1. Introduction
With the rapid development of drug delivery field, drug delivery system as an important controlled release delivery method has attracted much attention. Traditional delivery systems face a series of challenges, such as drug stability, drug release speed and delivery effectiveness. Therefore, it is an important research task to find new delivery vectors to solve these problems.
Alginate, as a kind of polysaccharide biopolymeric extracted from Marine algae, has the advantages of wide source, renewable raw material, easy preparation, biocompatibility, biodegradability and non-toxicity, and has been widely used in the field of drug delivery. Alginate can be gelated and crosslinked through the exchange of sodium ions and polyvalent cations, and the resulting crosslinked hydrogels can be used for controlled release of bioactive molecules, tumor modelling and tumor localization. In addition, alginate is bioinert and structurally stable, and the mechanical properties of the resulting gels can be precisely adjusted by means of cross-linking with ions, so that the efficacy of anti-tumor drugs can be better predicted. Alginate saline gel has superior biocompatibility and water absorption and moisture retention. Using its good biocompatibility and degradability, the drug can be effectively delivered to the designated lesion site to achieve the purpose of killing tumor cells with high local drug concentration. The nanomaterial constructed by cell adhesion peptide and alginate can effectively and quickly stop bleeding, and can increase the adhesion and mobility of NIH3T3 fiber cells, and speed up wound repair. Of course, alginate also has some disadvantages, such as high hydrophilicity will affect cell adhesion and proliferation, which also limits its use for cell delivery. Therefore, we need to develop more advantageous alginate composite hydrogels in the application of drug delivery [1].
Based on the basic properties of alginate and the preparation of cross-linking methods, this study discussed the alginate brine gel composite system and its application status in cancer treatment and wound dressing, in order to provide reference for the development and research of alginate composite hydrogels for drug delivery in the future.
2. Preparation of alginate brine gel
Sodium alginate is a linear anion polypolysaccharide commonly found in sargassum, macroalgae and other brown algae. Sodium alginate is a linear copolymer with the molecular formula (C6H7O6Na)n and the structure: α-L-gulonuronic acid (G unit) and β-D-mannouronic acid (M unit). Sodium alginate has good film forming properties, and the film obtained after drying water has high mechanical properties and stability. The basic characteristics of sodium alginate are not only regulated by its molecular weight and G/M ratio, but also the external environment is a decisive factor. For example, when the heat is high, sodium alginate decomposes quickly, and under low temperature conditions, its stability is better. When affected by ultraviolet light or oxidizing agents, it is easy to cause degradation and other reactions [2].
During the preparation of alginate gel, the composition characteristics of alginate are one of the important factors to consider. Alginate is a polymer compound containing a large number of functional groups, and its molecular chains are filled with a large number of carboxyl and hydroxyl groups, which gives it excellent water absorption and water solubility. These functional groups can interact with each other through hydrogen bond or electrostatic force, so as towrap and adsorb drug molecules and further enhance the stability of drugs.
As shown in Table 1, the preparation methods of sodium alginate hydrogels mainly include: chemical cross-linking and physical cross-linking. The most commonly used is the physical ion crosslinking method, where most of the crosslinking agents are non-toxic and harmless, conducive to use in the human body, and basically not limited by the natural environment. While the ion crosslinking method is simple, convenient and fast. However, the performance of sodium alginate hydrogels is relatively single, covalent crosslinked sodium alginate hydrogels increase the plasticity of injectable hydrogels, and can be modified by chemical modification to regulate the gelation time, gelation method to give sodium alginate gel more functions.
Table 1. Comparative analysis of different preparation methods [3-8].
