1. Introduction
Waterbody refers to the general term for surface water, groundwater, and the sediment and aquatic organisms it contains. Water is one of the main substances for human survival. On the one hand, water consumption is rising as the global population continues to rise and industry and agriculture develop quickly. On the other side, there are big problems with pollution control. The most often found substances among them include pharmaceutically active compounds (PHACs), personal care products (PCPs), endocrine-disrupting chemicals (EDCs), artificial sweepers (ASWs), etc. These substances are frequently referred to as emerging contaminants (ECs) due to the dearth of toxicological data and strong theoretical backing [1]. ECs in sewage are usually since the sewage treatment process of sewage plants can only remove specific pollutants, while emerging contaminants cannot be removed due to their low concentration. ECs are typically released into surface water and eventually enter sediments, soil, groundwater, and oceans [2]. BDue to their hydrophobicity, ECs can disrupt both human and animal endocrine systems and transmit antimicrobial resistance by bioaccumulating in lipid-rich tissues of organisms. Endocrine disrupting chemicals (ECs) have been discovered as being responsible for endometriosis, prostate, testicular, and breast cancers as well as serious problems with both human and animal reproductive health. They have decreased the quantity of human sperm, caused the development of fragile eggs, and damaged aquatic wildlife's immune systems [3]. For instance, the common plasticizer Bisphenol A (BPA) may interfere with the endocrine system. It explains a number of endocrine problems in many systems, including changes in the thyroid gland, male reproductive system, and salivary glands. BPA also harms plant growth, cell division, and the nervous system [4]. Based on the above hazards, the removal technology of emerging contaminants is particularly important. Currently, the methods used mainly include membrane technology, advanced oxidation process, and adsorption technology using various adsorbents, including activated carbon, modified Biochar, carbon nanotubes, and Graphene [5]. However, many issues have not been resolved in the research process of new pollutants in wastewater. For example, there is a lack of primary data on the toxicity and other properties of new pollutants in sewage, the migration and transformation process of new pollutants in the natural environment and sewage treatment system, and the removal mechanism and effect of new pollutants are unclear. The existing problems have brought significant challenges to controlling new pollutants. Therefore, further research on new pollutants in wastewater is fundamental.
2. Information on emerging contaminants
2.1. Emerging contaminants
"True or truly new" emerging contaminants, new compounds, or molecules that were either previously unknown or recently emerged in scientific literature are all examples of what the term "emerging contaminants" can be used to describe. Ii. newly discovered contaminants that pose a threat to the environment even when they are known to exist. Iii. In cases when new information makes it difficult for us to grasp the threats that such legacy pollutants pose to the environment and human health, we also want to address such issues [6]. Emerging contaminants are chemicals and microbes created by people from natural or manufactured sources. They frequently have negative effects on ecological and human health once they are released into the environment. These pollutants are present daily in personal care products, drugs, detergents, surfactants, insecticides, etc. Until the 1990s, most of these pollutants were not identified by environmental monitoring projects because their production and use unintentionally had harmful effects on the environment and organisms. However, these pollutants themselves have problems, and their metabolic/degradation products also have problems. There are many emerging contaminants, including household and industrial chemicals, hospital drugs, and agricultural chemicals. All these compounds are persistent in the environment (water, soil, sediment), can be widely spread, easily Bioaccumulation, and toxic [7].
2.2. Classification
ECs aren't always recently created compounds. Three main categories are covered by this word. Compounds that have recently entered the environment, such industrial additives, fall under the first category. The second kind is made up of substances that may have been present in the environment for a very long time in the past, but whose existence was only recently recognised and whose significance only just started to attract people's attention (such as narcotics). The third category consists of substances (like hormones) that have been around for a while but may have had negative impacts on people and the environment [8].
The classification of emerging contaminants are as follows [7]:
Table 1. The classification of emerging contaminants.
