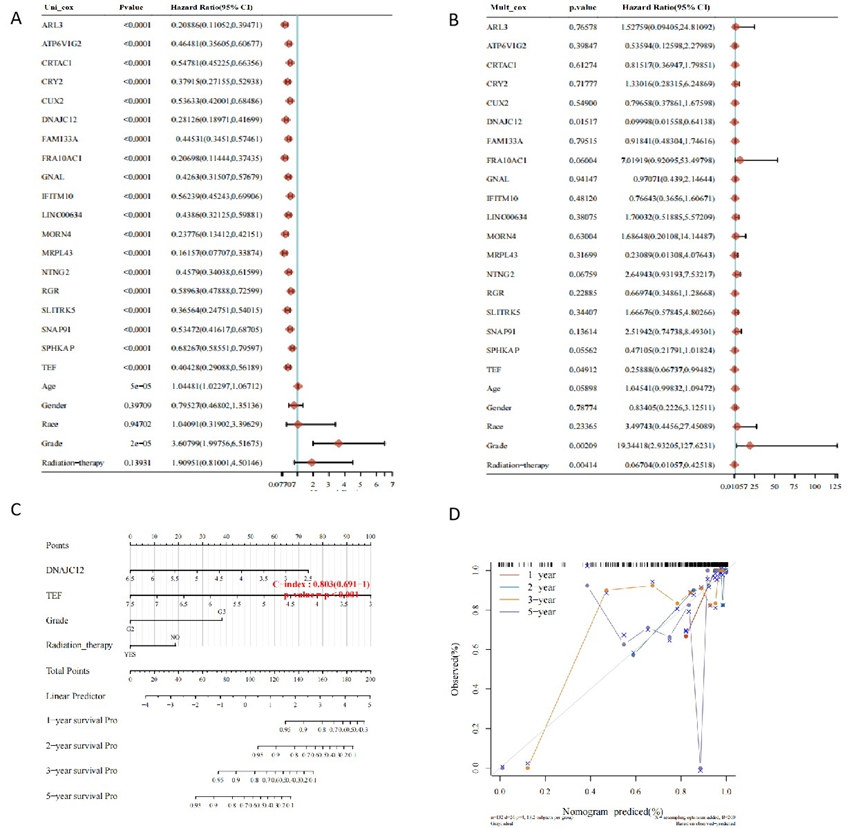
To explore how VTCN1 affects the prognosis of LGG, batch survival analysis was performed on data from samples with high VTCN1 expression. Based on the expression level of each individual gene, univariate Cox analysis was used to evaluate the prognostic significance and analyze prognostic differences, and the top 20 most significant protective factors were obtained (Figure 3A). Subsequently, predictive multivariate Cox analysis (Figure 3B) and construction of a prognostic model (Figures 3C and 3D) were performed. The results showed that in LGG, when VTCN1 was highly expressed, DNAJC12 (P<0.05, HR (high group) =0.09998, 95%CI=0.01558-0.64138) and TEF (P<0.05, HR (high group) =0.25888, 95%CI=0.06737-0.99482) were also protective factors. We believe that TEF and DNAJC12 may be involved in the process by which VTCN1 affects the prognosis of LGG.
3.3. Analysis of VTCN1 expression and immune cell infiltration
Due to the different relationships between VTCN1 and immune responses, the TIMER database was used to analyze the relationship between VTCN1 expression and the infiltration levels of 38 immune cell subtypes. The results showed that in LGG, VTCN1 was negatively correlated with plasma B cells, mast cells, CD4⁺ Th1 T cells, CD4⁺ Th2 T cells, naive CD8⁺ T cells, and gamma delta T cells, and positively correlated with common lymphoid progenitors, hematopoietic stem cells, macrophages, M2 macrophages, non-regulatory CD4⁺ T cells, natural killer (NK) T cells, and regulatory T cells (Tregs) (Figure 4).
