1. Introduction
Metals are functional materials that are widely used in industries, buildings’ structures, and vehicles. Corrosion is a problem that metals are always facing, it changes the mechanics and the other characteristics of the materials by reactions, causing problems such as economic loss, safety issues, and damage to the environment. The “China Corrosion Investigation Report”, which was done by the Chinese Engineering Academy, pointed out that the annual cost of corrosion in China accounts for about 5% of GDP, and its cost is greater than the total loss of all-natural disasters in the year [1]. To solve the problem, there are three main methods, which are the Corrosion inhibitor method [2-3], the Electrochemical Protection [4-6], and the Coating method [7]. The coating method has the advantage of low cost and a large range of usage, widely used to protect metals from corrosion [7]. To improve the coating, people introduced graphene. This paper mainly reviews the ways of application of graphene in organic coatings, by summarising research achievements, listing out the solution to the problems that will be met in the application, and talking about the advantages of introducing graphene, to provide inclusive information for researchers who are starting the graphene anti-corrosion coating area.
2. Combination of graphene with anti-corrosion paints
2.1. Working with epoxy materials
In organic materials, epoxy resin is the most popular one to combine with graphene, due to its characteristics like good adhesion with both metals and non-metal materials, chemical resistance, and it is compatible with a wide range of resins, fillers, and additives [8]. So it’s widely used as the material of anti-corrosion coatings, and usually the base paint when adding graphene in. Researchers have studied the synergistic gain of graphene-epoxy more deeply than that of polyurethane and acrylic acid systems [9].
2.2. Acting as filler to plug the pores
As is well known that coatings made of epoxy resin aren’t perfect. Micro may be produced during the curing process cracked pores, causing corrosive media like chlorine ions, to enter the coating through the pores, resulting in coating failure and matrix corrosion [8]. Researchers found that graphene can be mixed with the resin and filled in those pores by the advantage of its micro size, stopping corrosion molecules and dielectric going through the coating and reaching the metal base to cause corrosion. In addition, the addition of graphene can extend the path of the corrosion molecules that are going through the coating, instead of going straightly, causing a maze-like effect, which improves the ability of anti-corrosion of the coat (Figure 1).
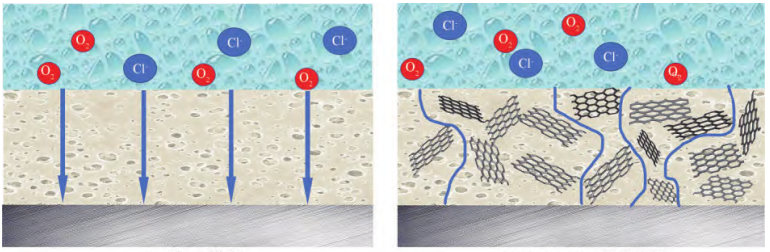
Figure 1. Schematic diagram of the anti-corrosion mechanism of pure Eps (left) coating and TRGO/Eps (right) composite coating [10]
According to Tu’s group’s research [11], the effect of graphene-modified epoxy coating on 10% hydrochloric acid solution for 72h was tested. With the increase of graphene content, the mass loss rate of the epoxy coating protected by the epoxy coating during the acid leaching process decreased, and when the graphene was mixed with 50% mass fraction, the mass loss rate of the metal substrate was 0.48%, which was 41% less than that of the pure epoxy coating without graphene addition. This led to the result that graphene not only can higher the mechanical property of the coat but also improve the corrosion resistance ability by constructing a multi-dimensional labyrinth structure to extend the intrusion path of erosive media.
2.3. Composite with corrosion inhibitors and making self-healing coating
In the realistic, coatings often face unpredictable mechanical damage, which breaks the coat and allows media from outside to pass through easier or direct contact with the metal base, causing corrosion. Corrosion inhibitors are used to stop or slow the reaction of corrosion, by forming passivation film on the metal base. The large specific surface area of graphene and its derivatives allows it to support a wide range of corrosion inhibitors, when the coating breaks, the corrosion inhibitor is released from the graphene surface and passivates the surface of the exposed metal substrate, enabling self-healing [9]. Another way to carry corrosion inhibitors is to use capsules.
Wang and his group [12] introduced a corrosion-inhibiting WEP anticorrosive coating with the triple anti-corrosion function was prepared by introducing polyotoluidine (POT) microcapsules on the surface of the modified graphene oxide (GO) and further encapsulating the corrosion inhibitor 2-mercaptobenzothiazole (MBT) inside the modified GO (Figure 2).
