1. Introduction
Nowadays the global warming becomes a serious issue since there are more greenhouse gas emissions, and carbon dioxide occupies 65% of that [1]. Hence, it is crucial to reduce the emission of carbon dioxide. 33.6 giga tons of CO2 was emitted in 2019 and 8.2 giga tons of that was due to the consumptions of fuels in transportations [2]. In the sector of transportations, on road vehicles consume the largest portion of the fuel, for example, around 54.2% of fuel was consumed by passenger vehicles in the US [3] and the road transport accounts for more than half of fuel demand in the UK [4]. Moreover, there are 1.44 billion cars around the world in 2021 [5], and the number is still raising with a increment of 3% between 2020 and 2021 [6]. Therefore, it is important to improve the fuel economy of vehicles in order to reduce the CO2 emission. Making the vehicles lighter by using lightweight new materials could achieve the goal. Up to 55% of the weight in car body are made of steel [7]. Steel is suitable for building a vehicle due to the mechanical properties of high strength, toughness, and stiffness. However, some traditional steels such as mild steel is relatively heavier than some new materials. In the direction of having lighter vehicles, some alternative materials can replace the traditional steels of the car such as new high-strength steel, aluminum alloy, magnesium alloy, and carbon fiber composites [8-11].
Aluminum alloy is used in a wide range of automotive applications including but not limited to frames, pistons, brackets, and wheels. Compared with steel, the density of aluminum is only 1/3 [12] of that of steel. Generally speaking, the weight of the body in the whole car is about 30% [12]. If the whole body is changed from steel to aluminum, the maximum weight will be reduced by about 40%. In terms of strength alone, the strength of aluminum is not as high as that of steel, but we can increase the strength by thickness for a total lower weight. As mentioned before, the density of aluminum is only 1/3 of that of steel, so under the same strength, aluminum can be lighter. Another very overlooked advantage of aluminum is that it is easily oxidized, which can be used to form a protective film against corrosion. However, aluminum alloy body also has many problems. First of all, the welding difficulty is very high, and the general welding method can not be used. Secondly, the maintenance difficulty and cost are very high, once the damage is serious, it can only be recycled.
Due to its excellent properties, magnesium has been used more and more in vehicles. Magnesium is the lightest of all engineered materials, with a density of 1.74 g/cm3 [13, 14]. It is 35% lighter than aluminum (2.7 g/cm3) and more than 400 % lighter than steel (7.86 g/cm3). Secondly, in addition to its high electrical and thermal conductivity, it has outstanding rigidity and a strength-to-weight ratio. It can withstand high operating temperatures and has good and excellent elasticity [13]. Despite these benefits, magnesium also possesses low strength and toughness, a high tendency to electrochemical rusting when in touch with different materials or electrolytes [14], difficulty in deformation by cold working, and a tendency to burn with oxygen. Therefore, there are many considerations in the manufacture and use of magnesium alloys to meet the basic requirements of vehicles and components.
The term "carbon fibre" refers to a type of fibre that has a high strength and high modulus and contains more than 90% carbon. Raw materials such as acrylic fibre and viscose fibre are oxidised at a high temperature and turned into carbon by using these two types of fibre. When compared to the tensile strength of steel, which is only 330 MPa, the tensile strength of carbon fibre can reach up to 3500 MPa, which is equivalent to an eightfold increase. Carbon fibre has a very high strength, but it is a material that breaks easily when subjected to a transverse force. This contrasts with steel, which has a constant tensile strength in all directions. As was just mentioned, the specific strength of carbon fibre is significantly greater than that of steel. This is because the density of carbon fibre is significantly lower than that of steel; in fact, the density of carbon fibre is only 1/5 of that of steel, which results in a significantly greater specific strength. In addition, carbon fibre has very good chemical performance in terms of resistance to acid and alkali, and it still has an advantage in being able to use it in a harsh environment. On the other hand, steel is easy to oxidise on rainy days, so simply looking at corrosion resistance, carbon fibre materials have higher performance advantages. In addition, there is the cost involved after the utilisation of the materials, which must be at or below the price of steel. At the same time, its density is typically quite low, and among the known engineering materials, its specific strength and specific modulus are the highest.