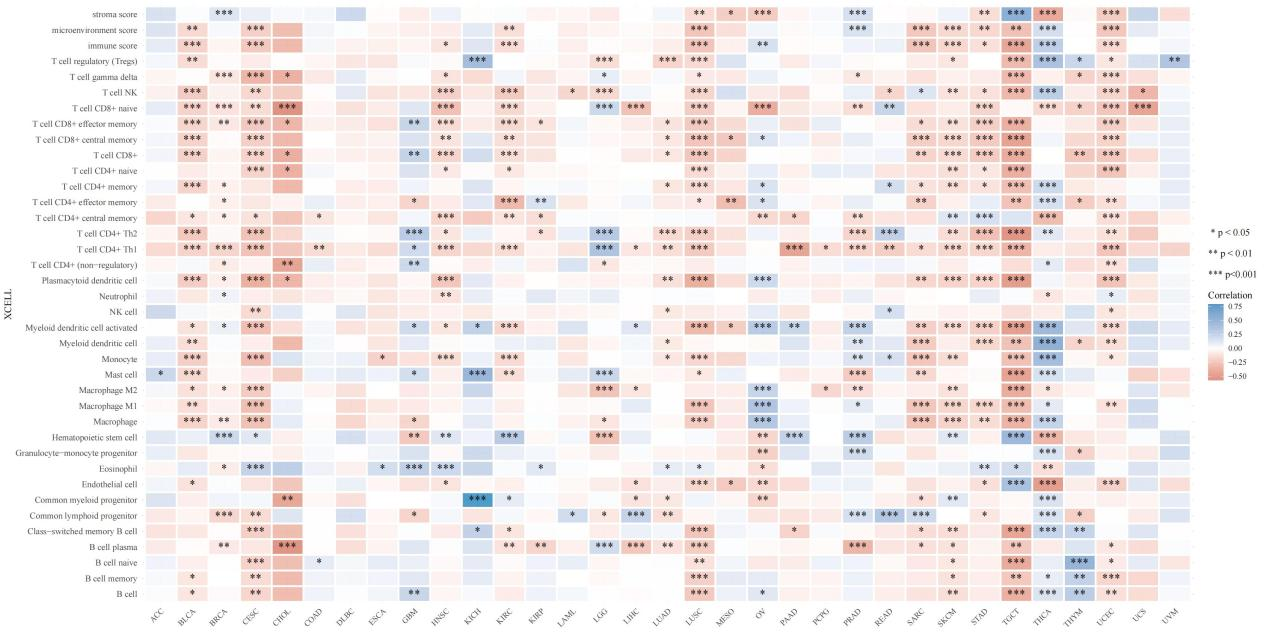
Figure 4. The correlation between VTCN1 expression and 38 immune cell subtypes with infiltration in LGG.
TMB and MSI are two emerging biomarkers associated with immune therapy response. As one of the immune checkpoints, it is necessary to explore the relationship between VTCN1 expression and the two immune regulators TMB and MSI. The results showed that the expression level of VTCN1 was significantly correlated with TMB in LGG (Figure 5).
_HrLRBf3.png)
3.4. Analysis of VTCN1 expression and ferroptosis-related genes
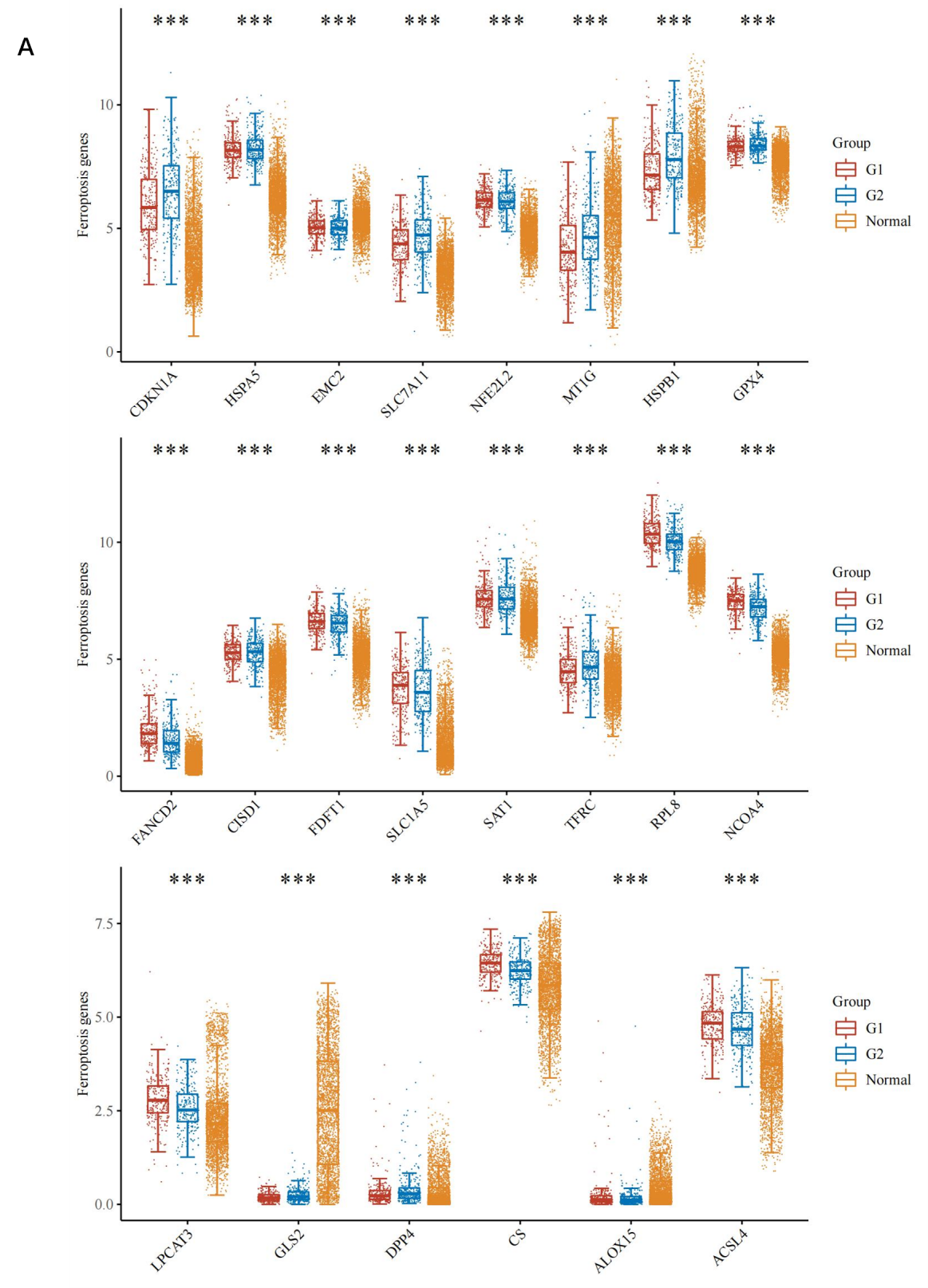
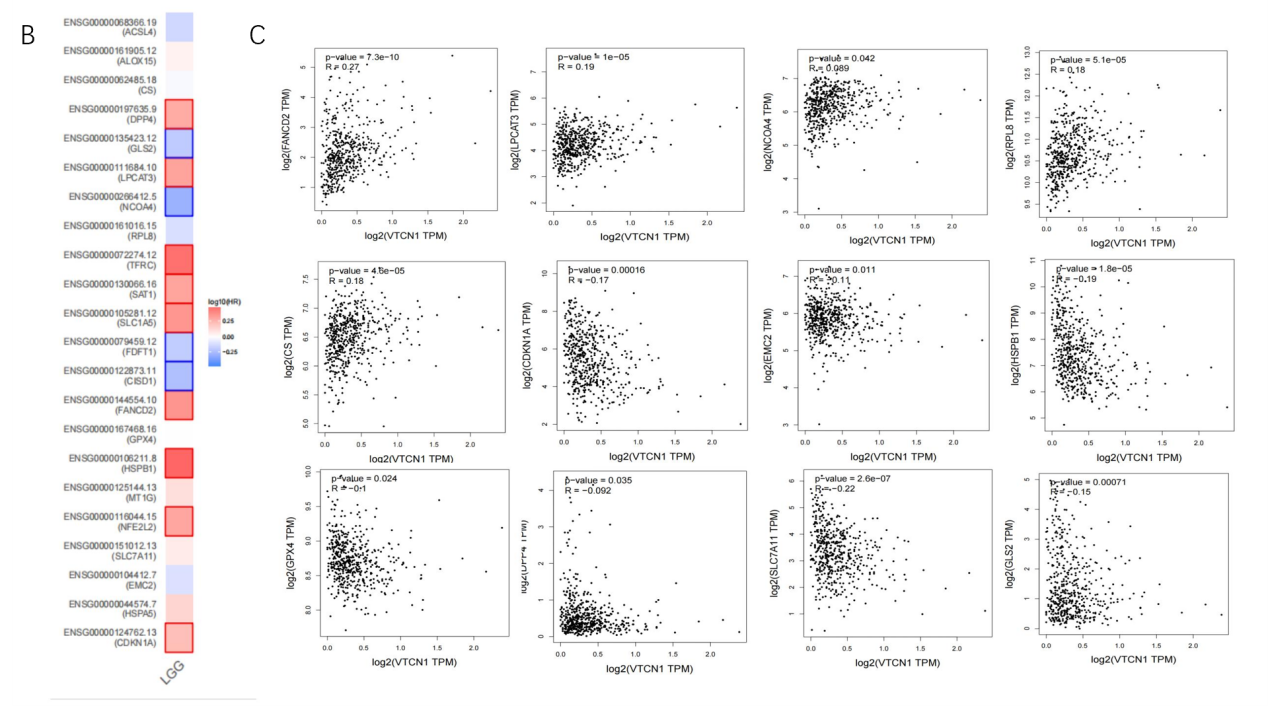
Ferroptosis is associated with various metabolic disorders such as iron metabolism, lipid metabolism, and antioxidant metabolism. To explore whether VTCN1 expression is associated with ferroptosis in LGG, LGG samples were divided into high VTCN1 expression group, low VTCN1 expression group, and combined with the normal group to perform differential analysis on the expression levels of 24 ferroptosis-related genes (Figure 6A). The results showed that 22 genes (CDKN1A, HSPA5, EMC2, SLC7A11, NFE2L2, MT1G, HSPB1, GPX4, FANCD2, CISD1, FDFT1, SLC1A5, SAT1, TFRC, RPL8, NCOA4, LPCAT3, GLS2, DPP4, CS, ALOX15, ACSL4) showed significant differences. Prognostic analysis of these 22 genes identified 4 genes (GLS2, NCOA4, FDFT1, CISD1) that had a positive impact on prognosis (Figure 7A). Finally, combined with correlation analysis, genes positively correlated with VTCN1 were screened out (Figure 7B). The results showed that the NCOA4 gene had significant differences between the high and low VTCN1 expression groups, and high NCOA4 expression was associated with higher overall survival rate of LGG; meanwhile, NCOA4 was positively correlated with VTCN1. In conclusion, we predict that NCOA4 plays a role by influencing ferroptosis in the process of VTCN1 affecting LGG.
