1. Introduction
Food safety is related to the physical health and life safety of the masses. And food testing is one of the significant ways to ensure food safety. The importance of food safety testing is that it can detect various chemical or biological contaminants that may adversely affect human health in food and avoid the occurrence of diseases. The main harmful factors in food are pathogenic bacteria, heavy metals, residual chemicals, and natural toxins.
Food safety testing technologies include chromatography, spectroscopy, and biotechnology. People's expectations for food safety are rising along with their standard of living, and they want to buy reassuring food. This requires on-site rapid testing in supermarkets, fields, and other areas to contain risks as soon as possible. There are several common detection methods, such as atomic absorption spectrophotometry (AAS), high-performance liquid chromatography (HPLC), isotope tracing method (ITT), and gas chromatography (GC). Compared with these traditional detection methods, biosensors have great sensitivity and selectivity. In addition, biosensors also have the characteristic of real-time detection, which can help people detect harmful substances in food in a timely manner and take corresponding measures. In addition, it does not require sample pre-treatment and is easy to operate, which can greatly reduce testing costs and improve testing efficiency.
Electrochemical detection method can be used in biomedical, environmental monitoring, and food safety. In these application processes, it is necessary to determine the electrochemical properties of the substance to be tested in the solution or sample, and select appropriate electrode materials and instrument operating conditions based on the characteristics of the substance to be tested, ultimately converting the concentration of the substance to an electrochemical signal. Therefore, studying electrochemical detection technology is of great significance for the determination of trace substances in biological or environmental specimens. The main methods of electrochemical detection include square wave voltammetry (SWV), cyclic voltammetry (CV), chronoamperometry (CA), linear sweep voltammetry (DPV), and linear sweep voltammetry (LSV). Different detection methods have different characteristics and application scenarios. The CV method is mainly used to study the reaction mechanism of the target substance on the surface of the electrode, while the DPV method is often utilized to examine the electrochemical process at the interface between metal and electrolyte solutions. The LSV, SWV, and CA methods have high analytical sensitivity, fast measurement speed, and are also widely used.
Electrochemical sensors can convert chemical reactions into electrical signals. Electrochemical biosensors utilize biological recognition elements such as enzymes, antibodies, etc. to recognize and measure specific molecules. Significant advancements have been made in electrochemical detection of food safety through cross integration with nanotechnology, metal organic frameworks (MOFs), intelligent technology, optics, and other technologies recently.
In food testing, biological factors include food hygiene and microbial limits, while chemical factors include pollutants, additives, dietary supplements, residues of agricultural and veterinary drugs, and food contact materials. Depending on the kinds of analytes of interest, this review covers numerous electrochemical biosensors for detecting several kinds of food pollutants. The author focuses on the research and development of electrochemical biosensors applied to detect contaminants, including pesticide residues, heavy metal elements, food additives, and mycotoxins. The recent five years' work will be primarily focused on, and insights on how the researchers might seek solutions and overcome future obstacles will be summarized. These electrochemical biosensors are described and contrasted in terms of electrode materials, modification techniques, electrochemical methodologies, target analyte, linear range, and limit of detection (LOD). To compare the selectivity, repeatability, and sensitivity of these electrochemical biosensors in order to evaluate their benefits and drawbacks.
2. Detection of harmful substances in food
2.1. Pesticides
For the fruit and vegetable planting industry, in order to ensure yield and fruit quality, pesticides are inevitably used for protection during the planting process. However, pesticides pose great harm to human health. Sometimes, even after repeatedly rinsing fruits and vegetables, pesticides remain on them and enter the body through human ingestion. The conventional methods for pesticide residue detection in the past were not only expensive but also time-consuming and labour-intensive. Therefore, electrochemical detection of pesticide residues emerged as the times require. Electrochemical sensor detection has also proposed corresponding detection methods for different types of pesticides, as shown in Table 1.
