1. Introduction
Carbon dioxide is a very abundant carbon resource on Earth, accounting for about 0.04% of the atmosphere. Carbon dioxide produced under natural conditions is a necessary resource for plant growth and other biological functions. Under ideal conditions, carbon dioxide production and consumption should reach a dynamic balance [1]. However, carbon dioxide is also a greenhouse gas. Excessive carbon dioxide can change the thermal balance of the atmosphere, absorb the infrared radiation of the earth, and cause the temperature of the atmosphere near the ground to increase, thus causing the greenhouse effect. With the development of society, energy consumption is increasing day by day. A large proportion of today's energy generation and consumption comes from burning chemical fuels, but the carbon dioxide produced by combustion leads to increasing concentrations of carbon dioxide in the air [2]. At the beginning of the 19th century, the concentration of carbon dioxide in the atmosphere was about 270 ppm, but it had increased to 401.3 ppm by July 2015. What is more worrying is that it is expected to reach 600 ppm by the end of the century. Studies have shown that the safety limit for atmospheric \( C{O_{2}} \) concentration is about 350 ppm, and this uncontrolled increase in \( C{O_{2}} \) concentration has caused widespread concern [3]. Therefore, the capture and conversion of carbon dioxide into useful products will become the focus of scientific and technological development now and in the future.
Electrochemical carbon dioxide reduction reaction ( \( C{O_{2}}RR \) ) is a viable carbon dioxide utilization process and is an important component of energetic oxygen and carbon cycles (Figure 1). However, the conversion efficiency of this conversion is not considerable, and the product-free energy obtained by electrochemical carbon dioxide in the reduction process can only reach 30%-40% of the free energy of the product consumed [4]. At the same time, because the kinetic chemical potential required for the reduction reaction of carbon dioxide is too large, it is difficult to control the selectivity of chemical products and the stability of the reaction. Especially in the energetic oxygen and carbon cycles, it will compete with the hydrogen evolution reaction in the water environment, reducing the atomic conversion rate of energy conversion [2]. Therefore, researchers try to find catalysts to improve the efficiency and stability of the reaction. Studies have shown that low-cost copper (Cu) catalysts are a viable option. However, Cu increases competition with hydrogen evolution and requires a large overpotential to reduce carbon dioxide to hydrocarbons [5]. Therefore, scientists have tried to improve the efficiency of catalysts, and Cu-based nano-material catalysts have become a better choice. This paper mainly focuses on Cu-based nano-material catalysts and explores how the development of such catalysts in recent years has improved the efficiency and selectivity of \( C{O_{2}}RR \) .
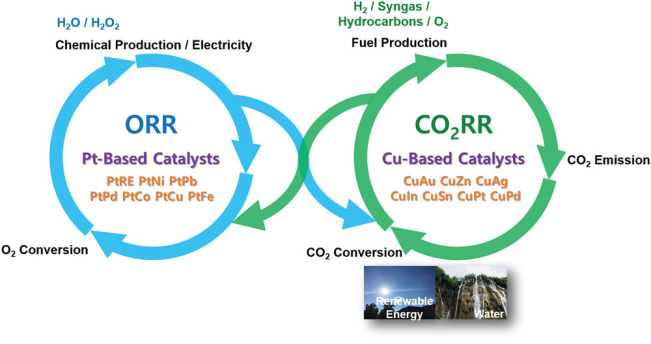
Figure 1. Energetic oxygen and carbon cycles [2].
Oxygen reduction reaction (ORR) and carbon dioxide reduction reaction ( \( C{O_{2}}RR \) ) are important electrocatalytic processes that occur on the gas diffusion electrode of hydrogen fuel cells and \( C{O_{2}} \) electrolyzers, respectively.
2. Electrochemical carbon dioxide reduction reaction
In the past ten years, various carbon dioxide reduction methods have been widely studied, including: electrochemical, photochemical, biochemical and thermochemical methods, among which the electrochemical carbon dioxide reduction reaction ( \( C{O_{2}}RR \) ) can store renewable energy in chemical form has been widely concerned. However, in order for the \( C{O_{2}}RR \) to be feasible in industry, four requirements must be considered: (1) The chemical potential of the reduction is low enough to provide energy conversion efficiency. (2) Improve the Faraday efficiency (FE), which can save energy and reduce the cost of separating byproducts; (3) Allow the carbon dioxide to react completely as much as possible, reducing the amount of carbon dioxide in the air. (4) Electrochemical cells should be scaled up to wear out other industrial processes [6].