Categories | Content | ||
Drugs | Illegal and prescription drugs | ||
Personal care products | Cosmetics, surfactants, disinfectants, household fungicides, food additives | ||
Industrial chemicals | Food additives, pesticides, polychlorinated biphenyls, flame retardants, antibacterial substances | ||
Disinfection products | From water treatment plant: Nitrosamine, halogenated Nitromethane, halo acetonitrile, Trihalomethane, Haloacetic acids | ||
Algal toxin | Toxicity released by some algae: cyanobacterium toxin, microcystin | ||
Fungicides and their metabolites | Plant and agricultural preventive agents [insecticides] | ||
Bioterrorism and sabotage devices | Biological and chemical weapons | ||
2.3. Source and pathway
As mentioned earlier, the main sources of emerging contaminants are wastewater discharged from factories, domestic wastewater, medical wastewater, and wastewater generated from agricultural production activities(As shown in Figure 1). These wastes generated due to people's production and daily life are unavoidable, and the composition of wastewater generated from different sources is different.
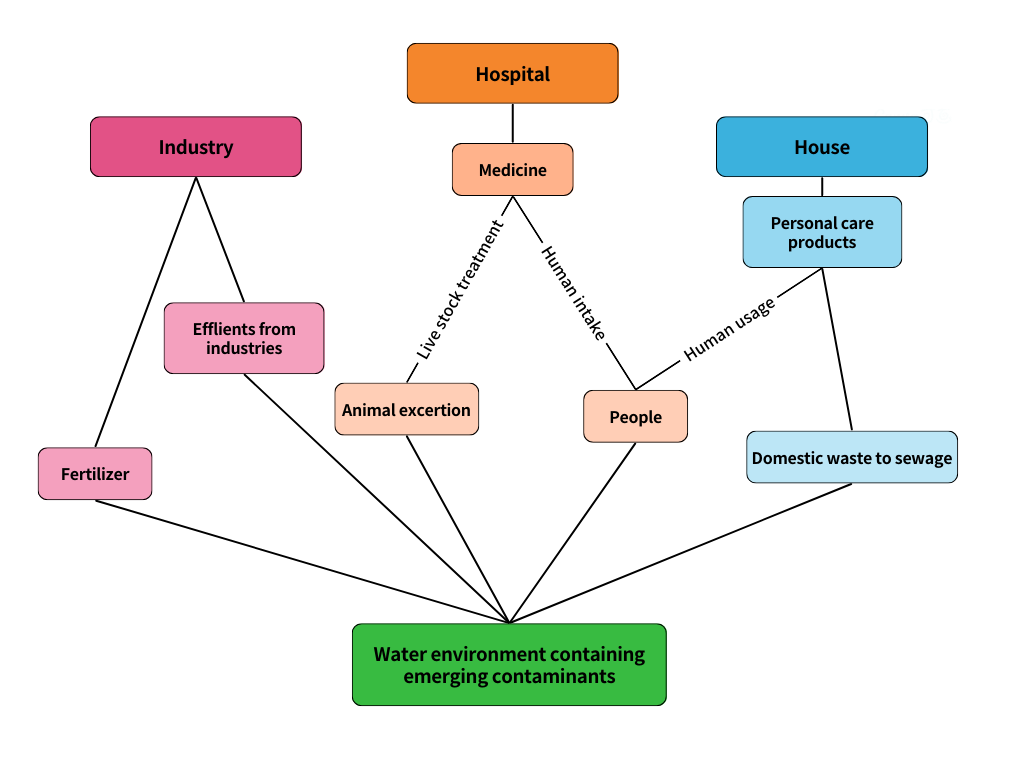
Figure 1. Source and pathway of ECs.
2.3.1. Industry. Industrial production is a major source of sewage generation, and the pollution generated by different types of manufacturing and processing industries has its specificity. For example, the pharmaceutical industry manufactures, extracts, processes, purifies, and packages chemical and biological materials into solid and liquid forms for use as drugs for humans and animals. Drug synthesis and formulation wastewater is frequently produced by the pharmaceutical sector. The majority of raw materials used in global distribution are produced through chemical synthesis using biological, inorganic, and organic reactions. The amount of wastewater produced will rise as a result of the reactors and separators used in the multi-product pharmaceutical sector frequently being large or inefficient and not being designed based on capacity. Chemical synthesis, fermentation, and natural or biological extraction are the three divisions of pharmaceutical engineering. Because of this, the wastewater produced by the pharmaceutical industry primarily consists of a lot of volatile solvents, a lot of unused raw materials like nutrient solutions, metal salts, starch, nitrates, and phosphates, as well as a lot of metal and halogen impurities [9].