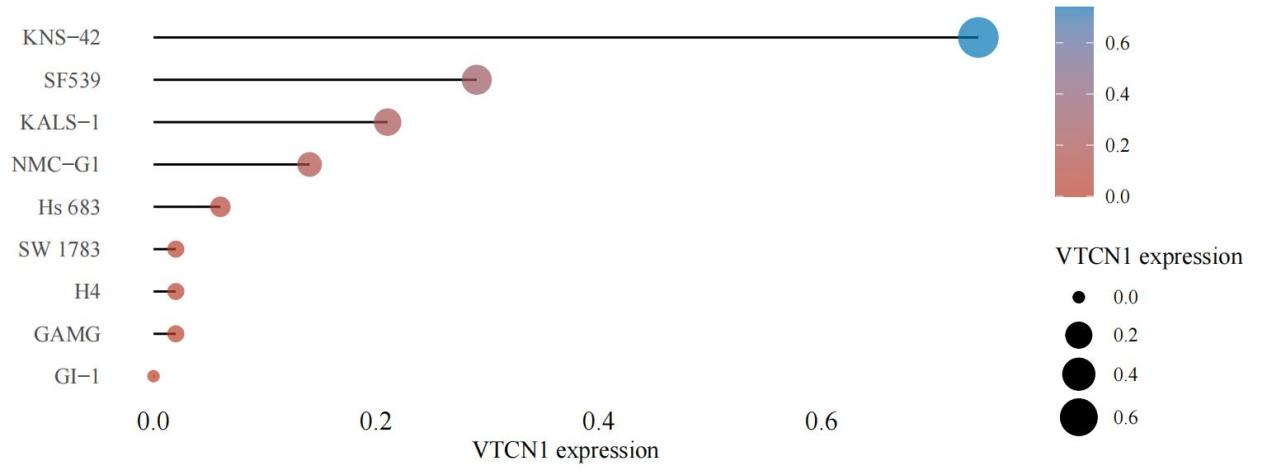
3.5. Expression of VTCN1 in various LGG cell lines
The expression of VTCN1 in various LGG cell lines was predicted based on the CCLE database (Figure 8). VTCN1 expression was the highest in KNS-41 cells, which is suitable for constructing knockout/knockdown models. VTCN1 expression was the lowest in G1-1 cells, which is suitable for constructing VTCN1 overexpression models, providing a direction for further verifying the mechanism by which VTCN1 regulates cancer progression in LGG.
_MCW8Hbt.png)
3.6. Correlation between VTCN1 and various pathways
The correlation between VTCN1 and various pathways in LGG was predicted based on enrichment scores. The results showed that VTCN1 had the most significant positive correlation with MYC_targets and the most significant negative correlation with Reactive Oxygen Species (ROS), providing bioinformatics prediction information for further research on the mechanism.
4. Discussion
As one of the immune checkpoints, VTCN1 is a hot topic in recent tumor immunotherapy. Previous studies have suggested that VTCN1 acts as a risk factor for glioma and provides a target for glioma immunotherapy [14,15]. Due to the low early diagnosis rate, the main research focus has been on advanced high-grade gliomas. Therefore, research on LGG is urgent.
Prognostic analysis revealed that high VTCN1 expression may act as a protective factor affecting the progression of LGG. Expression analysis showed that there was no significant difference in VTCN1 expression between LGG tumor tissues and normal tissues, and there was also no significant difference in VTCN1 expression among different tumor stages, grades, ages, or genders (Supplementary Figure 1), which is partially consistent with previous studies [13,14], but is related to TP53 mutation. The differences in VTCN1 expression and prognosis in LGG have attracted our attention, and this study predicts the potential regulatory mechanisms of high VTCN1 expression in LGG.