Table 1 shows the equilibrium electrode potentials against the standard hydrogen electrode for \( C{O_{2}}RR \) to different products. According to these data, the equilibrium electrode potentials of \( C{O_{2}}RR \) are very close to the equilibrium electrode potentials of \( {H_{2}} \) evolution from water. Because hydrogen is mainly found in acidic media, and carbon dioxide molecules are difficult to exist in alkaline media, the vast majority of \( C{O_{2}}RR \) occurs in neutral media. Therefore, the formation of carbon monoxide, formate, methanol or other hydrocarbons through electrochemical reduction reactions is a viable way to use carbon dioxide.
Table 1. Equilibrium potentials for CO2 reduction reactions at pH=7 against water reduction reaction [6].
\( C{O_{2}}RR PH=7 \) | Equilibrium potential/V vs SHE |
\( C{O_{2}}+{H_{2}}O+2{e^{-}}⇌CO+2O{H^{-}} \) \( C{O_{2}}+{H_{2}}O+2{e^{-}}⇌HCO{O^{-}}+O{H^{-}} \) \( C{O_{2}}+5{H_{2}}O+6{e^{-}}⇌C{H_{3}}OH+6O{H^{-}} \) \( C{O_{2}}+8{H_{2}}O+12{e^{-}}⇌{C_{2}}{H_{4}}+12O{H^{-}} \) \( C{O_{2}}+9{H_{2}}O+12{e^{-}}⇌{C_{2}}{H_{5}}OH+12O{H^{-}} \) \( C{O_{2}}+13{H_{2}}O+18{e^{-}}⇌{C_{3}}{H_{7}}OH+18O{H^{-}} \) \( C{O_{2}}+6{H_{2}}O+8{e^{-}}⇌C{H_{4}}+8O{H^{-}} \) | -0.52 -0.43 -0.39 -0.34 -0.33 -0.32 -0.25 |
Water reduction reaction PH=7 | Equilibrium potential/V vs SHE |
\( {H_{2}}O+2{e^{-}}⇌{H_{2}}+2O{H^{-}} \) | -0.31 |
3. Cu-based nano-material catalysts
The products of CO2RR reactions on different metal surfaces can generally be divided into three categories: (1) reduction of \( C{O_{2}} \) to CO, (2) reduction of \( C{O_{2}} \) to formic acid (HCOOH), and (3) reduction of CO or carbon oxides to hydrocarbons or alcohols. The scientists added hydrogen, the likely main product, to the classification. Metals for the electrochemical reduction of carbon dioxide are classified: as products other than \( {H_{2}} \) , CO, formic acid and CO*. CO* represents that after CO is generated in the reaction, CO will be further reduced to hydrocarbons, where the asterisk indicates that the CO intermediate will be adsorbed on the metal surface.
Figure 2 shows a portion of the periodic table, and different colors represent the end products and Faraday efficiencies of different monometallic catalysts in \( C{O_{2}}RR \) . The main product classification of the metal catalysts used in the experiment for the electric reduction of carbon dioxide, the figure shows only a part of the periodic table of chemical elements, marked with different colors, and shows the Faraday efficiency of the main product. Four groups were identified: \( {H_{2}} \) (red), formic acid (yellow), CO (blue) and CO* (cyan), which will be applied throughout the work. Since Ga does not follow the product trend of the periodic table, metals are omitted, while Ti - due to strong binding - is represented by arrows in the classification diagram below [7].
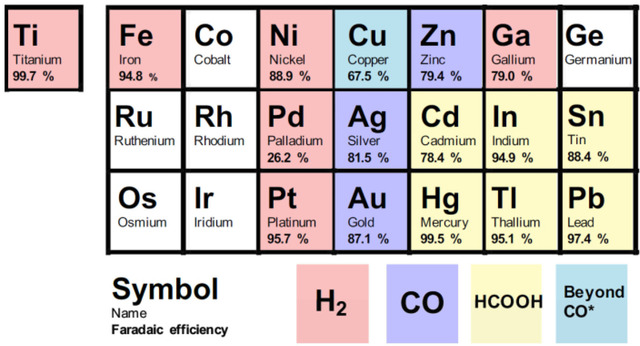
Figure 2. The end products and Faraday efficiencies of different monometallic catalysts in \( C{O_{2}}RR \) [7].
Obviously, copper is the most eye-catching element, because copper is the only metal that binds CO on the catalyst surface, it is neither too strong nor too weak, thus further reducing it to several hydrocarbon.