Wang et al. developed a DNA nanomachine based on a specifically built dual-recognition aptazyme beacon (DRAB), which utilizes the high throughput of DNA to combine the aptamer with the structure of DNAzyme and obtain a dual-recognition probe [1]. After recognizing chlorpyrifos, this nanomachine was activated, followed by an enzyme-free cycle where the signal probe continuously cut on the electrode surface. The sensor had a low detection limit of 0.178 µM in the linear chlorpyrifos concentration range from 0.5 nM to 0.5 µM. Through comparative and repeated experiments, it has been proven that the sensor has great selectivity, repeatability, and reproducibility. With good potential for practical usage, this sensor could simultaneously detect pesticides and heavy metal ions.
Acetylcholinesterase (AchE) biosensors have developed over the last few decades to provide reliable and sensitive electrochemical detection of acetylcholine and neurotoxic chemicals. These biosensors' ability to detect substances is improved even more by immobilizing enzymes and incorporating nanoparticles. Wei et al. combined cell culture with electrochemical detection to create a three-dimensional electrochemical biosensor based on cells for the evaluation of neurotoxicity and applied it to the exposure to organophosphorus pesticides (OPs) [2]. OPs inhibited the activity of AChE in cells, and the enzymatic product 1-naphthol (1-N) decreased over time. They selected three typical types of organophosphorus (chlorpyrifos, dimethoate, and isothion) as models to evaluate the potential of the sensor to measure organophosphorus neurotoxicity. The screen-printed carbon electrode (SPCE), which was enhanced with carbon nanotube electrode modification materials (ZIF-67@LDH/MWCNT), increased the sensor's sensitivity. The linear range of 1-N was determined to be 0.5-150 µM, while the detection limit was 0.148 µM. This sensor had high sensitivity, reliability, and simplicity.
When MOFs materials are applied in the sensing field, they are often combined with secondary electrochemical active materials to form composite materials with a core-shell structure, improving electron transfer ability and electrochemical sensing stability. Duan et al. exchanged the Me2NH2+ in Zn-bio-MOF-1 with cobalt ions to obtain Co@Zn-bio-MOF-1 [3]. The CoS/ZnS composite material was synthesized using the obtained material as the precursor and was utilized to modify the glassy carbon electrode (GCE). Then, a novel electrochemical sensor was designed and constructed by combining this material with molecularly imprinted polymers (MIPs) for the rapid and accurate identification of the organochlorine pesticide chloroneb. The sensor exhibited linear detection of chloroneb between 0.003 to 0.2 μM and 0.2 to 3.2 μM under optimal conditions, with a detection limit of 0.87 nM (S/N=3). It exhibited good repeatability, stability, and selectivity.
Table 1. Pesticide detection with electrochemical biosensors.
Analytes | Electrode materials | Electrochemical methods | Range of linearity | LOD | |
Chlorpyrifos | GE | CV | 0.5 nM~0.5 µM | 0.178 µM | [1] |
OPs | SPCE | DPV | 0.5~150 µM | 0.148 µM | [2] |
Chloroneb | GCE | DPV | 0.003~0.2 \( μM \) 0.2~3.2 \( μM \) | 0.87 nM | [3] |
2.2. Heavy metals
The common heavy metal pollutants in food include lead, mercury, cadmium, and chromium, which mainly come from industrial emissions and environmental pollution. After heavy metals accumulate in the human body, they can cause physiological toxicity by interfering with the transportation of proteins and enzymes in the body. Therefore, in order to ensure the safety of food, heavy metal detection is crucial. Electrochemical sensing technology has high sensitivity and fast response speed to heavy metal ions, and can achieve simultaneous detection of multiple metal ions in samples, as shown in Table 2. Improving electrode performance or developing new electrode forms through functional nanomaterials can break through the limitations of electrode structure on the further application of rapid detection technology.
Wang et al. developed a novel biosensor based on geobacter-dominated biofilms using mixed microbial communities for Cd2+, Ni2+, Pb2+, and Cu2+ low-concentration heavy metal detection [4]. The author analyzed the responsiveness and durability of biofilms under stress from heavy metal poisoning by analyzing the microbial population both prior to and after continuous impact. Geobacterium was the genus predominated in anodic biofilm, which had a stable community structure after 22 days of nonstop toxic shock. This biosensor had a sensitivity of 109.7 μA∙μM-1∙cm-2 to Cd2+ and 161.7 μA∙μM-1∙cm-2 to Pb2+, which was higher than the sensitivity of conventional heavy metal detection biosensors. The detection limits for Cd2+ and Pb2+ were 5.75 μM and 1.3 μM, respectively. It might work well as an alternate biosensor for analyzing groundwater and other things.