After performing batch survival analysis on data from LGG samples with high VTCN1 expression, we predict that TEF and DNAJC12 may be involved in the process by which VTCN1 affects LGG prognosis. TEF is a member of the PAR bZIP family and has anti-apoptotic potential dependent on its DNA-binding activity [16] and the ability to participate in cell cycle control [17]. DNAJC12 is a member of the heat shock protein family, and its role in promoting cancer progression in various types of cancers has been reported [18]; among them, some studies have pointed out that high DNAJC12 expression is a poor prognostic marker for human brain glioma [19], but experimental support is still lacking. The biological functions of DNAJC12 and TEF in the development and metastasis of LGG are still unclear, and their regulatory role with VTCN1 needs further verification.
In addition to the intrinsic effects of tumor cells, the development of malignant brain tumors is partially attributed to the immunosuppressive components of the brain tumor microenvironment [20]. Studies have shown that in LGG, glioma cells spread and invade the surrounding brain tissue and are closely mixed with non-tumor cell types [21]; therefore, it is crucial to identify non-tumor cell changes in LGG. Correlation analysis between VTCN1 expression and immune cell infiltration in LGG showed that high VTCN1 expression was positively correlated with the infiltration of lymphoid progenitors, hematopoietic stem cells, macrophages, M2 macrophages, non-regulatory CD4⁺ T cells, natural killer cells, and regulatory T cells, and was also significantly positively correlated with TMB. As a quantitative biomarker, TMB is used to reflect the total number of mutations carried by tumor cells. Tumor cells with high TMB have higher levels of neoantigens [22], which is believed to help the immune system recognize tumors and stimulate the proliferation of anti-tumor T cells and anti-tumor responses [23]. In conclusion, we predict that the process by which high VTCN1 expression inhibits LGG progression may be related to TMB and the infiltration of relevant immune cells.
Ferroptosis is a recent hot research direction, and it is associated with various metabolic disorders such as iron metabolism, lipid metabolism, and antioxidant metabolism. The connection between high-grade gliomas and iron metabolism has been reported [24]. Some studies have pointed out that transferrin receptor-mediated iron accumulation [25,26] and iron metabolism-related genes are involved in pathological processes of glioma such as proliferation, metastasis, epithelial-mesenchymal transition, necrosis, autophagy, apoptosis, P53 degradation, cytotoxicity caused by reactive oxygen species, and chemotactic monocyte recruitment [24]. At present, the relationship between LGG and ferroptosis has not been extensively studied, and its molecular mechanism still needs further exploration. Whether ferroptosis-related indicators can be used to assist in the diagnosis of LGG and whether ferroptosis can be targeted to guide LGG treatment have not yet reached a clear conclusion. Through the analysis of VTCN1 expression and ferroptosis-related genes in this study, we predict that nuclear receptor coactivator 4 (NCOA4), which is positively correlated with VTCN1, may be involved in regulating the effect of VTCN1 on LGG prognosis. NCOA4 mediates the selective autophagic degradation of ferritin (an intracellular iron storage complex), thereby playing a key role in intracellular and systemic iron homeostasis [27]. NCOA4 depletion inhibits the delivery of ferritin to lysosomes, leading to impaired ferritin degradation; the reduction in ferritin turnover results in a significant decrease in intracellular iron, thereby altering intracellular iron homeostasis [28]. We predict that high VTCN1 expression induces high NCOA4 expression, maintaining intracellular and systemic iron homeostasis, which provides a new direction for studying the role of ferroptosis in LGG.
5. Conclusion
Based on bioinformatics methods, this study infers that VTCN1 has certain reference value in the development and research of LGG prognostic biomarkers. High VTCN1 expression affects the cell cycle, apoptosis, and oxidative stress through DNAJC12 and TEF, and influences iron homeostasis through NCOA4, thereby participating in the occurrence and development of LGG. In addition, high tumor mutational burden and the infiltration of relevant immune cells may also play important roles.
References
[1]. Zhang, W. J., Liu, Q. R., Zhang, M., et al. (2017). Research progress on DNA damage repair and glioma resistance to temozolomide.West China Journal of Pharmaceutical Sciences,32(5), 555-558. https: //doi.org/10.13375/j.cnki.wcjps.2017.05.034
[2]. Ma, J. X., Wu, P. F., Yang, W., et al. (2018). Study on macrophage infiltration in prognostic prediction of low-grade glioma.Chinese Journal of Cells and Stem Cells (Electronic Edition), 8(4), 204-211.
[3]. Ma, X. T., & Guo, Z. H. (2021). Functions of co-signaling molecules and their applications in tumor therapy.Chinese Journal of Cancer Biotherapy, 28(12), 1219-1226.