3.1. Cu-based nanoparticle (NP) effect
Most \( C{O_{2}} \) reduction electrocatalysts fall into three broad categories: metallic, non-metallic, and molecular catalysts (Figure 3). Among them, nanomaterials in the classification of metal catalysts have received extensive attention. Compared to bulk metals, nanomaterial metals have better particle size distribution, drug release, stability and particle shape [8]. At the same time, because of the increased surface area of the nanomaterial, it can provide more surface active sites than the bulk metal materials. Moreover, nanostructured catalyst surfaces usually contain a large number of marginal/low coordination sites, indicating that different catalytic reactions may occur than the planar/full coordination sites in the block [9].
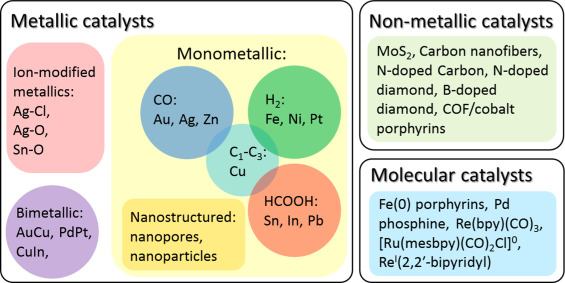
Figure 3. Three major categories of electrocatalysts for \( C{O_{2}} \) reduction [8].
The effect of catalyst is affected by the size of the Cu NP. On the surface of nanomaterials, the energy of chemical interaction is different because of the different coordination number of angular atoms, edge atoms and crystal surface atoms. Because of the low coordination number of the metal surface, it is easier to form chemical bonds to reduce the surface free energy. The strong interaction between the metal surface and the adsorbed molecules will promote the cracking and recombination of chemical bonds to form new substances. Then, if the force between the metal nuclei is relatively small, the product will break away from the metal catalyst and proceed to the next reaction [10].
The scientists placed the Cu (100), Cu (110) and Cu (111) electrodes in a solution of 0.1M \( KHC{O_{3}} \) and performed the \( C{O_{2}}RR \) reaction on the electrodes with a constant current density of 5 \( mA c{m^{-2}} \) . The experimental results show that \( {C_{2}}{H_{4}} \) is mainly generated on Cu (100) and tends to \( C{H_{4}} \) on Cu (111), while Cu (110) is selective to intermediate products (Table 2) [11].
Table 2. The efficiency of products of \( C{O_{2}}RR \) at Cu single crystal electrodes at different planes [11].
Electrode | Potential(V) vs NHE | \( C{H_{4}} \) | \( {C_{2}}{H_{4}} \) | CO | \( HCO{O^{-}} \) | MeCHO | EtOH | \( {H_{2}} \) | Total |
Polycrystal | -1.44 | 33.3% | 25.5% | 1.3% | 9.4% | 1.1% | 5.7% | 20.5% | 103.5% |
(100) | -1.42 | 25.0% | 31.7% | 0.0% | 5.1% | 1.9% | 9.8% | 23.3% | 96.9% |
(110) | -1.55 | 49.5% | 15.1% | 0.0% | 6.6% | 3.1% | 7.4% | 18.8% | 100.4% |
(111) | -1.56 | 38.9% | 4.7% | 0.0% | 4.8% | 0.0% | 0.9% | 56.5% | 105.7% |
In addition to size and crystal plane of the Cu-based NP, the effect of catalyst will be also determined by Cu-alloy, Cu-based core/shell, support on the Cu NP catalysis and the shape of the Cu-based NP [9]. These aspects are worthy of attention and further study.
3.2. Cu-based nanoalloys catalysts
Single metallic NP catalysts have certain limitations. The main product of gold (Au) and silver (Ag) in \( C{O_{2}}RR \) reaction is CO [12, 13]. This is because the molecular force between the two metals and CO is relatively small, and the degree of intermolecular bonding is low, so CO molecules are relatively easy to escape from the surface of nanomaterials and there is no way to carry out the next reaction. At the same time, gold (Au) and silver (Ag) are both precious metals, which require high costs and cannot be applied in industry. When the metal catalyst is platinum (Pt), nickel (Ni) or palladium (Pd), the reaction is more inclined to produce hydrogen, because \( C{O_{2}}RR \) is at a disadvantage in the competition with hydrogen evolution reaction, hydrogen atoms are more easily combined with the surface of the metal NP, reaction. Therefore, chemical selectivity, catalyst activity and ability to compete with hydrogen evolution reactions are all challenges faced in the selection of \( C{O_{2}}RR \) catalysts. Hence, trying to combine copper (Cu) with another metal to make a nanoalloy is a feasible way to enhance the catalyst
3.2.1. Cu-In Alloy. Indium (In) mainly generates formate in the reaction of \( C{O_{2}}RR \) , and studies have shown that this metal can inhibit the hydrogen evolution reaction to a large extent, and the adsorption amount of hydrogen atoms on the surface of metallic NP materials can be ignored [14]. Therefore, it is a feasible method to alloy copper (Cu) and indium (In) to limit the occurrence of hydrogen evolution reaction.