2.3.2. Domestic sewage. Common domestic wastewater has the most varied contaminant makeup, though. These wastewaters contain phenol, acetone, benzene, petroleum products, nitrogen compounds, various resin acids (such as bitter acid), phenolic compounds typically found in the paper industry, even low concentrations of heavy metals, and corrosive Mineral acid, which are substances typically found in the wastewater of the petroleum industry. Sulphide compounds and organic compounds are the main contaminants in home wastewater. It should be noted that several wastewater treatment methods (such as chemical oxidation, distillation, etc.) include heating and evaporating liquids. Numerous theoretical and experimental studies have demonstrated that variations in the concentration of contaminants (chemical and mechanical) in water may considerably alter the nature and intensity of heat and mass transfer, impacting the effectiveness of wastewater treatment [10].
2.3.3. Agricultural wastewater. Pesticide residue is the primary cause of contamination in agricultural production, and numerous studies have shown that pesticides are dangerous to both individuals and the environment. Before combining with household wastewater, pesticide production effluent must undergo extensive treatment. As a result of the high concentration and tenacity of water sources, research into pesticide treatment in water sources is essential. Changes in influent composition, pesticides' physical makeup, and pesticide-contaminated water's pH range from highly acidic (0.5) to very alkaline (14) present obstacles for pesticide treatment in water.The literature also states that the biological oxygen demand (BOD) and chemical oxygen demand (COD) of effluent from pesticide manufacture range from 30 to 11590 mg/L and 150 to 33750 mg/L, respectively. Pesticide concentrations in different water sources range from 0.1 to 107 mg/L [11].
3. Health hazards
We are aware that the main sources of emerging contaminants (ECs) are drug residues, flame retardants, insecticides, sunscreens, disinfection by-products, sediments, organic carbon, pathogens, metals, and surfactants. As shown in Figure 2, ECs have the potential to enter the environment and have a negative impact on human health or other biological processes [12].
Pesticides have been linked to a number of illnesses, including cancer, poor reproductive results, peripheral neuropathy, neurobehavioral disorders, weakened immune systems, and allergic reactions, particularly skin allergies. The threshold of acute poisoning of these pesticides was slowed down by the cumulative suppression of cholinesterase activity caused by long-term and low-dose exposure to organophosphorus chemistry. Dehydration and malnutrition also lower the toxicity threshold of these insecticides. In some occupational groups exposed to certain pesticides, such as farmers or pesticide manufacturers, epidemiological studies have shown an increased risk of lung cancer, lymphatic cancer, and possibly other forms of cancer. In addition, ingestion of certain insecticides may lead to cancer risk [13].
Specific components of personal care products (PCPs) are also associated with various health issues, such as cancer, kidney and kidney damage, nephrotic syndrome or impaired kidney function, and fetal nerve, kidney, and skin damage. The biological buildup of these metals in the human body can be changed by physiological changes. Exogenous chronopathy, damage to wounds during healing and dehiscence, fish flavour syndrome, nephrosis, steroid addiction syndrome, susceptibility to infection, Corticosteroid broad-spectrum skin, and endocrine complications, including inhibition of the hypothalamus pituitary adrenal axis, are some side effects connected to some PCP components, particularly skin-whitening components [14].
One of the most widely used plasticizers in the world is bisphenol A. It causes changes to the thyroid, male genetic system, and many other endocrine diseases in many different systems. In addition to having harmful effects on hormones, bisphenol A raises the incidence of breast cancer in people and, as an antiandrogen, results in male feminization [15].