Zhang et al. loaded a substantial amount of antimonene sheet on the surface of ZIF-67 MOF and prepared a method built upon ZIF-67@antimonene (AMNFs) ultra-sensitive electrochemical sensors for nanocomposites [5]. AMNFs was a type of two-dimensional sp2 bonded honeycomb lattice material with good stability and hydrophilicity. Functional ZIF-67@AMNFs exhibited an exceptional layered structure, resulting in a material with a large active surface area, high adsorption efficiency, and excellent conductivity. The author used cyclic voltammetry to test the performance of sensing layers made of metal-organic skeleton material. The sensor electrode has enhanced its adsorption capacity for Pb2+, Hg2+, and Cu2+. The detection limits for Pb2+, Hg2+, and Cu2+ were 0.031 pM, 0.042 pM, and 0.01 pM, respectively. This sensor was used to simultaneously measure Pb2+, Hg2+, and Cu2+ in samples of rice, corn, sorghum, honey, milk, and tea.
In order to develop electrochemical biosensors for a highly sensitive and accurate detection of Pb2+, Liu et al. constructed a unique three-dimensional (3D) DNA nanostructure using methylene blue (MB) as a signal molecule [6]. Through the examination of Polyacrylamide-gel electrophoresis (PAGE) and Atomic force microscopy (AFM), the author confirmed the formation of 3D DNA structure. The relationship between the logarithm of Pb2+ concentrations and SWV response currents was linear in the range of 1 pM to 100 μM, with a detection limit of 2.61 pM (S/N=3). The three-dimensional DNA nanostructures exhibited excellent performance.
Table 2. Heavy metal detection with electrochemical biosensors.
Analytes | Electrode material | Electrochemical methods | Range of linearity | LOD | |
Cd2+ Pb2+ | CP | CV | 5.75 μM 1.3 μM | [4] | |
Pb2+ Hg2+ Cu2+ | SPCE | CV | 0.1~200 pM | 0.031 pM 0.042 pM 0.01 pM | [5] |
Pb2+ | GCE | CV | 1-100 μM | 2.61 pM | [6] |
2.3. Illegal additives
There are many types of food additives, mainly including antioxidants, preservatives, colorants, thickeners, sweeteners, etc. The difference between food additives and illegal additives is that food additives can be reasonably added under legal provisions, while illegal additives are additives that violate the law and are absolutely not allowed to be added to any food. In order to enhance the color of food, pigments are frequently utilized in the food industry, but some pigments, as industrial dyes, are mostly organic compounds synthesized from benzene, naphthalene, and other raw materials through a series of reactions such as diazotization and sulfonation, such as Sudan Red and Rhodamine B. These prohibited pigments have adverse effects on the human body, including carcinogenicity, genotoxicity, and cytotoxicity. As shown in Table 3, electrochemical sensing methods have been used for the detection of different food additives.
Sun et al. designed a sensitive electrochemical sensor to detect Sudan red I and sunset yellow (SY), and AuNPs/zr-MOF-Graphene were used as electrode modification materials [7]. Graphene had excellent nanoscale structure and electron transfer efficiency, and incorporating graphene into composite materials could help improve sensitivity. Under optimal conditions, the detection ranges of SY and Sudan I were 0.1~1000 μM and 0.1~800 μM, respectively, with detection limits of 0.1 μM and 0.1 μM. The application of modified AuNPs/zr-MOF-Graphene electrodes in the detection of azo dyes in food had the benefits of being inexpensive, having a broad detection range, having good reproducibility, and being stable.