The experimental results show that the \( C{u_{11}}I{n_{9}} \) nano-alloy catalyst is more inclined to produce CO in \( C{O_{2}}RR. \) Figure 4 shows that In is preferentially located at the edge of Cu atoms rather than the angular or plane positions, and the electronic properties of d orbitals In Cu are not affected, but the absorption of hydrogen atoms by Cu atoms adjacent to In atoms is weakened, so the reaction is more inclined to produce CO with efficiency to be 95% [15]. Meanwhile, other studies have shown that when the ratio of copper (Cu) to Indium (In) is 94:6, the reaction tends to produce CO, but when the ratio is 25:75, the main product of the reaction is formate [16].
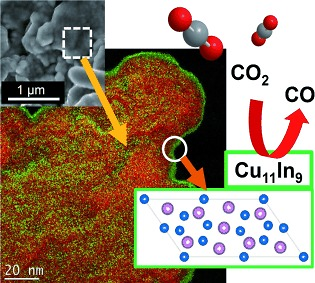
Figure 4. Electron and geometric arrangement on \( C{u_{11}}I{n_{9}} \) catalyst surface [15].
3.2.2. Cu-Zn Alloy. Cu-Zn is a kind of excellent metallic nanomaterials catalyst to reduce \( C{O_{2}} \) to CO or \( \gt 2{e^{-}} \) products, and most reports on Cu-Zn alloy catalysts have proved that the catalyst has high selectivity for 2e - transfer products such as CO [17]. Figure 5 displays that the Cu-Zn catalyst is a kind of “two-site” catalyst where the Zn site produces CO, after that CO spillovers and inserts into Cu site for further reduced [2]. With this special catalyst structure, the main product of the reaction is ethanol, and the Faradaic efficiency is increased to 29%.
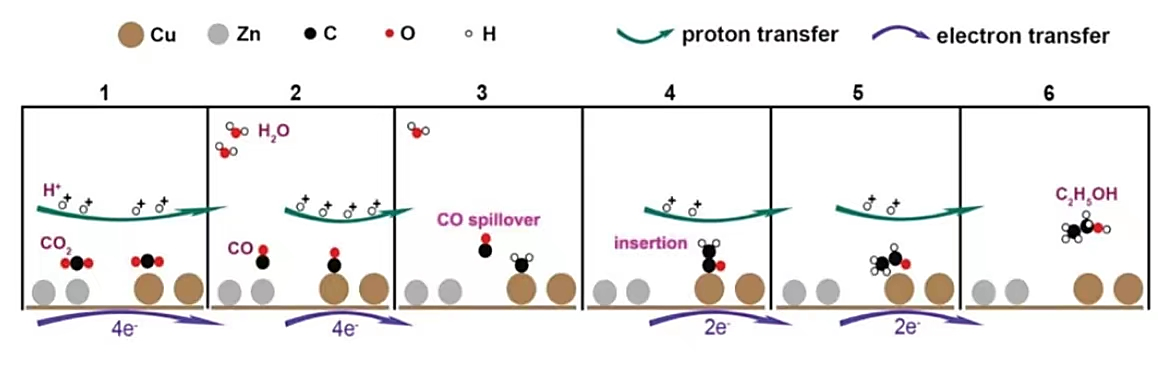
Figure 5. Mechanism for a Cu-Zn system [2].
In addition, Cu-Zn catalyst can also increase the yield of formate. Yin et al used Cu-Zn-modified \( CaF{e_{2}}{O_{4}} \) (CFO) as the cathode and \( W{O_{3}} \) as the anode to construct a Z-scheme system. The yield of HCOOH was significantly improved, and the Faraday efficiency reached 53.6-56.1%, in which the oxygen produced by the anode was almost the average of the reduction products (Figure 6). This proves that the Z-scheme system is driven by water molecules providing electrons to drive carbon dioxide reduction [18].
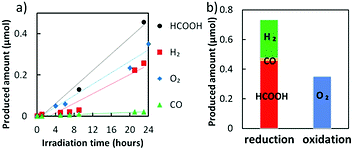
Figure 6. The oxygen produced by the anode (a) Mass production of HCOOH, H2, O2 and CO by Cu-Zn NPs under visible light irradiation with Z-scheme and − 0.5V bias potential. (b) The reduction products detected on the cathode side and the total amount of O2 detected on the anode side after 24 h. [18].