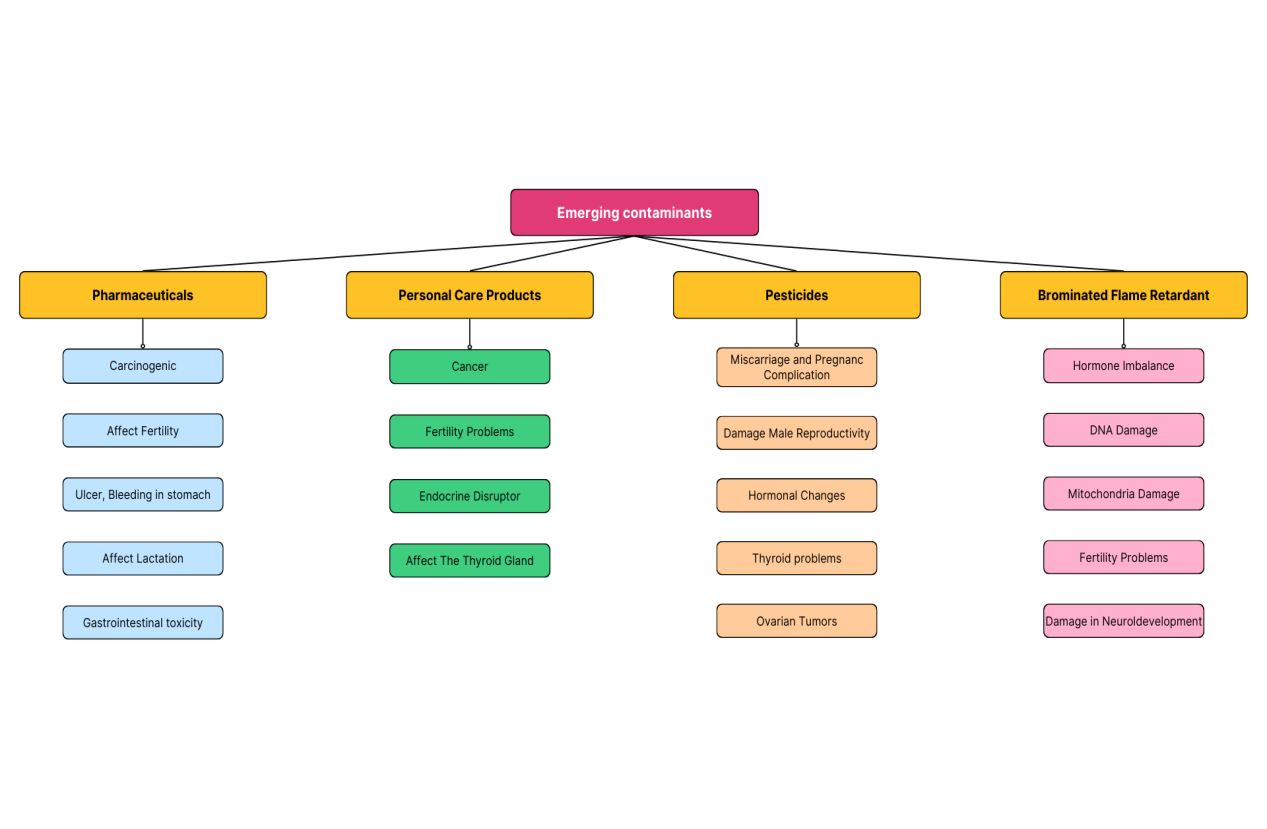
Figure 2. The impact of emerging contaminants on humans [15].
4. Removal process method
4.1. Adsorption process
The advanced sewage treatment methods mainly include reverse osmosis, diffusion, filtration, and dilution [16], among which adsorption is one of the most suitable methods for removing organic and inorganic pollutants. Adsorption refers to the attachment of molecules of a gas, liquid, or dissolved solid to a surface. This term also refers to treating waste where activated carbon removes organic compounds from wastewater.