[4]. Yue, C. H., Yang, M., Jiang, J. T., et al. (2016). Mechanism and application progress of co-stimulatory molecule B7-H4 in tumor immunotherapy.Chinese Journal of Clinical Laboratory Science, 34(11), 850-853. https: //doi.org/10.13602/j.cnki.jcls.2016.11.15
[5]. Tang, J., Ma, X., Chen, X., et al. (2022). Strengthening of antitumor effects in breast cancer from a novel B7-H4- and CD3-targeting bispecific antibody by an oncolytic virus.Annals of Translational Medicine, 10(14), 805. https: //doi.org/10.21037/atm-22-3423
[6]. Song, X., Zhou, Z., Li, H., et al. (2020). Pharmacologic Suppression of B7-H4 Glycosylation Restores Antitumor Immunity in Immune-Cold Breast Cancers.Cancer Discovery, 10(12), 1872-1893. https: //doi.org/10.1158/2159-8290.CD-20-0402
[7]. Kim, N. I., Park, M. H., Kweon, S. S., et al. (2020). B7-H3 and B7-H4 Expression in Breast Cancer and Their Association with Clinicopathological Variables and T Cell Infiltration.Pathobiology, 87(3), 179-192. https: //doi.org/10.1159/000505756
[8]. Kim, N. I., Park, M. H., Kweon, S. S., et al. (2022). [Retraction] The Coexpression and Clinical Significance of Costimulatory Molecules B7-H1, B7-H3, and B7-H4 in Human Pancreatic Cancer.OncoTargets and Therapy, 15, 699-700. https: //doi.org/10.1159/000505756
[9]. Yang, J., Tian, Z., Gao, H., et al. (2022). Clinical significance and correlation of PD-L1, B7-H3, B7-H4, and TILs in pancreatic cancer.BMC Cancer, 22(1), 584. https: //doi.org/10.1186/s12885-022-09639-5
[10]. Zhu, Y., Chen, J., Liu, Y., et al. (2022). Prognostic values of B7-H3, B7-H4, and HHLA2 expression in human pancreatic cancer tissues based on mIHC and spatial distribution analysis.Pathology Research and Practice, 234, 153911. https: //doi.org/10.1016/j.prp.2022.153911
[11]. Dong, L., Gu, Y., Zhou, Y., et al. (2022). Expression of B7 family checkpoint proteins in cervical cancer.Modern Pathology, 35(6), 786-793. https: //doi.org/10.1038/s41379-021-00979-4
[12]. Chen, L., Dong, J., Li, Z., et al. (2022). The B7H4-PDL1 classifier stratifies immuno-phenotype in cervical cancer.Cancer Cell International, 22(1), 3. https: //doi.org/10.1186/s12935-021-02423-8
[13]. Yao, Y., Ye, H. X., & Zhou, L. F. (2011). Research progress on B7 family negative co-stimulatory molecules and glioma.National Medical Journal of China, (43), 3093-3095.
[14]. Liu, Y. T. (2014). Expressions of PDCD4 and B7-H4 in pediatric optic nerve glioma and their clinical significance.International Eye Science,14(8), 1391-1393.
[15]. Chen, Y., Li, Z. Y., Zhang, X. H., et al. (2013). Expression of B7-H4 in human glioma tissues and its clinical significance.Chinese Journal of Pathophysiology, 29(5), 810-814.
[16]. Inukai, T., Inaba, T., Dang, J., et al. (2005). TEF, an antiapoptotic bZIP transcription factor related to the oncogenic E2A-HLF chimera, inhibits cell growth by down-regulating expression of the common beta chain of cytokine receptors.Blood, 105(11), 4437-4444. https: //doi.org/10.1182/blood-2004-08-2976
[17]. Saleh, A. A., Gohar, S. F., Hemida, A. S., et al. (2020). Evaluation of ASPM and TEF Gene Expressions as Potential Biomarkers for Bladder Cancer.Biochemical Genetics, 58(3), 490-507. https: //doi.org/10.1007/s10528-020-09962-1
[18]. Li, Y., Li, M., Jin, F., et al. (2021). DNAJC12 promotes lung cancer growth by regulating the activation of βcatenin.International Journal of Molecular Medicine, 47(6), 105. https: //doi.org/10.3892/ijmm.2021.4938
[19]. Sun, H., Zou, H. Y., Cai, X. Y., et al. (2020). Network Analyses of the Differential Expression of Heat Shock Proteins in Glioma.DNA and Cell Biology, 39(7), 1228-1242. https: //doi.org/10.1089/dna.2020.5425
[20]. Klemm, F., Maas, R. R., Bowman, R. L., et al. (2020). Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells.Cell, 181(7), 1643-1660.e17. https: //doi.org/10.1016/j.cell.2020.05.007
[21]. Torres, D., & Canoll, P. (2019). Alterations in the Brain Microenvironment in Diffusely Infiltrating Low-Grade Glioma.Neurosurgical Clinics of North America,30(1), 27-34. https: //doi.org/10.1016/j.nec.2018.08.001
[22]. Shi, S. S., Li, X., Ding, Y., et al. (2022). Roles of tumor mutational burden and tumor-infiltrating immune cells in prognosis and progression of colorectal cancer.Journal of Clinical and Pathological Research, 42(7), 1543-1551.