In this paper, the automotive sector as well as uses of magnesium, aluminum alloys, and carbon fibre composites for the automotive industry are discussed in terms of their potential contribution to the reduction of vehicle weight and improvement of fuel economy. In this section, information regarding the benefits, technical limitations, and prospects of each newly developed lightweight material for use in the automotive industry is presented. A comparison of the mechanical properties of different materials, such as their strength, hardness, and stiffness as well as their manufacturability factors, with their suitability and cost in order to select the best materials to be added to different body parts in order to reduce vehicle weight and ultimately achieve improved fuel economy.
2. Discussion
2.1. Steel
2.1.1. Traditional steel. Low-carbon steel, which is often referred to as mild steel, is one of the types of steel that is used the most frequently in the manufacturing of machinery and automobiles. Low-carbon steel can have a maximum carbon content of 0.30% [12], and its strength can range anywhere from 241 MPa to 2475 MPa [13]. A36 steel is one of the low-carbon steels that is frequently used for automotive applications. Its strength is between 400 and 550 MPa, and its composition is Fe-98C-0.25Mn-1.03Cu0.2P-0.04Si-0.28S-0.05 [14]. On the other hand, A36 has a comparatively high density of 7850 kilogrammes per cubic metre. As a result, in order to enhance the efficiency with which a vehicle burns gasoline, a number of researchers have investigated how some new types of high-strength steel can reduce the weight of a vehicle by making use of less material while maintaining the vehicle's level of safety.
2.1.2. New High-strength steels. Because austenite has the ability to participate in a wide variety of strengthening mechanisms, steels that are based on Fe-Mn-Al-C alloy systems and either austenitic or ferrite austenitic dual-phase microstructures are being considered for high-strength applications. S. Sohn and colleagues conducted research on a lightweight steel with the chemical composition Fe–0.7C–12Mn–5.5Al. Both its tensile and yield strengths are exceptionally high, coming in at 1.4 GPa and 1.3 GPa, respectively. Because it contains between 5 and 6% aluminum, the high-strength steel has a density that is 7.02 g/cm3, which is 10% lower than the density of ordinary steel [13]. After being homogenised at a temperature of 1200 °C for an hour and then hot-rolled between a temperature range of 1100 °C and 900 °C, this material is put to the test. After that, it spends one hour being cooled in a furnace. Steel sheet with a thickness of 1 millimeter can be produced by rolling steel sheet with a thickness of 3 millimeters at room temperature. At this point, it undergoes an annealing process at 640 degrees Celsius for ten minutes before being cooled at ambient temperature. The researchers detected peaks of austenite, ferrite, and -carbide in the steel's microstructures. The austenite grains are equally distributed, and finely flaking secondary phases may be found at the grain boundaries [15]. This material demonstrates an extremely high strength, which is greater than twice that of the A36 standard. When applied to cars, this might result in a reduction of weight of up to fifty percent. However, due to the high cost, low manufacturability, and high brittleness of high strength steel, it is not expected that high strength steel would completely replace standard steel in automobiles. Therefore, additional research is necessary to find a solution to these issues.
2.2. Aluminum alloy
2.2.1. Definition of aluminum alloy. Aluminum alloy is the non-ferrous metal structural material that is used the most frequently in industry. It has found widespread application in a variety of fields, including aviation, aerospace, automobile manufacture, machinery manufacturing, shipbuilding, and the chemical sector. Because of the recent rapid advancements in science and technology within the industrial economy in recent years, there has been an increase in demand for welded structural parts made of aluminum alloys. As a consequence of this, there has been a proliferation of research on the weldability of aluminum alloys. An aluminum alloy is the product of combining a number of different metals, and the end product of this process can vary greatly depending on which metals are combined.
The primary reason why we don't directly use aluminum is due to the fact that it is a highly pricey metal, which would result in a significant rise in the overall cost. Next, aluminum has a low melting point in comparison to other metals, which makes it difficult to weld, and it also has a high thermal expansion rate, thus welding aluminum is difficult. In addition to this, aluminum is a material that readily reacts with oxygen, which means that during the welding process, we need also remove the oxides.