4.1.1. Activated carbon adsorption (AC). When molecules are exposed to activated carbon, they adhere to one another by cohesion, which ranges from strong valence bonds to weak van der Waals attraction. Specific fluid molecules can be captured when they come into contact with the surface thanks to the attraction forces that are satisfied in the interior molecules of the solid phase. The adsorption of wastewater constituents on carbon is based on the Van der Waals force, and carbon has been activated to maximise the interface accumulation of liquid constituents on the solid interface surface [17]. In order to remove ethinylestradiol (EE2), for instance, Rovani et al. prepared various forms of activated carbon (AC) from a variety of agricultural wastes, including coffee waste, eucalyptus sawdust, soybean oil, and lime chemical activation. These industrial and agricultural wastes are pyrolyzed at 800 °C to remove the endocrine disruptors 17-- Ethinylestradiol (EE2) and 17--Estradiol (E2). The pH range of 2.0 to 11.0 is ideal for the first adsorption. The temperature of 25 ° C was adequate for the Sips Contour line#Temperature and associated issues model. [18] Granular activated carbon is the most effective adsorbent, removing 7–7% (initial concentration is 184mg/L) in the pH range of 80–96.6, according to a separate study by Wasay et al [19].
4.1.2. Modified biochar (MBC). Unlike traditional charcoal, which is commonly used for fuel, it is a charcoal used as an agricultural soil conditioner and for carbon collection and storage. It is used in agriculture to help plant growth but does not affect soil granulation [20]. The application of Biochar is very diverse, from thermoelectric production, flue gas purification, metallurgical application, agriculture and animal husbandry, and building materials to medical use. In order to reduce greenhouse gas emissions, it has become increasingly popular in some applications as a substitute for fossil carbon carriers in the past few years [21].
For instance, Yao et al. investigated the adsorption of sulfamethoxazole by Biochar using four commonly used materials: bamboo (BB), Brazilian pepperwood (BP), bagasse (BG), and hickory (HW). The outcomes demonstrated that each Biochar sample tested had some capacity to eliminate SMX that was present in water. The mobility and bioavailability of SMX in Biochar enhanced soil are shown to be lower than those in unmodified soil. The range of Biochar's solid water distribution coefficient (Kd) is 2-104 L/kg. Soil improvement with biochar should be encouraged in locations that receive irrigation from recycled or wastewater [22].
4.1.3. Carbon nanotubes. Carbon nanotubes (CNTs) are carbon allotropes with a structure akin to graphite. Depending on the degree of bending, how the original sheet was formed, the diameter, internal geometry, physical and chemical properties, and the synthesis process used, different adsorption characteristics are displayed [23]. Even when the same contaminants are present, the surface area of carbon nanotubes typically relies on the presence of single-wall or multi-wall structures, which may result in varying removal rates. Special adsorbents that can extract medicines and antibiotics from water are on the horizon thanks to carbon nanotubes [24]. Ji et al. investigated the role of aqueous solution chemistry in the tetracycline adsorption process on carbon nanotubes, and the findings indicated that it was crucial to the adsorption process.
This difference can be due to the accessibility of adsorption sites of Humic acid which is controlled by the porosity of adsorbent. Dissolved Humic acid greatly inhibited the adsorption of tetracycline on MWNT but had no effect on the adsorption of tetracycline on SWNT. Additionally, this study suggests that unintentional or accidental release of carbon nanotubes into the environment may have a major impact on the bioavailability and toxicity of antibiotic drugs with high adsorption affinity.
4.2. Membrane bioreactor(MBR)
A membrane bioreactor (MBR) combines biological wastewater treatment procedures (activated sludge process) with membrane processes like microfiltration or ultrafiltration. In the treatment of municipal and industrial wastewater, it is currently widely employed. The submerged membrane bioreactor (SMBR) and the side flow membrane bioreactor are the two fundamental MBR variants. The membrane is positioned inside the bioreactor and submerged in the wastewater in the SMBR setup. In contrast, the membrane is situated outside the reactor in a side flow membrane bioreactor as an additional step following biological treatment [25]. The feasibility of using a membrane bioreactor to treat commercial organophosphorus pesticides and their active ingredients was examined by Avik J et al. using five pesticides as raw materials: Wilson Malathion 50 EC Liquid Insectide, Lorsban-4E Insectide, Sniper Azinphos metal Insectide, Diazinon 500 E Insectide, and Thimet 15-G Soil&Systemic Granular Insectide. In the first two days following the introduction of specifications, the C e/C i of azinphos methyl increased significantly to slightly above 50% before progressively declining to a baseline level of 0.82 mg/L. This response showed an 83% removal efficiency for azophos methyl once the MBR system stabilised. The outcomes demonstrate that organophosphorus insecticides can be effectively biodegraded in a membrane bioreactor [26].