Clenbuterol hydrochloride (CLB) is a widely used β-Adrenergic agonists. Its working principle is to stimulate specific receptors in human muscle tissue, thereby promoting protein synthesis and increased liposolubility. This process ultimately leads to sustained growth of muscle fibers. Using clenbuterol in feed can promote pig growth, reduce fat content, and increase lean meat percentage, but consuming pork containing clenbuterol is hazardous to human beings. Lin et al. proposed a molecular imprinting-based electrochemical sensor for high-sensitivity CLB detection [8]. To manufacture the improved electrode, the author used a composite material made of MnFe2O4 and carbon quantum dots (CQDs). Using CLB as the template molecule, the molecularly imprinted polymer (MIP) film was electropolymerized with the cyclic voltammetry technique to obtain a MIP/MnFe2O4-CQDs-CS/GCE sensor. The sensor was easy to build, highly sensitive, had a broad linear range, and responded specifically to CLB. The built-in sensor could detect CLB with a 10 nM~10 mM concentration range under ideal circumstances, and the predicted LOD is 0.041 μM.
Melamine has been used as a "pseudo protein" added to milk powder, and its toxicity is relatively low. However, the homologues produced by its hydrolysis can enhance its toxicity. Mohebbi et al. used cyclic voltammetry (CV) to electrodeposit the synthesized g-C3N4 onto a carbon paste electrode (CPE), and used dip coating technology to electropolymerize OPD [9]. Finally, functionalized the g-C3N4/POPD nanocomposite with EDTA, and modified the surface of CPE using this nanocomposite material to prepare a high-performance melamine electrochemical sensor. POPD was an excellent conductive host and substrate for g-C3N4, and the two together overcame the drawbacks of POPD's poor mechanical strength and g-C3N4's poor conductivity. G-C3N4, EDTA, and POPD had synergistic effects, which could enhance the performance of electrochemical sensors. The oxidation peak currents of ferrocyanide were linearly proportional to the amount of melamine present in the range of 0.01~ 0.1 μM, with a detection limit of 9.0 nM. Using the sensor, melamine in dairy products has been determined.
Table 3. Illegal additives detection with electrochemical biosensors.
Analytes | Electrode materials | Electrochemical methods | Range of linearity | LOD | |
Sudan red I Sunset yellow | GCE | CV | 0.1~800 μM 0.1~1000 μM | 0.1 μM 0.1 μM | [7] |
Clenbuterol Hydrochloride | GCE | CV | 10 nM~10 mM | 0.041 μM | [8] |
Melamine | CPE/g-C3N4/POPD/EDTA | DPV | 0.01~ 0.1 μM | 9.0 nM | [9] |
2.4. Mycotoxin
Mycotoxins are metabolic products produced by fungi that grow in food or feed. When humans or animals ingest untreated or incompletely treated crops, the toxins can enter the animals or human body, endanger the kidneys and other immune organs, and even have strong carcinogenicity and teratogenicity. Aflatoxins (AFs), ochratoxins (OTs), trichothecenes, fumonisins (FBs), and zearalenone are the main species. As shown in Table 4, electrochemical induction methods can be used for the detection and analysis of these substances.
Aflatoxins (AFs) exist in soil, animals, plants, and various nuts, and are prone to contaminating grain and oil products such as peanuts, corn, rice, and soybeans. They are the most toxic type of fungal toxins and pose significant risks to the health of individuals. As shown in Figure 1, Dai et al. developed an accurate voltammetric electrochemical immunosensor for detecting aflatoxin B1 (AFB1) utilizing an type of nano-composite material (CCM@ZIF-8/PDA/IgG) labeled anti-mouse immunoglobulin IgG as a signal probe [10]. Package CCM in ZIF-8 to create a CCM@ZIF-8 composite material, then modified the surface of the composite material with PDA, and used a signal probe made from modified IgG to obtain CCM@ZIF-8/PDA/IgG. Due to its unique chemical structure, Curcumin (CCM) exhibited a significant electron transfer rate on the surface of gold electrodes. Meanwhile, the CCM that was enclosed in ZIF-8 performed better under load and was more stable due to ZIF-8's large specific surface area. The linear range was 0.5 pg/mL~10 ng/mL, and the LOD is 0.11 pg/mL. With acceptable recovery rates, this immunosensor might be utilized to detect AFB1 in samples of rice as well as wheat flour.
To find evidence of total AFs in pistachios, Pérez-Fernández et al. proposed a new competitive immunosensor based on SPCE [11]. They also described how to use the immunosensor's cross validation approach. The sensor had good reproducibility (RSD: 2%) in the linear range of 0.01~2 μg/L. The detection limits in PBS buffer and pistachio matrix were 0.017 μg/L and 0.066 μg/kg, respectively. Compared to alternatives to LC-MS/MS and ELISA-based standard analytical techniques, this immunosensor was simpler, cheaper, faster, and equally sensitive.