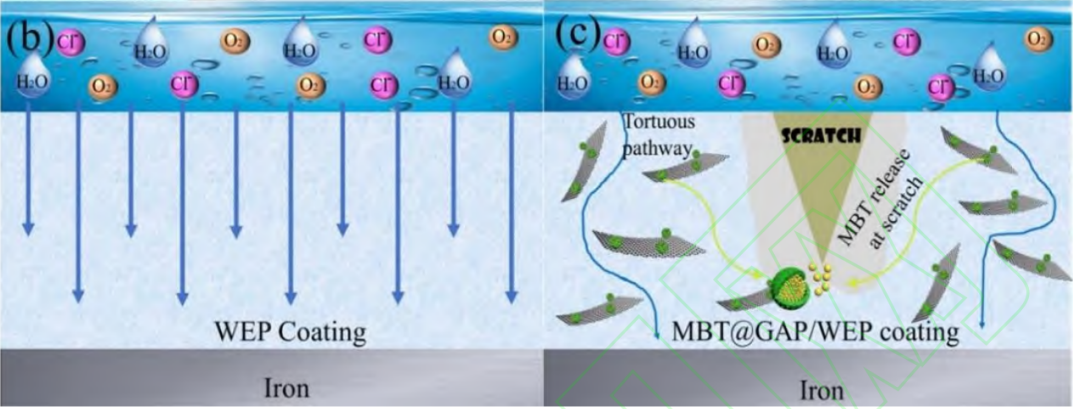
Figure 2. Corrosion resistance mechanism diagram of (b) WEP and (c) MBT@GAP/WEP coatings [12]
In Wang’s experiment, mechanical damage was simulated by creating scratches with a razor blade on control samples, which were then left to stand in a room-temperature environment. After 10 hours, the scratches were largely healed. The results demonstrate the self-healing ability of the layers and exhibit their corrosion protection properties.
And from Ma’s group’s research [13], A polyurea prepolymer was obtained by adding the GO dispersion to a mixture of isophorone diisocyanate and polyetheramine D2000, which was polymerized at the interface with 1,6-hexanediamine to produce monolayer or bilayer microcapsules with a diameter of only 0.5mm, which was thermally stable below 325 °C and had a self-healing efficiency of up to 80% for epoxy coatings with a mass fraction of 3% [9].
3. Problems when applying graphene to anti-corrosion coat
Firstly, because of the existence of Van Der Waals force between graphene layers, graphene becomes harder to separate as layers and agglomerate together. This effect negatively to the anti-corrosion ability of the coat, because graphene won’t be able to act as shielding to stop molecules going through the coat, even causing damage to the original shielding performance.
Secondly, because of the good electrical conductivity of graphene, the coating forms a galvanic couple with the metal substrate after breakage, which can easily accelerate metal corrosion [14-16].
Finally, the range of graphene in the coat. The corrosion resistance of graphene depends not only on whether it can be evenly dispersed in the coating but also closely related to the arrangement [9]. If the direction of graphene layers is not arrangeable, then it would reduce the ability of corrosion resistance.
4. Solutions to the common problems existed
4.1. Modification of graphene
When making composite coatings, graphene is not added and mixed to the base coat straightly. The most common way to process graphene before is modification. Different kinds of modifications to graphene, commonly seen as graphene oxides (GO), can give different characteristics that can lower the Van Der Waals forces between layers and improve superficial amphiphilia [7], therefore allowing it to be used in further steps.
In Wang and his group’s research [12], graphene oxide (GO), 3-aminobenzene sulfonic acid (ASA) and o-toluidine (OT) were used as raw materials to prepare polyotoluidine-oxygen by inorganic-organic hybridization on the surface of modified GO Graphene-based (GAP) composites. The successful reaction of OT on the modified GO surface was demonstrated by FTIR, XPS, and SEM tests, showing it increased the interlayer spacing of GO and improved the two-dimensional filler’s susceptibility to agglomeration.
According to Liu and his group’s research [17], graphene oxide was prepared by Hummers’ method and successfully modified the graphene surface with amine functional groups and amine content after reductive modification. The resulting graphene showed enhanced thickness, reduced wrinkles, improved dispersion, and reduced agglomeration. The conductivity of the amine-modified graphene was greatly reduced, weakening the corrosion-promoting effect after coating breakage.