2.2.2. Problems with aluminum alloy. Weldability is the primary concern for aluminum alloys because the primary properties of aluminum are incompatible with the welding process. As was discussed earlier, aluminum has a lower melting point, a higher thermal expansion, and an easier time reacting with oxygen. These characteristics make aluminum an unsuitable material for welding. In addition, some types of aluminum alloy present additional challenges. For instance, the strength of an aluminum alloy would often be lower if it has a good weldability, but this is not always the case.
2.2.3. Solution to this problem. There are certain distinctions to be made between the various aluminum alloys. wrought aluminum alloys and cast aluminum alloys both exist, but the differences between the two are relatively minor. For instance, the cast alloy can contain more other metals while the wrough alloy does not. Additionally, there are variations in melting point, thickness tensile strength, defects, and temperature tolerance. These variations allow us to use cast or wrought aluminum alloy in different places during welding.
In addition, there is a distinction to be made between the various components that can be mixed with aluminum to generate different types of aluminum alloy. For instance, if we add magnesium to aluminum in the form of the series called 5xxx, we will get moderate-to-high strength, good weldability, and good corrosion resistance; if we make an aluminum alloy with zinc in the form of the series called 7xxx, the alloy will be moderate-to-very high strength; and if we choose to add nickel to aluminum and form an aluminum alloy, the alloy will have a lower coefficient of expansion, making the process of welding easier. However, if we choose an aluminum alloy with magnesium or zinc, we cannot be certain of the weldability, changeability, or deformation quantity. On the other hand, if we choose aluminum and add nickel, we cannot be certain of the strength; consequently, we can use different types of aluminum alloy in different parts to ensure the lowest weight and highest strength. In addition, in order to make sure that we are able to reduce the overall weight, we can make the thickness of the aluminum alloy metal that we are using greater while still maintaining its strength.
2.3. Magnesium alloys
2.3.1. Application and background of magnesium alloy. During the Second World War, several aircraft began using magnesium alloys, which allowed for a reduction in weight [16]. Magnesium alloys are often only used for components that are related to the engine in industrial and commercial aircraft. This is due to the fact that magnesium alloy components are seen as being dangerous in the event of a fire or corrosion. The percentage of applications that make use of magnesium alloys in the automotive industry has been steadily climbing [17].
Magnesium (Mg) is the eighth most common element. It can happen either by the electrolysis of magnesium chloride melt in seawater or through the thermal metal reduction of magnesium oxide with silica. Both of these processes use magnesium. Each litre of water has 1.29 grammes (0.3%) of magnesium already present. In addition, the extraction of magnesium can be accomplished through calcination of MgCO3, the Pidgeon process (thermal reduction), or the Dow method (electrolysis) [18]. However, these techniques are costly, which makes the cost of refining an impediment and contributes to his lack of widespread use.
Recent research and discoveries on magnesium and magnesium alloys have mostly focused on lightening the load, cutting energy consumption, and minimising negative effects on the environment [19]. These days, more and more automobiles are being built with components like magnesium transmission boxes, high-performance engine blocks, and auto body structures made of magnesium. The exploration and development of magnesium alloys have been put to good use by companies that specialise in the manufacturing of automobiles. The Volkswagen firm was a pioneer in the use of magnesium alloys in the automotive industry. For its Beetle model, Volkswagen used 22 kg of magnesium in each vehicle. In 1928, Porsche debuted their first engine made of magnesium. The average consumption of magnesium per vehicle was 3 kilogrammes in 2005, 20 kilogrammes in 2010, and 50 kilogrammes in 2015, according to projections for growth. In order to satisfy these requirements, it is necessary to satisfy not only the technical, ecological, and economic requirements, but also the fundamental requirements for automotive components that are outlined in Figure 1.