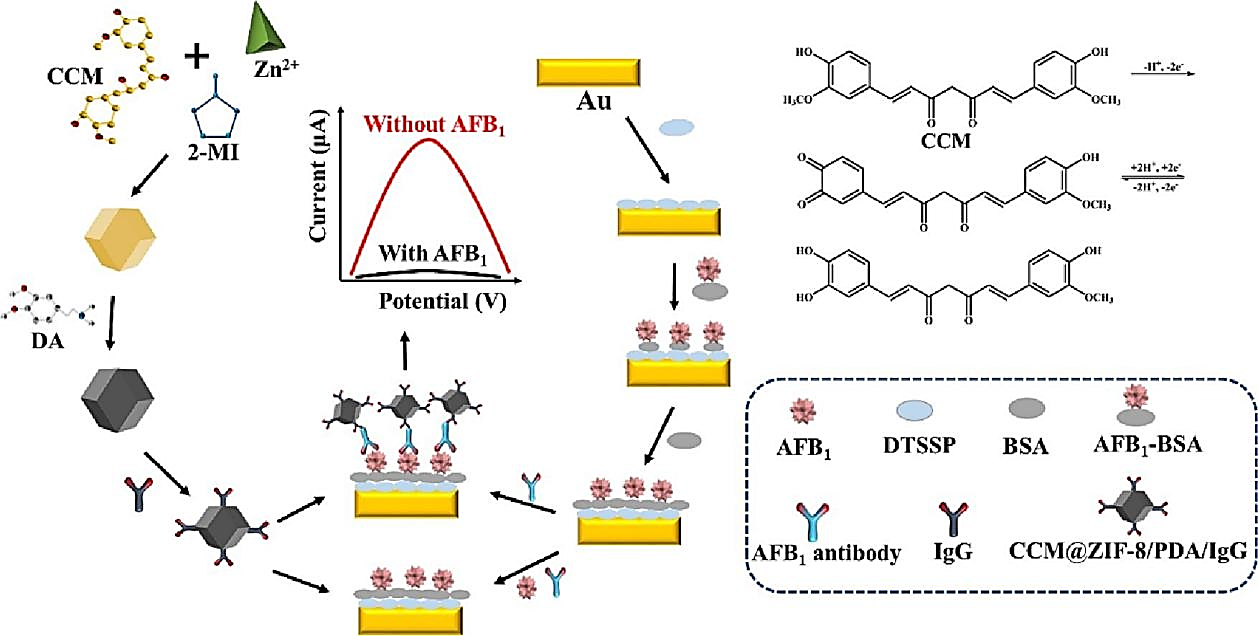
Figure 1. Schematic illustration of the electrochemical immunosensor for detecting AFB1 [10].
Ochratoxin A (OTA) consists of seven structurally similar compounds, with the highest toxicity and production of OTA. It is most common in moldy grains, feed, etc. To detect OTA, a fungal toxin with nephrotoxicity and carcinogenicity, Oliveira et al. designed a label-free impedance immunosensor. A BK7 glass substrate with a thin gold track covering it served as the foundation for the electrode construction [12]. Cysteamine was used as a thiol ligand in the formation of self-assembled monolayers (SAMs), which immobilized anti-OTA antibodies in the fixed area of the antibody through carboxyl activation. The author evaluated the performance of the biosensor using electrochemical impedance spectroscopy (EIS). The change in impedance value displayed a linear relationship with OTA concentration between 0.5 and 100 ng/mL, with a detection limit of 0.15 ng/mL. The recovery analysis of a coffee sample with OTA showed that the biosensor can be used for actual samples.
Table 4. Mycotoxin detection with electrochemical biosensors.