In Li’s group’s research [18], modified graphene oxide (MGO) was generated by modifying GO with [3-(2-aminoethyl) aminopropyl] trimethoxysilane. The laminar structure of MGO remained unchanged during cross-linking with the resin and the layer spacing was increased. So the prepared organic coating has a “maze structure” corrosion path, while the low conductivity of MGO is favorable to improve the corrosion resistance of the composite material [7]. By different modifications, common problems like agglomeration and acceleration of the corrosion can be solved.
4.2. Arranging graphene by magnetic field
The ability of corrosion protection of graphene composite coating is also closely related to the length of the path that molecules need to go through the coat. And the arrangement of graphene is the main factor in this. The better the graphene is arranged, the longer the path, and the greater the anti-corrosion ability.
According to Li’s group’s research paper [9], the method of using a magnetic or electric field to induce graphene was introduced by reviewing. Ding and his group’s research [19] was mentioned Li’ group that the modified polyol-supported Fe2O3 graphene material applies a certain horizontal magnetic torque to the magnetic graphene micro-nano sheet, which can make it rotate and arrange in an orderly manner in the epoxy coating (Figure 3).
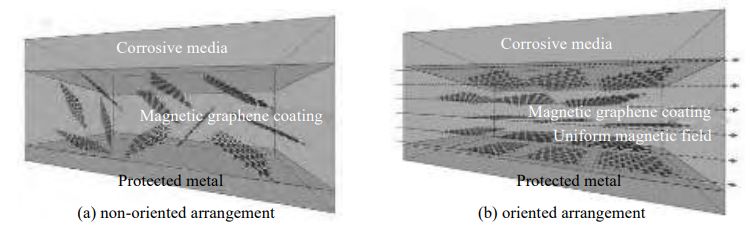
Figure 3. Non-oriented and oriented arrangement of magnetic graphene in uniform magnetic field [18]
5. Advantages of the introduction of graphene into anti-corrosion coatings
5.1. Environmental friendly and unreactive
Zhou and his research group [8] pointed out that graphene not only has good chemical inertness, oxidation resistance, and barrier properties but also is a non-toxic and harmless environmentally friendly material.
Graphene, as a special 2D material, is only layers of graphics, which is damaging less to the environment. Usually, anti-corrosion coatings are used in bridge structures and offshore environments. Structurally, in addition to the σ bonds connected to form a hexagonal ring layer structure, the vertical layer forms multi-atom connected π bonds, which are similar to benzene six-membered rings, and the bonds are very stable, so graphene has high chemical stability [20].
5.2. Ability to carry corrosion inhibitors
The self-healing coating is the one that can fix the damaged region itself. Without human intervention, a dense catalytic passivation film or slow-release adsorption film can be formed on the bare metal surface of the coating defect site, and the physical barrier function of the coating can be automatically restored by sealing the defect or inhibiting the corrosion reaction at the defect [9]. Even though it’s all done by corrosion inhibitors, sometimes used with capsules to carry, it can exert better performance when compositing with graphene.
As mentioned by Li’s group’s research paper [9], Ye’s group from Ningbo Institute of Materials, Chinese Academy of Sciences [21] prepared silylated aniline trimer (SAT) can form a cross-linking network with graphene after hydrolysis under certain conditions, which ensures the non-destructive dispersion of graphene in water and epoxy coatings, and the thickness of the modified graphene is only 1.4~1.6nm (4~5 layers), and the barrier effect is obvious. SAT, on the other hand, retains the intact electrical activity of aniline oligomers and acts as a corrosion inhibitor to inhibit corrosion reactions at minor defects. The SAT-G/EP coating is capable of forming Fe2O3, and Fe3O4 passivation films on the metal substrate, thus exhibiting self-healing and long-term protection. At the same time, the research group obtained a GO-containing porous backbone (8-PG) by chemical grafting of octaaminosesquioxane and then loaded benzotriazole (BTA) to prepare graphene-based nano containers. It was found that the corrosion and diffusion under the pure EP coating were very severe with the soaking time, while the 8-PG-BTA/EP composite layer was relatively slight and gradually weakened. This is because the release of BTA molecules not only acts as corrosion inhibition but also increases the density of the graphene coating and inhibits the vertical propagation of the corrosive medium [9].
5.3. Improving the adhesion capacity of the coat
Other than higher the anti-corrosion ability of the coat, graphene can also improve the adhesion of the coat to the metal base.