3.3. CuNCN type of catalysis
The team of scientists synthesized CuNCN by precipitation using ammonia water as a precipitator, and decomposed the material into \( C{u_{3}}N \) or \( {C_{x}}{N_{y}} \) loaded Cu NPs at different temperatures. The free energy of \( C{u_{3}}N \) in CuNCN-300 is low in the C-C atom coupling process. In the solution of 500 mA \( {cm^{-2}} \) and 1M KOH, the efficiency of \( {C_{2}}{H_{4}} \) in the reaction reaches 48.5%. The \( C{H_{4}} \) production efficiencies of CuNCN-500 and CuNCN-600 at 300 mA \( {cm^{-2}} \) and 1 M KOH were 66.3% and 66.3%, respectively (Figure 7) [19]. All these indicate that CuCNC NP exhibits better selectivity in the response of CO2RR.
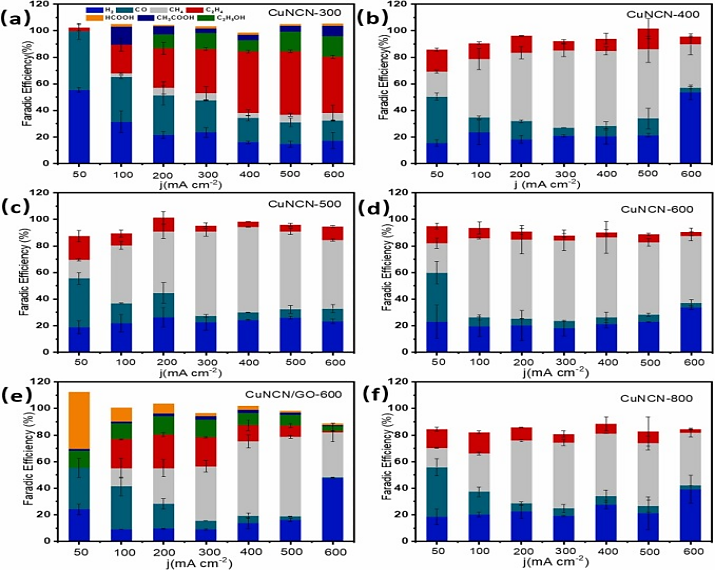
Figure 7 Faraday efficiency of (a) CUNCN-300, (b) CUNCN-400, (c) CUNCN-500, (d) CUNCN-600, (e) CuNCN/GO-600 and (f) CUNCN-800 products in a flow cell with 1m KOH as electrolyte [19].
3.3.1. CuO NPs. As mentioned earlier, different structural surfaces affect the selectivity of the efficiency of the reaction. Chi et al obtained five different CuO NPs by changing the surface structures and morphologies. Then, scientists put the five materials in 0.2 M KI solution and a constant cathode potential of −1.7 V for carbon dioxide reduction reaction. The test found that the main product of the five different structures was ethanol, and the efficiency was more than 95%. This proves that CuO NPs can improve the efficiency and selectivity of the reaction (Figure 8) [20]. At the same time, Hsu et al also found that the PH of the reaction environment also affected the catalytic efficiency of CuO NPs [21].
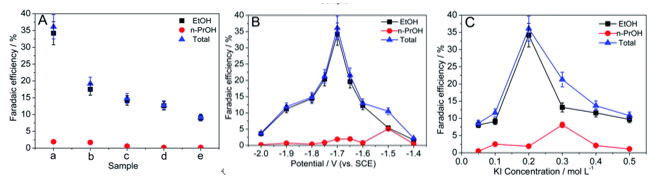
Figure 8. \( C{O_{2}}RR \) products catalyzed by five different CuO NP (A) different electrode effects, −1.7 V, 0.2 M KI; (B) Potential effect, 0.2 M KI; (C) Effect of KI concentration, −1.7 V [21].
CuO NPs can also be used as mesoporous nanosheets to participate in the catalytic reaction of other NPS to \( C{O_{2}}RR \) , so as to accurately control the reaction potential of the catalyst to improve the efficiency. Zhang et al. combined CuO mesoporous nanosheets with AgNPs. In this special structure, CuO mesoporous nanosheets are conducive to gas penetration, and improve the carbon dioxide adsorption activity on Ag NPs, in which case the optimal efficiency of CO formation can reach 91.2% (Figure 9) [22].