4.3. Advanced oxidation processes
Advanced oxidation processes (AOPs) are a group of chemical processes used to treat wastewater and water in order to remove organic (and occasionally inorganic) contaminants by oxidation in response to hydroxyl radicals (OH). However, the word typically refers more precisely to a subset of chemical methods that make use of ozone (O3), hydrogen peroxide (H2O2), and/or ultraviolet radiation in practical wastewater treatment applications. The term "in situ chemical oxidation" refers to one sort of technique. Hanadi et al. investigated the removal of heavy metal ions using advanced oxidation (UV/H2O2) technology. The methylene blue dye was photolyzed using an advanced oxidation technique (UV/H2O2). The outcomes demonstrated that mixing UV light and hydrogen peroxide might considerably shorten the time it took for MB dye to completely fade [27].
Nishiwak et al. looked at the use of sophisticated oxidation techniques (Fenton) to remove heavy metals (lead) from aqueous solutions. The findings demonstrated that the highest lead removal % occurred at 30 mg/l. The Fenton method for removing heavy metals such as lead is influenced by variables such as oxidant concentration, catalyst, pollutant concentration, pH value, and reaction duration. At a concentration of 30 mg/l, the lead removal rate is approximately 90%. As a result, lead can be successfully removed from aqueous solutions using Fenton oxidation technique when the pH is low [28].
5. Conclusion
Pharmaceuticals, personal care items, pesticides, industrial chemicals, and microplastics are just a few of the numerous compounds that have been identified as emerging pollutants in water, soil, and the atmosphere.
The treatment of emerging contaminants is a significant challenge, and research is ongoing to develop effective strategies. Here are some potential future prospects based on current trends and areas of research.
Advanced Treatment Technologies: Scientists and engineers are continuously working on developing and improving advanced treatment technologies to remove emerging contaminants from water and wastewater. These technologies include advanced oxidation processes like ozonation, ultraviolet (UV) irradiation, and advanced filtration techniques like activated carbon, nanofiltration, and reverse osmosis. Ongoing research aims to enhance these technologies' efficiency, cost-effectiveness, and scalability.
Bioremediation and Microbial Degradation: Biological approaches, such as bioremediation and microbial degradation, hold promise for the treatment of emerging contaminants. Certain bacteria and fungi can break down or metabolize specific contaminants, reducing their concentration. Researchers are exploring the use of microbial consortia and genetically modified microorganisms to enhance degradation capabilities and optimize treatment processes.
Green and Nature-Based Solutions: There is increasing interest in green and nature-based solutions for treating emerging contaminants. Constructed wetlands, biofiltration systems, and phytoremediation techniques involve using natural processes and vegetation to remove or degrade contaminants. These eco-friendly approaches have the potential to be cost-effective, sustainable, and aesthetically pleasing.
Advanced Monitoring and Detection: The detection of emerging contaminants in environmental matrices (wastewater) is particularly challenging due to the low detection limit required, complex sample properties, some methods being too expensive, time-consuming, requiring trained personnel, and difficulty in separating these compounds from interference. Therefore, it is necessary to continuously research advanced extraction and purification technologies, coupled with improvements in instrument technology, to provide the required sensitivity and specificity for accurate measurement.
References
[1]. Parida, V.K., et al., Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. 2021. 9(5): p. 105966.
[2]. Taheran, M., et al., Emerging contaminants: Here today, there tomorrow!. 2018. 10: p. 122-126.
[3]. Ahmed, S.F., et al., Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. 2021. 416: p. 125912.
[4]. Pereira, L.C., et al., 2015. 22(18): p. 13800-13823.
[5]. Rathi, B.S., P.S. Kumar and P. Show, A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. 2021. 409: p. 124413.
[6]. Sauvé, S. and M. Desrosiers, 2014. 8(1): p. 15.