Analytes | Electrode materials | Electrochemical methods | Range of linearity | LOD | |
Aflatoxin B1 | Au | CV | 0.5 pg/mL ~10 ng/mL | 0.11 pg/mL | [10] |
Aflatoxins | SPCE | 0.01~2 μg/L | 0.017 μg/L | [11] | |
Ochratoxin A | Au | EIS | 0.5~100 ng/mL | 0.15 ng/mL | [12] |
3. Conclusion
In order to prevent the occurrence of food safety incidents, more advanced technologies have been developed to effectively monitor food safety. Electrochemical technology is widely used in food detection which benefits from great sensitivity, faster detection speed, and accurate detection results. This paper introduces the application of electrochemical biosensors to detect pesticides, heavy metals, illegal additives, and mycotoxin. Although these detection techniques have been proven effective, further promotion of their use in food safety testing still requires addressing deficiencies in material preparation and experimental processes. In the selection of modified electrode materials, researchers can composite more types of materials with strong conductivity and novel characteristics, such as metal nanoparticles, carbon materials, etc., and simplify the synthesis process of composite materials. Research has shown that detection methods based on nanotechnology exhibit significant advantages in sensitivity, sample consumption, and operational difficulty. Future electrochemical biosensors will become more prevalent and have better detection performance as a result of the development of novel nanomaterials, microfluidics, and intelligent sensing strategies.
References
[1]. Wang W, Xu Y, Cheng N, Xie Y, Huang K and Xu W 2020 Sens. Actuators B Chem. 321 128598
[2]. Wei X et al. 2023 Sens. Actuators B Chem. (PB) 376 132941
[3]. Duan D, Ye J, Cai X and Li K 2021 Microchim Acta 188 111
[4]. Wang J, Yang X, Cui M, Liu Y, Li X, Zhang L and Zhan G 2022 Biosens. and Bioelectron. 206 114146
[5]. Zhang Y, Xu Y, Li N, Liu X, Ma Y, Yang S, Luo H, Hou H and Huo D 2023 Food Chem. 421 136131
[6]. Liu Y, Kong L, Han Z, Yuan R and Chai Y 2023 Sens. Actuators B Chem. 382 133486
[7]. Sun R et al. 2022 Food Control 145 109491
[8]. Lin C and Li Y 2023 Int. J. Electrochem. Sci. 18 100178
[9]. Mohebbi M, Ghanbari K and Nejabati F 2023 J. Electroanal. Chem. 946 117757
[10]. Dai H et al. 2023 Microchem. J. 191 108852
[11]. Pérez-Fernández B, Maestroni B, Nakaya S, Bussalino S, Vlachou C and Escosura-Muñiz A 2023 Food Control 152 109859
[12]. Oliveira J, Burgos-Flórez F, Sampaio I, Villalba P and Zucolotto V 2023 Talanta 260 124586
Cite this article
Luo,J. (2024). Detection of food harmful substances based on electrochemical biosensors. Applied and Computational Engineering,59,192-198.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Wang W, Xu Y, Cheng N, Xie Y, Huang K and Xu W 2020 Sens. Actuators B Chem. 321 128598
[2]. Wei X et al. 2023 Sens. Actuators B Chem. (PB) 376 132941
[3]. Duan D, Ye J, Cai X and Li K 2021 Microchim Acta 188 111
[4]. Wang J, Yang X, Cui M, Liu Y, Li X, Zhang L and Zhan G 2022 Biosens. and Bioelectron. 206 114146
[5]. Zhang Y, Xu Y, Li N, Liu X, Ma Y, Yang S, Luo H, Hou H and Huo D 2023 Food Chem. 421 136131
[6]. Liu Y, Kong L, Han Z, Yuan R and Chai Y 2023 Sens. Actuators B Chem. 382 133486
[7]. Sun R et al. 2022 Food Control 145 109491
[8]. Lin C and Li Y 2023 Int. J. Electrochem. Sci. 18 100178
[9]. Mohebbi M, Ghanbari K and Nejabati F 2023 J. Electroanal. Chem. 946 117757
[10]. Dai H et al. 2023 Microchem. J. 191 108852
[11]. Pérez-Fernández B, Maestroni B, Nakaya S, Bussalino S, Vlachou C and Escosura-Muñiz A 2023 Food Control 152 109859
[12]. Oliveira J, Burgos-Flórez F, Sampaio I, Villalba P and Zucolotto V 2023 Talanta 260 124586