[23]. Li, X., Hu, Y., & Zhang, Q. Y. (2021). Research progress of tumor mutational burden as an emerging biomarker for breast cancer immunotherapy.Journal of Practical Oncology, 35(6), 568-571.
[24]. Xu, S., Wang, Z., Ye, J., Mei, S., & Zhang, J. (2021). Identification of Iron Metabolism-Related Genes as Prognostic Indicators for Lower-Grade Glioma.Frontiers in Oncology, 11, 729103.
[25]. Chirasani, S. R., Markovic, D. S., Synowitz, M., Eichler, S. A., Wisniewski, P., Kaminska, B., Otto, A., Wanker, E., Schäfer, M., Chiarugi, P., Meier, J. C., Kettenmann, H., & Glass, R. (2009). Transferrin-receptor-mediated iron accumulation controls proliferation and glutamate release in glioma cells.Journal of Molecular Medicine (Berlin), 87(2), 153-167.
[26]. Rosager, A. M., Sørensen, M. D., Dahlrot, R. H., Hansen, S., Schonberg, D. L., Rich, J. N., Lathia, J. D., & Kristensen, B. W. (2017). Transferrin receptor-1 and ferritin heavy and light chains in astrocytic brain tumors: Expression and prognostic value.PLOS ONE, 12(8), e0182954.
[27]. Quiles Del Rey, M., & Mancias, J. D. (2019). NCOA4-Mediated Ferritinophagy: A Potential Link to Neurodegeneration.Frontiers in Neuroscience, 13, 238.
[28]. Santana-Codina, N., & Mancias, J. D. (2018). The Role of NCOA4-Mediated Ferritinophagy in Health and Disease.Pharmaceuticals (Basel), 11(4), 114.
Cite this article
Fan,W.;Han,X.;Liu,H. (2025). Bioinformatics analysis of VTCN1 in brain low-grade glioma. Journal of Clinical Technology and Theory,3(3),41-51.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Clinical Technology and Theory
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zhang, W. J., Liu, Q. R., Zhang, M., et al. (2017). Research progress on DNA damage repair and glioma resistance to temozolomide.West China Journal of Pharmaceutical Sciences,32(5), 555-558. https: //doi.org/10.13375/j.cnki.wcjps.2017.05.034
[2]. Ma, J. X., Wu, P. F., Yang, W., et al. (2018). Study on macrophage infiltration in prognostic prediction of low-grade glioma.Chinese Journal of Cells and Stem Cells (Electronic Edition), 8(4), 204-211.
[3]. Ma, X. T., & Guo, Z. H. (2021). Functions of co-signaling molecules and their applications in tumor therapy.Chinese Journal of Cancer Biotherapy, 28(12), 1219-1226.
[4]. Yue, C. H., Yang, M., Jiang, J. T., et al. (2016). Mechanism and application progress of co-stimulatory molecule B7-H4 in tumor immunotherapy.Chinese Journal of Clinical Laboratory Science, 34(11), 850-853. https: //doi.org/10.13602/j.cnki.jcls.2016.11.15
[5]. Tang, J., Ma, X., Chen, X., et al. (2022). Strengthening of antitumor effects in breast cancer from a novel B7-H4- and CD3-targeting bispecific antibody by an oncolytic virus.Annals of Translational Medicine, 10(14), 805. https: //doi.org/10.21037/atm-22-3423
[6]. Song, X., Zhou, Z., Li, H., et al. (2020). Pharmacologic Suppression of B7-H4 Glycosylation Restores Antitumor Immunity in Immune-Cold Breast Cancers.Cancer Discovery, 10(12), 1872-1893. https: //doi.org/10.1158/2159-8290.CD-20-0402
[7]. Kim, N. I., Park, M. H., Kweon, S. S., et al. (2020). B7-H3 and B7-H4 Expression in Breast Cancer and Their Association with Clinicopathological Variables and T Cell Infiltration.Pathobiology, 87(3), 179-192. https: //doi.org/10.1159/000505756
[8]. Kim, N. I., Park, M. H., Kweon, S. S., et al. (2022). [Retraction] The Coexpression and Clinical Significance of Costimulatory Molecules B7-H1, B7-H3, and B7-H4 in Human Pancreatic Cancer.OncoTargets and Therapy, 15, 699-700. https: //doi.org/10.1159/000505756
[9]. Yang, J., Tian, Z., Gao, H., et al. (2022). Clinical significance and correlation of PD-L1, B7-H3, B7-H4, and TILs in pancreatic cancer.BMC Cancer, 22(1), 584. https: //doi.org/10.1186/s12885-022-09639-5
[10]. Zhu, Y., Chen, J., Liu, Y., et al. (2022). Prognostic values of B7-H3, B7-H4, and HHLA2 expression in human pancreatic cancer tissues based on mIHC and spatial distribution analysis.Pathology Research and Practice, 234, 153911. https: //doi.org/10.1016/j.prp.2022.153911
[11]. Dong, L., Gu, Y., Zhou, Y., et al. (2022). Expression of B7 family checkpoint proteins in cervical cancer.Modern Pathology, 35(6), 786-793. https: //doi.org/10.1038/s41379-021-00979-4
[12]. Chen, L., Dong, J., Li, Z., et al. (2022). The B7H4-PDL1 classifier stratifies immuno-phenotype in cervical cancer.Cancer Cell International, 22(1), 3. https: //doi.org/10.1186/s12935-021-02423-8
[13]. Yao, Y., Ye, H. X., & Zhou, L. F. (2011). Research progress on B7 family negative co-stimulatory molecules and glioma.National Medical Journal of China, (43), 3093-3095.