According to Parhizkar’s group’s research [22], graphene can increase the roughness of the surface of the metal base, also, by forming N-H bonds with epoxy resin and forming Si-O-Fe bonds with the metal surface to improve the Adhesion between the epoxy resin coat and the metal base [8]. Also, graphene-modified acrylate structural adhesive forms strong adhesion with a wide range of colored metals by irradiating ultraviolet light of certain wavelengths [8].
Zhou’s group’s research [20] compared the surface topography and bond strength after the coating pull-out experiments of epoxy coat samples with and without graphene addition, resulting in 11.7MPa of strength when adding 0.5% of graphene-modified coatings. Showing that the addition of graphene improves the adhesion capacity of the paint film.
6. Conclusion
Based on the research on graphene anti-corrosion paint now, it’s a fact that the main focus of the area is the composition of graphene and organic paint, especially those epoxy materials with properties like good adhesion capacity, good corrosion resistance, and good compatibility with a wide range of fillers. In the composition paint, graphene acts as a filler, because of the size advantage, it can fill the pores that exist when producing the epoxy paint to stop the corrosive media from passing through the protection layers or to longer the path those media need to pass through, which is called the “maze effect”, improve the corrosion resistance of the paint. Another way to use graphene is to carry corrosion inhibitors or to composite with inhibitor carriers, to bring self-healing property to the paint. Furthermore, problems exist during the application. The common ones are agglomeration and the ranging of graphene, and accelerating the corrosion due to the good electrical conductivity of graphene. The agglomeration and conduction problems can be solved by modification to change its properties. And researchers found that the graphene pieces can be arranged in fields. In addition, graphene has unmarkable advantages applied to environmentally friendly anti-corrosion paints, ability to carry corrosion inhibitors, and being able to improve the adhesion capacity of the paint.
This paper is a review of the main trend of research in graphene anti-corrosion paint, the information will be provided to researchers and scholars who are interested in the area and looking for an overview of the whole situation. However, there are deficiencies. Because of the limitation of the author, on academic qualifications, there are barriers to the source of literature and limitation on understanding. So the understanding of literature may not be as deep in the paper.
References
[1]. Anonymous. Corrosion science and protection technology, 2004(01):65. DOI:CNKI:SUN:FSFJ.0.2004-01-019.
[2]. Sengupta S., Murmu M., Mandal S., et al. Competitive corrosion inhibition performance of alkyl/acyl substituted 2-(2-hydroxybenzylideneamino) phenol protecting mild steel used in adverse acidic medium: a dual approach analysis using FMOs/molecular dynamics simulation corroborated experimental findings [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 617: 126314.
[3]. Anitha R., Unnisa C.B.N., Hemapriya V., et al. Anti-corrosive potential of cyperus rotundus as a viable corrosion inhibitor for mild steel in sulphuric acid [J]. Pigment & Resin Technology, 2020, 49(4): 295-304.
[4]. Zhou Y., Zhang P., Yan F. Corrosion resistance of a nano-Mg modified silane conversion coating with cathodic protection on magnesium alloy AZ91D [J]. Materials Letters, 2021, 284(1): 128930.
[5]. Kaghazchi L., Naderi R., Ramezanzadeh B. Construction of a high-performance anti-corrosion film based on the green tannic acid molecules and zinc cations on steel: electrochemical/surface investigations [J]. Construction and Building Materials, 2020, 262: 120861.
[6]. Huang H.J., Fu Y., Mu X.J., et al. Molecular self-assembly of novel amphiphilic topological hyperbranched polymers for super protection of copper in extremely aggressive acid solution [J]. Applied Surface Science, 2020, 529: 147076.
[7]. Liwen Cao, Yao Li, Haojie Chen, Jiangang Qu. Research on the application of modified graphene oxide in anticorrosive coatings. New Chemical Materials. https://link.cnki.net/urlid/ 11.2357.TQ.20230914.2027.005
[8]. Maoqiang Zhou, Xiaotian Zhou, Wenhui Zhao. Anti-corrosion performance of graphene-modified fiber-reinforced high-performance resin composite coatings in marine environment [J]. New Building Materials, 2022, 49 (10): 37-39+64.
[9]. Tongsheng Li, Haocheng Zhan, Huiping Lan, et al. Progress in Graphene Oxide Modification and Its Application in Epoxy Anticorrosive Coating [J]. Journal of Chemical Engineering of Chinese Universities, 2023, 37(04):525-536.)