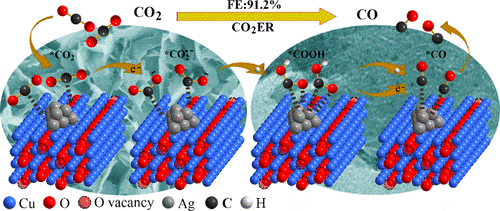
Figure 9. Mechanism of \( C{O_{2}}RR \) catalyzed by Ag/CuO [22].
4. Conclusion
Cu surface is a unique active catalyst for \( C{O_{2}}RR \) , and Cu-based alloys represent a diverse family of \( C{O_{2}}RR \) , whose structural sensitivity has not been adequately studied. The vast majority of Cu-based alloys in \( C{O_{2}}RR \) are favorable for chemical reduction products of double electron transfer processing. Another challenge involves the temporal stability of activity and selectivity. The Faraday efficiency changes in the first few hours of catalysis, and in most cases, the competitive hydrogen evolution reaction is superior to the \( C{O_{2}}RR \) treatment due to the deposition of metal ion pollutants in the electrolyte, which promotes hydrogen precipitation. In addition to copper-based nanoalloys, metal nitrogen-doped high-surface carbon catalysts, referred to the literature as MNC electrocatalysts, have emerged as another cost-effective, highly active and selective \( C{O_{2}}RR \) electrocatalyst for CO production and "beyond CO" products.
In the past decade, substantial experimental and theoretical progress has been made in the electrocatalysis of \( C{O_{2}}RR \) by Cu-based nanocatalysts. Through the precise control of the size, composition, morphology and structure of the nanocatellator, the catalytic effect of the nanocatellator is greatly enhanced than that of the traditional copper foil or bulk electrode. However, the FE and selectivity for products are still too low and require further improvement. Stability issues also need to be addressed. In order for these Cu catalysts to be useful for \( C{O_{2}}RR \) new stabilization strategies need to be developed and production scaled up, which is challenging.
References
[1]. Qiao, J., Liu, Y., Hong, F. and Zhang, J. (2014). A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev., 43(2), pp.631–675.
[2]. Kim, C., Dionigi, F., Beermann, V., Wang, X., Möller, T. and Strasser, P. (2018). Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO_2 RR). Advanced Materials, 31(31), p.1805617.
[3]. Xie, H., Wang, T., Liang, J., Li, Q. and Sun, S. (2018). Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today, 21, pp.41–54. doi:https://doi.org/ 10.1016/j.nantod.2018.05.001.
[4]. Hori, Y. in Modern Aspects of Electrochemistry, Vol. 42, eds Vayenas C. G., White R. E., Gambao-Aldaco M. E. 89–189, Springer (2008).
[5]. Mistry, H., Varela, A.S., Bonifacio, C.S., et al. (2016). Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nature Communications, [online] 7(1). doi:https://doi.org/10.1038/ncomms12123.
[6]. Martínez-Huitle C.A., Rodrigo, M.A. and Scialdone, O. (2018). Electrochemical water and wastewater treatment. Oxford: Butterworth-Heinemann, an imprint of Elsevier.
[7]. Bagger, A., Ju, W., Varela, A.S., Strasser, P. and Rossmeisl, J. (2017). Electrochemical CO2 Reduction: A Classification Problem. ChemPhysChem, [online] 18(22), pp.3266–3273.
[8]. Neelesh Kumar Mehra, Srivastava, S., Madan, J. and Pankaj Kumar Singh (2022). Multifunctional Nanocarriers. Elsevier.
[9]. Lu, Q. and Jiao, F. (2016). Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy, 29, pp.439–456.
[10]. Li, Q.X. and Sun, S. (2016). Recent advances in the organic solution phase synthesis of metal nanoparticles and their electrocatalysis for energy conversion reactions. 29, pp.178–197.
[11]. Hori, Y., Wakebe, H., Tsukamoto, T. and Koga, O. (1995). Adsorption of CO accompanied with simultaneous charge transfer on copper single crystal electrodes related with electrochemical reduction of CO2 to hydrocarbons. Surface Science, 335, pp.258–263.
[12]. Mistry, H., Reske, R., Zeng, Z., Zhao, Z.J., Greeley, J., Strasser, P. and Cuenya, B.R. (2014). Exceptional Size-Dependent Activity Enhancement in the Electroreduction of CO2 over Au Nanoparticles. Journal of the American Chemical Society, 136(47), pp.16473–16476.
[13]. Kim, C., Eom, T., Michael Shincheon Jee, Jung, H., Kim, H., Byoung Koun Min and Yun Jeong Hwang (2017). Insight into Electrochemical CO2 Reduction on Surface-Molecule-Mediated Ag Nanoparticles. 7(1), pp.779–785.