Cross-linking methods | Cross-linking species | Gel mechanism |
Physical cross-linking | Ion cross-linking | Ion exchange reaction |
Electrolytic method | Mixed with chitosan in electrochemistry the composite gel was formed under the reaction | |
Covalent hydrogen bond | Alginate network was repaired by covalent hydrogen bonding | |
Chemical cross-linking | Crosslinking of hydroxyl groups | The active hydroxyl group is in a condensation reaction with a molecule containing a primary amine |
Covalent crosslinking | Sodium alginate is grafted with norbornene or tetrazine groups and cross-linked by the inverse electron demand reaction between the two groups | |
Schiff base reaction | The aldehyde group of oxidized alginates can cross-link Schiff base with amino substance |
3. Application of alginate saline gel in the biomedical field
3.1. The role of alginate gel in drug delivery
As an important drug delivery carrier, alginate gel has good encapsulation effect. The coating effect is mainly realized by electrostatic interaction, hydrogen bond and hydrophobic interaction. Alginate gel is electronegative and can combine with the cations in the drug to form a stable ionic complex, so as to realize the coating of the drug. This encapsulation improves the stability of the drug and prevents it from being broken down and inactivated in the body. The alginate gel has a porous structure inside, which can form cavities to contain drug molecules, thus achieving drug encapsulation. This encapsulation can improve the solubility of the drug and increase its stability in the body. In addition, alginate brine gels are able to achieve the encapsulation of drugs through hydrophobic interactions. Hydrophobic interaction is when the hydrophobic part of the hydrogel and the hydrophobic part of the drug combine with each other to form a hydrophobic interaction force, so as to achieve the coating of the drug. This encapsulation can improve the solubility of the drug and increase its bioavailability in the body. Injectable hydrogels avoid surgical injuries and reduce the systemic toxic side effects of drugs, showing great potential in the field of local drug delivery. Most gels cannot accurately control the proportion of different drugs during drug encapsulation and delivery, resulting in low synergistic therapeutic efficacy. The structure and synthesis process of some gels are complex, thus limiting their clinical translational applications.
To control drug release from alginate gel is one of its key roles in drug delivery. As the wrapping role of the drug is completed, alginate gel controls the rate and manner at which the drug is released by regulating its physical and chemical properties. This release control is important for improving drug efficacy, reducing side effects, and extending the duration of drug action. A common way to control release is through the physical structure of hydrogels. Alginate gels have a mesh structure and are porous, which allows the drug to be diffused through the internal voids of the hydrogel, allowing for progressive release. This release mode can be achieved by regulating the pore size and distribution of the gel, the fiber structure of the gel, and the hydrophilic and lipophilic properties of the gel. Another common form of release control is through chemical reactions. Alginate gels can regulate the rate of drug release by changing parameters such as their pH, ion concentration, and degree of crosslinking. For example, acid- base responsive release of drugs can be achieved by exploiting the acid-base response properties of the gel. In addition, the ion exchange properties of gels can be used to control the rate and manner of drug release. Alginate brine gels can also be used to achieve drug release control through a carrier drug delivery system. The system wraps the drug inside the gel to release the drug inside the body. By changing the properties and structure of the carrier, the rate of release of various drugs can be regulated and the duration of drug effects can be extended.
3.2. Cancer targeting prescription
In the process of tumor formation, spread, metastasis and recurrence, cancer stem cells (CSC), which have the function of self-recovery and can generate non-homogeneous tumor cells, play a key role. The development of a tunable scaffold system to customize the scaffold characteristics of CSCS from different tissues is essential for the study of tumors. Alginate composite hydrogels can enhance the characteristics of CSC, making it widely used in breast cancer treatment and research. Some researchers promoted the enrichment of breast cancer cells by producing alginate polycaprolactone carrier materials, and the collected cells showed high driness, favorable to epithelial- mesenchymal transformation, invasion, drug resistance and angiogenesis. Polycaprolactone is a commonly used scaffold fabrication biomaterial. The scaffold microenvironment can be controlled by adjusting the alginate/polycaprolactone fiber ratio, so that different cell populations can be selected according to its adhesion ability, thereby increasing the enrichment of multi-CSC. Moreover, the heterogeneous scaffold microenvironment can also help to isolate CSC from different tissues. Other researchers designed a uniform interpenetrating polymer network of collagen and alginate to measure the colony-forming ability of breast cancer, further enabling high-throughput in vitro screening of single-cell spheres, and reliable analysis of tumor cell viability, in hopes of screening and analyzing CSC, and increasing the study of tumor occurrence and development [1].
Liu et al. prepared alginate with pH and reduction response to tumor microenvironment, as shown in Figure 1. Using superparamagnetic Fe3O4 nanoparticles as a template, the surface of the superparamagnetic Fe3O4 nanoparticles was modified with double response alginate, and after Ca2+ crosslinking, it was used to support anti-tumor drug epirubicin hydrochloride. Degradability and superparamagnetic, under the guidance of the external magnetic field, it can effectively reach the designated lesion site, and then through the response to the low acidity and high glutathione concentration of the tumor, release its loaded epirubicin hydrochloride, in order to achieve a high local drug concentration to kill tumor cells, and reduce the damage of the drug to normal tissue. In addition, the nanoparticle can be positioned at the tumor site for nuclear magnetic imaging, and used for the integration of tumor diagnosis and treatment [9].