[7]. Nuro, A., Emerging contaminants. 2021: BoD–Books on Demand.
[8]. Stefanakis, A.I. and J.A. Becker, A review of emerging contaminants in water: Classification, sources, and potential risks. Impact of water pollution on human health and environmental sustainability, 2016: p. 55-80.
[9]. Gadipelly, C., et al., Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Industrial & Engineering Chemistry Research, 2014. 53(29): p. 11571-11592.
[10]. Vysokomornaya, O.V., E.Y. Kurilenko and A.A. Shcherbinina. Major contaminants in industrial and domestic wastewater. 2015: EDP Sciences.
[11]. Saleh, I.A., N. Zouari and M.A. Al-Ghouti, Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environmental Technology & Innovation, 2020. 19: p. 101026.
[12]. Pradhan, B., et al., Emerging groundwater contaminants: A comprehensive review on their health hazards and remediation technologies. Groundwater for Sustainable Development, 2023. 20: p. 100868.
[13]. World, H.O. and N.E.P. United, Public health impact of pesticides used in agriculture. 1990, World Health Organization; World Health Organization: Geneva.
[14]. Omenka, S.S. and A.A. Adeyi, Heavy metal content of selected personal care products (PCPs) available in Ibadan, Nigeria and their toxic effects. Toxicology Reports, 2016. 3: p. 628-635.
[15]. Bolong, N., et al., A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination, 2009. 239(1-3): p. 229-246.
[16]. Acero, J.L., et al., Coupling of adsorption, coagulation, and ultrafiltration processes for the removal of emerging contaminants in a secondary effluent. Chemical Engineering Journal, 2012. 210: p. 1-8.
[17]. Perrich, J.R., Activated carbon adsorption for wastewater treatment. 2018: CRC press.
[18]. Rovani, S., et al., Development of a new adsorbent from agro-industrial waste and its potential use in endocrine disruptor compound removal. Journal of Hazardous Materials, 2014. 271: p. 311-320.
[19]. Wasay, S.A., S. Barrington and S. Tokunaga, Efficiency of GAC for Treatment of Leachate from Soil Washing Process. Water, Air, and Soil Pollution, 1999. 116(3): p. 449-460.
[20]. Editor., W., Biochar [G/OL] Wikipedia. 2022.
[21]. Weber, K. and P. Quicker, Properties of biochar. Fuel, 2018. 217: p. 240-261.
[22]. Yao, Y., et al., Adsorption of sulfamethoxazole on biochar and its impact on reclaimed water irrigation. Journal of Hazardous Materials, 2012. 209-210: p. 408-413.
[23]. Rodriguez-Narvaez, O.M., et al., Treatment technologies for emerging contaminants in water: A review. Chemical Engineering Journal, 2017. 323: p. 361-380.
[24]. Ji, L., et al., Adsorption of tetracycline on single-walled and multi-walled carbon nanotubes as affected by aqueous solution chemistry. Environmental Toxicology and Chemistry, 2010. 29(12): p. 2713-2719.
[25]. Contributors, W., Membrane bioreactor. 2023.
[26]. Ghoshdastidar, A.J., et al., Membrane bioreactor treatment of commonly used organophosphate pesticides. Journal of Environmental Science and Health, Part B, 2012. 47(7): p. 742-750.
[27]. Mohammed, H.A., S.K. Ali and M.I. Basheer. Heavy metal ions removal using advanced oxidation (UV/H2O2) technique. 2020: IOP Publishing.
[28]. Moghadam, F. and A.G. Pourafshar, Investigation of heavy metal (Lead) removal from aqueous solution by using advanced oxidation process (Fenton). Chemical Science International Journal, 2019. 27(2): p. 1-7.
Cite this article
Entemake,Z. (2024). Review of emerging contaminants sources, effects, and removal methods. Applied and Computational Engineering,56,52-59.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Parida, V.K., et al., Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. 2021. 9(5): p. 105966.
[2]. Taheran, M., et al., Emerging contaminants: Here today, there tomorrow!. 2018. 10: p. 122-126.