[14]. Ding, C., Li, A., Shouqin Lü, Zhang, H. and Li, C. (2016). In Situ Electrodeposited Indium Nanocrystals for Efficient CO2 Reduction to CO with Low Overpotential. ACS Catalysis, 6(10), pp.6438–6443.
[15]. Rasul, S., Anjum, D.H., Jedidi, A., Minenkov, Y., Cavallo, L. and Takanabe, K. (2014). A Highly Selective Copper-Indium Bimetallic Electrocatalyst for the Electrochemical Reduction of Aqueous CO2 to CO. Angewandte Chemie International Edition, 54(7), pp.2146–2150.
[16]. Zhu, M., Tian, P., Li, J., Chen, J., Xu, J. and Han, Y.-F. (2019). Structure‐Tunable Copper–Indium Catalysts for Highly Selective CO2 Electroreduction to CO or HCOOH. Chemsuschem, 12(17), pp.3955–3959.
[17]. Wan, L., Zhang, X., Cheng, J., Chen, R., Wu, L., Shi, J. and Luo, J. (2022). Bimetallic Cu–Zn Catalysts for Electrochemical CO2 Reduction: Phase-Separated versus Core–Shell Distribution. ACS Catalysis, 12(5), pp.2741–2748.
[18]. Yin, G., Sako, H., Gubbala, R.V., Ueda, S., Yamaguchi, A., Abe, H. and Miyauchi, M. (2018). A Cu–Zn nanoparticle promoter for selective carbon dioxide reduction and its application in visible-light-active Z-scheme systems using water as an electron donor. Chemical Communications, 54(32), pp.3947–3950.
[19]. Li, H., Cao, S., Sun, H., Lu, Y., Zhang, Y., Lu, X., Zeng, J. and Yan, Z. (2023). CuNCN derived Cu-based/CxNy catalysts for highly selective CO2 electroreduction to hydrocarbons. Applied Catalysis B: Environmental, 320, p.121948.
[20]. Chi, D., Yang, H., Du, Y., Lv, T., Sui, G., Wang, H. and Lu, J. (2014). Morphology-controlled CuO nanoparticles for electroreduction of CO2 to ethanol. RSC Adv., 4(70), pp.37329–37332.
[21]. Hsu, J., Eid, A.H., Randall, C., Mohamed S.E. Houache, Yaser Abu-Lebdeh and Al-Abadleh, H.A. (2022). Mechanistic In Situ ATR-FTIR Studies on the Adsorption and Desorption of Major Intermediates in CO2 Electrochemical Reduction on CuO Nanoparticles. Langmuir, 38(48), pp.14789–14798.
[22]. Zhang, W., Zhu, N., Ding, L., Hu, Y. and Wu, Z. (2021). Efficacious CO2 Adsorption and Activation on Ag Nanoparticles/CuO Mesoporous Nanosheets Heterostructure for CO2 Electroreduction to CO. Inorganic Chemistry, 60(24), pp.19356–19364.
Cite this article
Zheng,X. (2024). Cu-based nano-material catalysts for electrochemical carbon dioxide reduction reaction (CO2RR). Applied and Computational Engineering,58,17-25.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Qiao, J., Liu, Y., Hong, F. and Zhang, J. (2014). A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev., 43(2), pp.631–675.
[2]. Kim, C., Dionigi, F., Beermann, V., Wang, X., Möller, T. and Strasser, P. (2018). Alloy Nanocatalysts for the Electrochemical Oxygen Reduction (ORR) and the Direct Electrochemical Carbon Dioxide Reduction Reaction (CO_2 RR). Advanced Materials, 31(31), p.1805617.
[3]. Xie, H., Wang, T., Liang, J., Li, Q. and Sun, S. (2018). Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today, 21, pp.41–54. doi:https://doi.org/ 10.1016/j.nantod.2018.05.001.
[4]. Hori, Y. in Modern Aspects of Electrochemistry, Vol. 42, eds Vayenas C. G., White R. E., Gambao-Aldaco M. E. 89–189, Springer (2008).
[5]. Mistry, H., Varela, A.S., Bonifacio, C.S., et al. (2016). Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nature Communications, [online] 7(1). doi:https://doi.org/10.1038/ncomms12123.
[6]. Martínez-Huitle C.A., Rodrigo, M.A. and Scialdone, O. (2018). Electrochemical water and wastewater treatment. Oxford: Butterworth-Heinemann, an imprint of Elsevier.