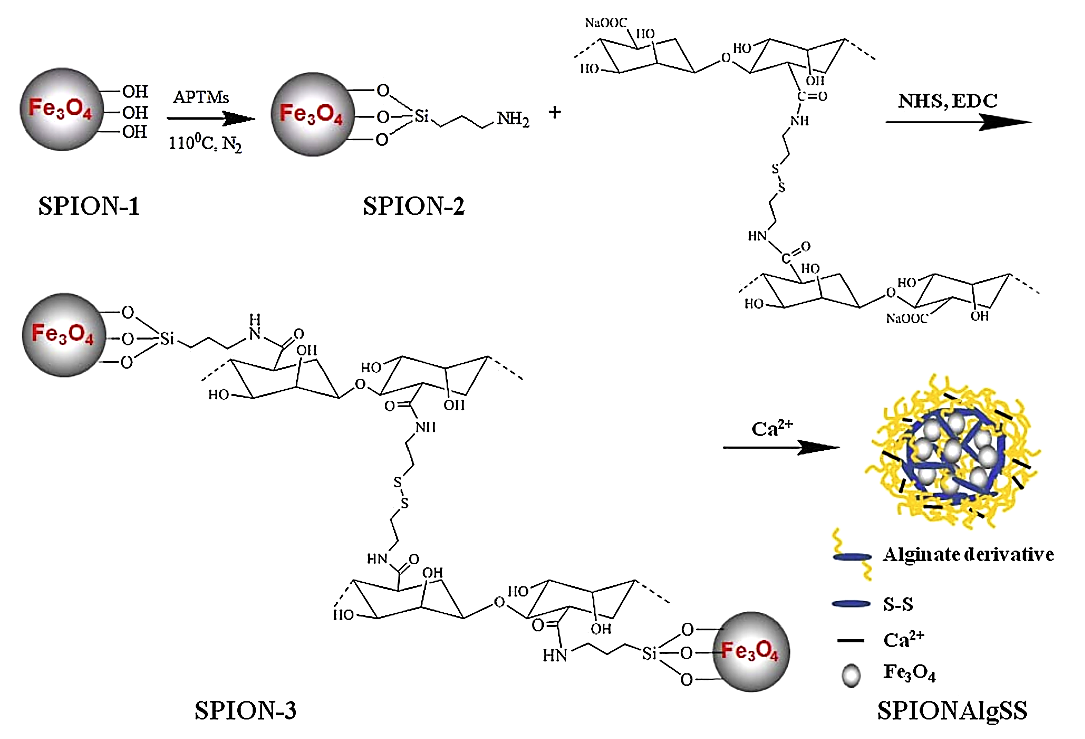
Figure 1. Pathways to prepare dual reaction alginate modified SPION [9].
Noninvasive and biocompatible targets based on mesoporous silica nanoparticles (MSN) are rapidly advancing toward DDS. In particular, controlled, leak-free, gated cavity entrances to MSN materials play an important and central role in ensuring the release of specific drugs and preventing premature leakage during transport before reaching their destination. Further research into the functional mechanisms of chensinin-1b lipopeptide on MCF-7 cells and the construction and use of its tumor-directed stimulus-response nanomedical drug delivery system will ensure that the gate material is consumed only in response to specific intrinsic or extrinsic stimuli. pH, redox potential, temperature, biomolecules, light, magnetic fields, and ultrasound, or a combination of these stimuli, are critical for precise therapeutic approaches and possible applications in the human body [10].
3.3. Wound dressing
Although alginate matrix biomaterials in combination with other bioactive substances contribute to the improvement of the skin repair process with the help of growth factors, the high price of growth factors hinders their widespread use. Therefore, the researchers innovatively used other bioactive substances at the same time to create an alginate-based hydrogel with hemostatic function and stable mechanical properties, which offers a new therapeutic potential for skin tissue engineering. For example, cell adhesion peptide and alginate isomorphism can combine into a nanomaterial, which proved that the supramolecular was effective and rapid in hemostasis, increased the adhesion and mobility of fibrocyte NIH3T3, and accelerated wound repair in a whole-skin injury experiment in mice. In addition, the existing research showed that micron-scale hydrogel containing Ac2-26 could improve the encapsulation rate, stability and release efficiency of Ac2-26, while significantly improving the wound repair speed. Completely covering the wound model within 24 hours, which may be a highly promising wound dressing [11].