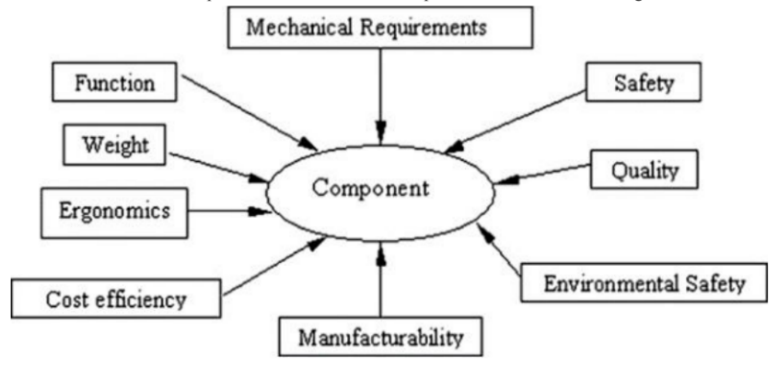
Figure 1. Basic requirements for vehicle components [15]
2.3.2. Magnesium alloys properties. Magnesium is believed to be the lightest of all designed metallic elements that are created as engineering alloys due to its density of 1.74 grammes per cubic centimetre. The hexagonal lattice structure that it possesses is what determines the fundamental qualities that it possesses. Magnesium has a density that is about the same as aluminum but only about one fifth of that of steel. Because of this, the push in the field to lower the body structure began to prefer magnesium as a means of improving some challenges that had previously been intractable. These difficulties include corrosion, resistance, and porosity. In Table 1, we compare the magnesium alloys and steels with regard to their representative mechanical parameters. In comparison to aluminum, the elasticity and noise and vibration-damping qualities of magnesium are significantly higher. It possesses remarkable physical and mechanical features such as high resistance, strength, and toughness, among others. These qualities include an improved ability to be manufactured, a longer life in service, and the prospect of faster solidification due to a lower latent heat. Because of this, a greater quantity of magnesium alloys may be produced in a shorter amount of time compared to aluminum alloys.
In addition to this, they are also more amenable to being machined [20]. In automotive applications where a reduction in weight is necessary, we can accomplish this goal by reducing the amount of energy required for rolling damping and acceleration, which in turn results in a reduction in fuel consumption. In addition to that, it is feasible to lower the amount of carbon dioxide that is released into the atmosphere. The high cost, on the other hand, makes the effective use of magnesium alloys in the automobile industry difficult to achieve. Magnesium does, however, have a few drawbacks, including the following: strength and toughness that do not meet the body manufacturing conditions; susceptibility to electrochemical corrosion when in contact with various metals or electrolytes; the possibility of using cold working; challenging deformations; and the tendency to oxygen combustion. Magnesium's strength and toughness do not meet the body manufacturing conditions. Magnesium's susceptibility to electrochemical corrosion when in contact with different metals or Table 1. Comparison of the mechanical properties of magnesium alloys and steel [21].
Table 1. Comparison of the mechanical properties of magnesium alloys and steel [21]
Density (r)g/cc | Tensile Strength, Yield MPa | Hardness | Tensile Strength, Ultimate MPa | Shear Modulus GPa | |
Magnesium (AM60A-F) | 1.80 | 131 | 65 | 241 | 17.0 |
Steel (A36) | 7.85 | 250 | 120 | 400-550 | 79.3 |
2.3.3. Magnesium alloying and casting. Magnesium alloys are the most common form in which magnesium's chemical composition can be found in nature. Magnesium in its purest form has a rather low mechanical strength, which enables it to be alloyed with other elements to improve its qualities. Magnesium can be strengthened with the addition of other alloying elements, which can also alter the material's chemical reactivity. In most cases, aluminum, zinc, manganese, silicon, copper, rare earth stacks, and zirconium are the other metals that are alloyed with magnesium. The majority of commercial and industrial magnesium alloys contain aluminum between 0.1 and 0.4 percent and manganese between 0.5 and 3 percent.