[3]. Ahmed, S.F., et al., Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. 2021. 416: p. 125912.
[4]. Pereira, L.C., et al., 2015. 22(18): p. 13800-13823.
[5]. Rathi, B.S., P.S. Kumar and P. Show, A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. 2021. 409: p. 124413.
[6]. Sauvé, S. and M. Desrosiers, 2014. 8(1): p. 15.
[7]. Nuro, A., Emerging contaminants. 2021: BoD–Books on Demand.
[8]. Stefanakis, A.I. and J.A. Becker, A review of emerging contaminants in water: Classification, sources, and potential risks. Impact of water pollution on human health and environmental sustainability, 2016: p. 55-80.
[9]. Gadipelly, C., et al., Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Industrial & Engineering Chemistry Research, 2014. 53(29): p. 11571-11592.
[10]. Vysokomornaya, O.V., E.Y. Kurilenko and A.A. Shcherbinina. Major contaminants in industrial and domestic wastewater. 2015: EDP Sciences.
[11]. Saleh, I.A., N. Zouari and M.A. Al-Ghouti, Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environmental Technology & Innovation, 2020. 19: p. 101026.
[12]. Pradhan, B., et al., Emerging groundwater contaminants: A comprehensive review on their health hazards and remediation technologies. Groundwater for Sustainable Development, 2023. 20: p. 100868.
[13]. World, H.O. and N.E.P. United, Public health impact of pesticides used in agriculture. 1990, World Health Organization; World Health Organization: Geneva.
[14]. Omenka, S.S. and A.A. Adeyi, Heavy metal content of selected personal care products (PCPs) available in Ibadan, Nigeria and their toxic effects. Toxicology Reports, 2016. 3: p. 628-635.
[15]. Bolong, N., et al., A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination, 2009. 239(1-3): p. 229-246.
[16]. Acero, J.L., et al., Coupling of adsorption, coagulation, and ultrafiltration processes for the removal of emerging contaminants in a secondary effluent. Chemical Engineering Journal, 2012. 210: p. 1-8.
[17]. Perrich, J.R., Activated carbon adsorption for wastewater treatment. 2018: CRC press.
[18]. Rovani, S., et al., Development of a new adsorbent from agro-industrial waste and its potential use in endocrine disruptor compound removal. Journal of Hazardous Materials, 2014. 271: p. 311-320.
[19]. Wasay, S.A., S. Barrington and S. Tokunaga, Efficiency of GAC for Treatment of Leachate from Soil Washing Process. Water, Air, and Soil Pollution, 1999. 116(3): p. 449-460.
[20]. Editor., W., Biochar [G/OL] Wikipedia. 2022.
[21]. Weber, K. and P. Quicker, Properties of biochar. Fuel, 2018. 217: p. 240-261.
[22]. Yao, Y., et al., Adsorption of sulfamethoxazole on biochar and its impact on reclaimed water irrigation. Journal of Hazardous Materials, 2012. 209-210: p. 408-413.
[23]. Rodriguez-Narvaez, O.M., et al., Treatment technologies for emerging contaminants in water: A review. Chemical Engineering Journal, 2017. 323: p. 361-380.
[24]. Ji, L., et al., Adsorption of tetracycline on single-walled and multi-walled carbon nanotubes as affected by aqueous solution chemistry. Environmental Toxicology and Chemistry, 2010. 29(12): p. 2713-2719.
[25]. Contributors, W., Membrane bioreactor. 2023.
[26]. Ghoshdastidar, A.J., et al., Membrane bioreactor treatment of commonly used organophosphate pesticides. Journal of Environmental Science and Health, Part B, 2012. 47(7): p. 742-750.
[27]. Mohammed, H.A., S.K. Ali and M.I. Basheer. Heavy metal ions removal using advanced oxidation (UV/H2O2) technique. 2020: IOP Publishing.
[28]. Moghadam, F. and A.G. Pourafshar, Investigation of heavy metal (Lead) removal from aqueous solution by using advanced oxidation process (Fenton). Chemical Science International Journal, 2019. 27(2): p. 1-7.