[10]. Yang He, Siying Li, Chuanqiang Li, et al. Anti-corrosion properties of thermally reduced graphene oxide/epoxy composite coatings [J]. Advances in Chemical Engineering, 2023, 42(04):1983-1994. DOI:10.16085/j.issn.1000-6613.2022-1178.
[11]. Xin Tu, Jianyi Deng, Wendong Wu, Yu Tong, Maodong Li, Ding Jinsen. Preparation and protective properties of graphene-modified epoxy resin coatings. 1002-7432(2023) 04-0040-05.
[12]. Haihua Wang, Mengyu Ye, Guiqiang Fei, et al. Preparation and properties of corrosion-inhibiting polyotoluidine-graphene oxide-based anticorrosive materials [J/OL]. Journal of Composite Materials, 1-13[2023-12-03]. https://doi.org/10.13801/j.cnki.fhclxb.20230817.006.
[13]. Ma Y.X., Zhang Y.R., Liu J.T., et al. GO-modified double-walled polyurea microcapsules/epoxy composites for marine anticorrosive self-healing coating [J]. Materials & Design, 2020, 189: 108547-108562.
[14]. Wenge Chen, Yixiao Yang, Qian Zhao, et al. Nanoscale mechanics of metal-coated graphene nanocomposite powders [J] Materials Today Communications. 2022(33): 104731.
[15]. Zhengqing Yang, Wen Sun, Lida Wang, et al. Tuning the oxygen reduction reaction activity of graphene through fluorination modification to inhibit its corrosion-promotion activity [J] Corrosion Science, 2020 (175): 108860.
[16]. Wen Sun, Lida Wang, Tingting Wu, et al. Synthesis of low electrical-conductivity graphene / pernigraniline composites and their application in corrosion protection [J] Carbon, 2014 (79): 605-614.
[17]. Kaixin Liu, Wen Sun, Lida Wang, Guichang Liu, et al. Amino modulation of graphene and its effect on corrosion protection properties of coating. 0253-4320( 2023) 11-0085-07.
[18]. Li H.Q., Xue C.H., Gao L., et al. “Labyrinthine structure” anticorrosive water-based composite coatings [J]. Progress in Organic Coatings, 2021, 150, 105974.
[19]. Ding R., Chen S., Zhou N., et al. The diffusion-dynamical and electrochemical effect mechanism of oriented magnetic graphene on zinc-rich coatings and the electrodynamics and quantum mechanics mechanism of electron conduction in graphene zinc-rich coatings [J]. Journal of Alloys and Compounds, 2019, 784: 756-768.
[20]. Zhongwei Zhou, Jianxiong Zhang, Shaobo Jin, et al. Application and performance of graphene in anticorrosive coatings [J]. Coating Protection, 2022, 43(11):60-64.
[21]. Ye Y.W., Zhang D.W., Liu T., et al. Superior corrosion resistance and self-healable epoxy coating pigmented with silanzied trianiline-intercalated graphene [J]. Carbon, 2019, 142: 164-176.
[22]. Parhizkar N., Shahrabi T., Ramezanzadeh B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film [J]. Corrosion Science, 2017, 123: 55-75.
Cite this article
Xiao,W. (2024). Applications of graphene in organic anticorrosive coatings. Applied and Computational Engineering,63,21-27.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Anonymous. Corrosion science and protection technology, 2004(01):65. DOI:CNKI:SUN:FSFJ.0.2004-01-019.
[2]. Sengupta S., Murmu M., Mandal S., et al. Competitive corrosion inhibition performance of alkyl/acyl substituted 2-(2-hydroxybenzylideneamino) phenol protecting mild steel used in adverse acidic medium: a dual approach analysis using FMOs/molecular dynamics simulation corroborated experimental findings [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 617: 126314.
[3]. Anitha R., Unnisa C.B.N., Hemapriya V., et al. Anti-corrosive potential of cyperus rotundus as a viable corrosion inhibitor for mild steel in sulphuric acid [J]. Pigment & Resin Technology, 2020, 49(4): 295-304.
[4]. Zhou Y., Zhang P., Yan F. Corrosion resistance of a nano-Mg modified silane conversion coating with cathodic protection on magnesium alloy AZ91D [J]. Materials Letters, 2021, 284(1): 128930.
[5]. Kaghazchi L., Naderi R., Ramezanzadeh B. Construction of a high-performance anti-corrosion film based on the green tannic acid molecules and zinc cations on steel: electrochemical/surface investigations [J]. Construction and Building Materials, 2020, 262: 120861.