Aluminum is the most efficient component for bringing about the desired improvements. Aluminum imparts some resistance to corrosion and contributes to the magnesium alloy's increased flexibility. On the other hand, Mn and Zn both contribute to an increase in the composite's electrical conductivity. The hardness and strength of an alloy can be increased without compromising its weldability when a little quantity of aluminum, along with trace amounts of zinc and manganese, is added to the mix. This also makes the alloy more responsive to heat treatment. The price, on the other hand, represents a reduction in the elasticity of the metal. After welding, magnesium alloys that contain more than 1.5% aluminum are required to have their stress relieved since they are susceptible to the effects of strain corrosion. The degree of hardness achieved by some of these can be increased with the application of heat treatment. Aluminum, manganese, and zinc are the three components that make up Mg-Al-Zn combination gold. These are the most frequent types of alloying elements that are utilised in a variety of applications. The addition of manganese to magnesium alloys makes them more resistant to salt and boosts their yield strength. At high temperatures, Th, Ce, and Zr are combined without the presence of Al in order to produce the Mg-Zn-Zr group [22]. Because iron, copper, and nickel induce a decrease in the corrosion resistance of magnesium alloys, these impurity components need to have as low of a concentration as possible in the alloy. Zinc and aluminum, when combined, have the ability to neutralise the corrosive effects of iron and nickel impurities that may be present in magnesium alloys. These impurities can cause damaging corrosion. A higher zinc percentage (more than one percent) might cause an increase in thermal short-circuiting, which in turn can cause weld cracking [22].
2.3.4. Present technological barriers and solutions for magnesium alloy applications in automotive industry. The disadvantages of magnesium alloys include a high level of reactivity in the molten state, worse resistance to galvanic corrosion and fatigue and creep when compared to steel, and poor fatigue and creep performance. The low melting point of magnesium alloys, which is 650 degrees Celsius, and their reactivity provide a concern when they are used (insufficient resistance to corrosion). When compared to steel, the risk of fire during the production and use of magnesium alloys is the primary difficulty. This risk is especially prevalent during the finishing and grinding processes, when the flakes produced can be ignited or even explode due to the low melting point of the magnesium alloys. Another issue is that magnesium alloys have a low yield strength and hardness, and therefore are unable to bear relatively high loads. This creates a unique risk for the vehicle's ability to maintain its standard level of safety performance. The economic difficulty is the third thing that needs to be taken into consideration; the trade price of magnesium once reached $2,000 per tonne. Magnesium, in contrast to chips, steel, and other materials, is easily oxidised when it comes into contact with air. Because of this, magnesium is often stored for no longer than three months. In addition, the cost of low-alloy steel, measured in tonnes, is approximately $600 per tonne.
Even though magnesium alloys have many flaws in the practical manufacturing and application of the material, there are still ways in which it is possible to improve it through technical means. Eliminating the possibility of starting a fire can be done in a number of different ways, including minimising fine and high-speed cutting, adding additional metal elements, employing coolants, etc. In addition, in order to strengthen the magnesium alloy's strength, hardness, and stability, we can raise the proportion of other metals present in the alloy, such as zinc, aluminum, manganese, and other metals. For example, elevating the percentage of aluminum metal present in magnesium alloy might result in that material exhibiting greater levels of hardness. The cost of magnesium alloy is also quite costly; therefore, we may ensure the economy by adding the appropriate quantity of magnesium alloy in the appropriate location or by carrying forward the outstanding performance of magnesium alloy in order to encourage its use. According to the aforementioned research, magnesium alloys, which are a novel lightweight material, have met the standards of application in the automobile body in terms of their strength, stiffness, and density. This means that they can replace the application of steel in the car body. But additional research is needed to figure out how to address the issue of magnesium alloy's high cost.
2.4. Carbon fibre
Carbon fibre, which is a material that consists of strong and thin crystalline filaments of carbon, is regarded as an effective solution to lower the weight of cars and is one of several alternative solutions that can be used to replace the materials that make up automobile bodies. The weight of the vehicle is a crucial role in the fuel efficiency of the vehicle, thus lowering the vehicle's weight can considerably cut the amount of fuel the vehicle needs. Despite the fact that this is a challenge, it is imperative that neither the quality nor the cost of automobiles be compromised in any way. It is anticipated that if carbon fibre were utilised in the construction of the majority of automotive parts, the overall weight of the vehicle might be lowered by as much as fifty percent. Carbon fibre is significantly more lightweight than the materials and components that are currently used in cars. There is a 35% improvement that may be made to the fuel efficiency. The results of this statistical analysis demonstrate that the weight of carbon fibres can flawlessly satisfy the requirements for weight reduction.