[14]. Liu, Y. T. (2014). Expressions of PDCD4 and B7-H4 in pediatric optic nerve glioma and their clinical significance.International Eye Science,14(8), 1391-1393.
[15]. Chen, Y., Li, Z. Y., Zhang, X. H., et al. (2013). Expression of B7-H4 in human glioma tissues and its clinical significance.Chinese Journal of Pathophysiology, 29(5), 810-814.
[16]. Inukai, T., Inaba, T., Dang, J., et al. (2005). TEF, an antiapoptotic bZIP transcription factor related to the oncogenic E2A-HLF chimera, inhibits cell growth by down-regulating expression of the common beta chain of cytokine receptors.Blood, 105(11), 4437-4444. https: //doi.org/10.1182/blood-2004-08-2976
[17]. Saleh, A. A., Gohar, S. F., Hemida, A. S., et al. (2020). Evaluation of ASPM and TEF Gene Expressions as Potential Biomarkers for Bladder Cancer.Biochemical Genetics, 58(3), 490-507. https: //doi.org/10.1007/s10528-020-09962-1
[18]. Li, Y., Li, M., Jin, F., et al. (2021). DNAJC12 promotes lung cancer growth by regulating the activation of βcatenin.International Journal of Molecular Medicine, 47(6), 105. https: //doi.org/10.3892/ijmm.2021.4938
[19]. Sun, H., Zou, H. Y., Cai, X. Y., et al. (2020). Network Analyses of the Differential Expression of Heat Shock Proteins in Glioma.DNA and Cell Biology, 39(7), 1228-1242. https: //doi.org/10.1089/dna.2020.5425
[20]. Klemm, F., Maas, R. R., Bowman, R. L., et al. (2020). Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells.Cell, 181(7), 1643-1660.e17. https: //doi.org/10.1016/j.cell.2020.05.007
[21]. Torres, D., & Canoll, P. (2019). Alterations in the Brain Microenvironment in Diffusely Infiltrating Low-Grade Glioma.Neurosurgical Clinics of North America,30(1), 27-34. https: //doi.org/10.1016/j.nec.2018.08.001
[22]. Shi, S. S., Li, X., Ding, Y., et al. (2022). Roles of tumor mutational burden and tumor-infiltrating immune cells in prognosis and progression of colorectal cancer.Journal of Clinical and Pathological Research, 42(7), 1543-1551.
[23]. Li, X., Hu, Y., & Zhang, Q. Y. (2021). Research progress of tumor mutational burden as an emerging biomarker for breast cancer immunotherapy.Journal of Practical Oncology, 35(6), 568-571.
[24]. Xu, S., Wang, Z., Ye, J., Mei, S., & Zhang, J. (2021). Identification of Iron Metabolism-Related Genes as Prognostic Indicators for Lower-Grade Glioma.Frontiers in Oncology, 11, 729103.
[25]. Chirasani, S. R., Markovic, D. S., Synowitz, M., Eichler, S. A., Wisniewski, P., Kaminska, B., Otto, A., Wanker, E., Schäfer, M., Chiarugi, P., Meier, J. C., Kettenmann, H., & Glass, R. (2009). Transferrin-receptor-mediated iron accumulation controls proliferation and glutamate release in glioma cells.Journal of Molecular Medicine (Berlin), 87(2), 153-167.
[26]. Rosager, A. M., Sørensen, M. D., Dahlrot, R. H., Hansen, S., Schonberg, D. L., Rich, J. N., Lathia, J. D., & Kristensen, B. W. (2017). Transferrin receptor-1 and ferritin heavy and light chains in astrocytic brain tumors: Expression and prognostic value.PLOS ONE, 12(8), e0182954.
[27]. Quiles Del Rey, M., & Mancias, J. D. (2019). NCOA4-Mediated Ferritinophagy: A Potential Link to Neurodegeneration.Frontiers in Neuroscience, 13, 238.
[28]. Santana-Codina, N., & Mancias, J. D. (2018). The Role of NCOA4-Mediated Ferritinophagy in Health and Disease.Pharmaceuticals (Basel), 11(4), 114.