[7]. Bagger, A., Ju, W., Varela, A.S., Strasser, P. and Rossmeisl, J. (2017). Electrochemical CO2 Reduction: A Classification Problem. ChemPhysChem, [online] 18(22), pp.3266–3273.
[8]. Neelesh Kumar Mehra, Srivastava, S., Madan, J. and Pankaj Kumar Singh (2022). Multifunctional Nanocarriers. Elsevier.
[9]. Lu, Q. and Jiao, F. (2016). Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy, 29, pp.439–456.
[10]. Li, Q.X. and Sun, S. (2016). Recent advances in the organic solution phase synthesis of metal nanoparticles and their electrocatalysis for energy conversion reactions. 29, pp.178–197.
[11]. Hori, Y., Wakebe, H., Tsukamoto, T. and Koga, O. (1995). Adsorption of CO accompanied with simultaneous charge transfer on copper single crystal electrodes related with electrochemical reduction of CO2 to hydrocarbons. Surface Science, 335, pp.258–263.
[12]. Mistry, H., Reske, R., Zeng, Z., Zhao, Z.J., Greeley, J., Strasser, P. and Cuenya, B.R. (2014). Exceptional Size-Dependent Activity Enhancement in the Electroreduction of CO2 over Au Nanoparticles. Journal of the American Chemical Society, 136(47), pp.16473–16476.
[13]. Kim, C., Eom, T., Michael Shincheon Jee, Jung, H., Kim, H., Byoung Koun Min and Yun Jeong Hwang (2017). Insight into Electrochemical CO2 Reduction on Surface-Molecule-Mediated Ag Nanoparticles. 7(1), pp.779–785.
[14]. Ding, C., Li, A., Shouqin Lü, Zhang, H. and Li, C. (2016). In Situ Electrodeposited Indium Nanocrystals for Efficient CO2 Reduction to CO with Low Overpotential. ACS Catalysis, 6(10), pp.6438–6443.
[15]. Rasul, S., Anjum, D.H., Jedidi, A., Minenkov, Y., Cavallo, L. and Takanabe, K. (2014). A Highly Selective Copper-Indium Bimetallic Electrocatalyst for the Electrochemical Reduction of Aqueous CO2 to CO. Angewandte Chemie International Edition, 54(7), pp.2146–2150.
[16]. Zhu, M., Tian, P., Li, J., Chen, J., Xu, J. and Han, Y.-F. (2019). Structure‐Tunable Copper–Indium Catalysts for Highly Selective CO2 Electroreduction to CO or HCOOH. Chemsuschem, 12(17), pp.3955–3959.
[17]. Wan, L., Zhang, X., Cheng, J., Chen, R., Wu, L., Shi, J. and Luo, J. (2022). Bimetallic Cu–Zn Catalysts for Electrochemical CO2 Reduction: Phase-Separated versus Core–Shell Distribution. ACS Catalysis, 12(5), pp.2741–2748.
[18]. Yin, G., Sako, H., Gubbala, R.V., Ueda, S., Yamaguchi, A., Abe, H. and Miyauchi, M. (2018). A Cu–Zn nanoparticle promoter for selective carbon dioxide reduction and its application in visible-light-active Z-scheme systems using water as an electron donor. Chemical Communications, 54(32), pp.3947–3950.
[19]. Li, H., Cao, S., Sun, H., Lu, Y., Zhang, Y., Lu, X., Zeng, J. and Yan, Z. (2023). CuNCN derived Cu-based/CxNy catalysts for highly selective CO2 electroreduction to hydrocarbons. Applied Catalysis B: Environmental, 320, p.121948.
[20]. Chi, D., Yang, H., Du, Y., Lv, T., Sui, G., Wang, H. and Lu, J. (2014). Morphology-controlled CuO nanoparticles for electroreduction of CO2 to ethanol. RSC Adv., 4(70), pp.37329–37332.
[21]. Hsu, J., Eid, A.H., Randall, C., Mohamed S.E. Houache, Yaser Abu-Lebdeh and Al-Abadleh, H.A. (2022). Mechanistic In Situ ATR-FTIR Studies on the Adsorption and Desorption of Major Intermediates in CO2 Electrochemical Reduction on CuO Nanoparticles. Langmuir, 38(48), pp.14789–14798.
[22]. Zhang, W., Zhu, N., Ding, L., Hu, Y. and Wu, Z. (2021). Efficacious CO2 Adsorption and Activation on Ag Nanoparticles/CuO Mesoporous Nanosheets Heterostructure for CO2 Electroreduction to CO. Inorganic Chemistry, 60(24), pp.19356–19364.