[6]. Huang H.J., Fu Y., Mu X.J., et al. Molecular self-assembly of novel amphiphilic topological hyperbranched polymers for super protection of copper in extremely aggressive acid solution [J]. Applied Surface Science, 2020, 529: 147076.
[7]. Liwen Cao, Yao Li, Haojie Chen, Jiangang Qu. Research on the application of modified graphene oxide in anticorrosive coatings. New Chemical Materials. https://link.cnki.net/urlid/ 11.2357.TQ.20230914.2027.005
[8]. Maoqiang Zhou, Xiaotian Zhou, Wenhui Zhao. Anti-corrosion performance of graphene-modified fiber-reinforced high-performance resin composite coatings in marine environment [J]. New Building Materials, 2022, 49 (10): 37-39+64.
[9]. Tongsheng Li, Haocheng Zhan, Huiping Lan, et al. Progress in Graphene Oxide Modification and Its Application in Epoxy Anticorrosive Coating [J]. Journal of Chemical Engineering of Chinese Universities, 2023, 37(04):525-536.)
[10]. Yang He, Siying Li, Chuanqiang Li, et al. Anti-corrosion properties of thermally reduced graphene oxide/epoxy composite coatings [J]. Advances in Chemical Engineering, 2023, 42(04):1983-1994. DOI:10.16085/j.issn.1000-6613.2022-1178.
[11]. Xin Tu, Jianyi Deng, Wendong Wu, Yu Tong, Maodong Li, Ding Jinsen. Preparation and protective properties of graphene-modified epoxy resin coatings. 1002-7432(2023) 04-0040-05.
[12]. Haihua Wang, Mengyu Ye, Guiqiang Fei, et al. Preparation and properties of corrosion-inhibiting polyotoluidine-graphene oxide-based anticorrosive materials [J/OL]. Journal of Composite Materials, 1-13[2023-12-03]. https://doi.org/10.13801/j.cnki.fhclxb.20230817.006.
[13]. Ma Y.X., Zhang Y.R., Liu J.T., et al. GO-modified double-walled polyurea microcapsules/epoxy composites for marine anticorrosive self-healing coating [J]. Materials & Design, 2020, 189: 108547-108562.
[14]. Wenge Chen, Yixiao Yang, Qian Zhao, et al. Nanoscale mechanics of metal-coated graphene nanocomposite powders [J] Materials Today Communications. 2022(33): 104731.
[15]. Zhengqing Yang, Wen Sun, Lida Wang, et al. Tuning the oxygen reduction reaction activity of graphene through fluorination modification to inhibit its corrosion-promotion activity [J] Corrosion Science, 2020 (175): 108860.
[16]. Wen Sun, Lida Wang, Tingting Wu, et al. Synthesis of low electrical-conductivity graphene / pernigraniline composites and their application in corrosion protection [J] Carbon, 2014 (79): 605-614.
[17]. Kaixin Liu, Wen Sun, Lida Wang, Guichang Liu, et al. Amino modulation of graphene and its effect on corrosion protection properties of coating. 0253-4320( 2023) 11-0085-07.
[18]. Li H.Q., Xue C.H., Gao L., et al. “Labyrinthine structure” anticorrosive water-based composite coatings [J]. Progress in Organic Coatings, 2021, 150, 105974.
[19]. Ding R., Chen S., Zhou N., et al. The diffusion-dynamical and electrochemical effect mechanism of oriented magnetic graphene on zinc-rich coatings and the electrodynamics and quantum mechanics mechanism of electron conduction in graphene zinc-rich coatings [J]. Journal of Alloys and Compounds, 2019, 784: 756-768.
[20]. Zhongwei Zhou, Jianxiong Zhang, Shaobo Jin, et al. Application and performance of graphene in anticorrosive coatings [J]. Coating Protection, 2022, 43(11):60-64.
[21]. Ye Y.W., Zhang D.W., Liu T., et al. Superior corrosion resistance and self-healable epoxy coating pigmented with silanzied trianiline-intercalated graphene [J]. Carbon, 2019, 142: 164-176.
[22]. Parhizkar N., Shahrabi T., Ramezanzadeh B. A new approach for enhancement of the corrosion protection properties and interfacial adhesion bonds between the epoxy coating and steel substrate through surface treatment by covalently modified amino functionalized graphene oxide film [J]. Corrosion Science, 2017, 123: 55-75.