4. Conclusion
This research conducted in-depth exploration on the application of alginate saline gel in biomedicine, and achieved certain research results. This research introduced the basic characteristics and preparation methods of alginate gel, as well as its key role in drug delivery. As a drug delivery carrier, alginate gel can effectively encapsulate drugs, achieve sustained release and targeted delivery of drugs, and expand the application field of drug delivery. This research also studied the release performance of alginate gel, and found that it has excellent biocompatibility and biodegradability, which provides the basis for the application of alginate gel in the field of drug delivery. To sum up, the alginate gel-based drug delivery system, as the core carrier of drug transportation, has great potential for application in the drug delivery industry. This study provides a basis for further development of alginate gel application in biomedicine, and points out future research directions and practical suggestions. However, there are still some shortcomings in this research. Understanding of the properties and delivery mechanism of alginate gel is not comprehensive enough, and further research is needed.
In the future, the application of alginate gel in the field of drug delivery can be further studied from the following aspects. Firstly, the influence of different preparation methods on the performance of alginate gel can be explored, and the synthesis process of alginate gel can be optimized to improve its delivery effect. Secondly, the release mechanism of alginate gel can be further studied to explore how to regulate and optimize the drug release behavior. In addition, practical applications can consider applying alginate gel to specific drug delivery systems and conducting clinical trials to verify its delivery effect and safety.
References
[1]. Huang LL, Huang CY, Xu MZ and Lin HQ. 2023 Modern Applied Pharmacy in China 40(16) 2295-2305
[2]. Chen X, Zheng G, Li M, et al. 2024 Chinese Journal of Tissue Engineering Research 28(5) 789
[3]. Zhang R, Xie L, Wu F, et al. 2022 Frontiers in Pharmacology 13 828896
[4]. Yang MN, Zhang N, Wang FY and Liu JG. 2021 Chinese journal of tissue engineering research 25(28)
[5]. Han Z, Wang P, Lu Y, et al. 2022 Science advances 8(8) eabl5066
[6]. Fan Z, Deng J, Li P Y, et al. 2019 Biomaterials 197 244-254
[7]. Yang Z, Zhao F, Zhang W, et al. 2021 Chemical Engineering Journal 419 129520
[8]. Fan C, Xu K, Huang Y, et al. 2021 Bioactive materials 6(4) 1150-1162
[9]. Peng N, Ding X, Wang Z, et al. 2019 Carbohydrate polymers 204 32-41
[10]. Liu Z A. Study on the mechanism of Chensinin- lb lipopeptides on MCF-7 cells and construction and application of tumor-targeted stimulus-response nanodrug delivery system. China academic journal electronic publishing house. 2019.
[11]. Zhang ZK, Li Z, Li Y, Wang YY, Wang YP, Zhou XK, Ma SS and Guan FX, 2020 Chinese Journal of Tissue Engineering Research 25(4)
Cite this article
Sun,C. (2024). Application of alginate saline gel for drug delivery, cancer treatment and wound dressing. Applied and Computational Engineering,60,165-169.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Huang LL, Huang CY, Xu MZ and Lin HQ. 2023 Modern Applied Pharmacy in China 40(16) 2295-2305
[2]. Chen X, Zheng G, Li M, et al. 2024 Chinese Journal of Tissue Engineering Research 28(5) 789
[3]. Zhang R, Xie L, Wu F, et al. 2022 Frontiers in Pharmacology 13 828896
[4]. Yang MN, Zhang N, Wang FY and Liu JG. 2021 Chinese journal of tissue engineering research 25(28)
[5]. Han Z, Wang P, Lu Y, et al. 2022 Science advances 8(8) eabl5066
[6]. Fan Z, Deng J, Li P Y, et al. 2019 Biomaterials 197 244-254
[7]. Yang Z, Zhao F, Zhang W, et al. 2021 Chemical Engineering Journal 419 129520
[8]. Fan C, Xu K, Huang Y, et al. 2021 Bioactive materials 6(4) 1150-1162
[9]. Peng N, Ding X, Wang Z, et al. 2019 Carbohydrate polymers 204 32-41
[10]. Liu Z A. Study on the mechanism of Chensinin- lb lipopeptides on MCF-7 cells and construction and application of tumor-targeted stimulus-response nanodrug delivery system. China academic journal electronic publishing house. 2019.
[11]. Zhang ZK, Li Z, Li Y, Wang YY, Wang YP, Zhou XK, Ma SS and Guan FX, 2020 Chinese Journal of Tissue Engineering Research 25(4)