Carbon fibre has many benefits, one of which is that it is extremely strong; in fact, it is just as strong as steel. Because of this, it is able to handle the strain that comes with car racing at such high speeds. It is possible for the vehicle to keep the occupants safe even in the event of a collision. It is a good alternative since carbon fibre can be formed into any shape, which means that it can satisfy the requirements of automobiles in a variety of different contexts. Carbon fibre has a high strength-to-weight ratio, which means that it can help a vehicle reach higher top speeds [23]. This is accomplished by fusing together more than 50,000 separate strands of carbon fibre, which results in the fibre being extremely robust and inflexible. Carbon fibre is able to function in difficult environments thanks to its resistance to corrosion and its extended lifespan.
In some applications, carbon fibre is being used in place of steel. Its specific gravity is significantly lower than that of steel by more than a quarter (figure 2). In general, the tensile strength of carbon fibre resin composite materials is higher than 3500Mpa. This is 7-9 times higher than the tensile strength of steel. The tensile elastic modulus ranges from 23000 to 43000 MPa, which is significantly greater than steel's value. However, one thing that is important to keep in mind is that the technology of carbon fibre is not yet fully developed, and it is only mastered by a few numbers of luxury automobile manufacturers.

Figure 2. Carbon fiber will be used in some sports cars
3. Conclusion
3.1. High strength steel
As discussed above, high strength steels are ideal for use in automobiles because of their high strength and lower density than conventional steels. However, high-strength steels also have some drawbacks, such as their high cost and difficulty of manufacture, as well as their high brittleness. Therefore, additional research is needed to solve these problems.
3.2. Aluminum alloy
One of the other solutions is replacing traditional steel by aluminum alloy. Many existing problems of aluminum alloys are discussed and how to solve them. The application of aluminum alloys in automotive components is also discussed in detail, as well as the solutions to the disadvantages of aluminum alloys. A compromise solution is proposed after discussing the different metal additions to aluminum alloys and using cast or wrought aluminum alloy. By using different aluminum alloys in different parts of the car and increasing the thickness, the strength of the car is guaranteed while the weight of the car is reduced, and the fuel economy is improved. However, the cost of aluminum steel is steel too high, more studies are required to solve this problem.
3.3. Magnesium alloy
In addition, the excellent performance of magnesium alloy with low density can reduce the body's weight and achieve the car's fuel economy. However, the problem of the high reactivity of magnesium alloy in the molten state and the strength of magnesium alloy is less severe than other alloys. The disadvantages of magnesium alloys are apparent, such as low strength and high reactivity in the molten state, which can be improved by extensive research on the properties and alloying of magnesium alloys, using methods such as increasing the proportion of other metal elements in magnesium alloys and using coolants. Enable magnesium alloys to replace other alloys in the automotive industry.
3.4. Carbon fiber
Therefore, carbon fiber is an ideal material to replace materials used for car body materials. Carbon fiber is an important part for weight reduction and fuel efficiency improvement. In addition, it has good strength to protect drivers and passengers in the car. While the cost of carbon fiber is one disadvantage that should be overcome before it is put into mass production. To solve this problem more research needs to be done.
In conclusion, since all of the materials discussed are relatively expensive, so price will not be taken into account when comparing which of them has the most potential for further development to use on vehicles. The aim of this article is to improve the fuel economy by reducing the weight of the car while ensuring that it is safe, in other words maintain the strength. Therefore, the strength to density ratios of the materials are compared to decide which material can achieve the goal. As the result, the carbon fiber has the greatest strength to density ratio, which is the most suitable material to reduce the car weight in order to improve the fuel economy. Therefore, more studies of carbon fiber can be done to solve the problem of high cost and low manufacturability.
References
[1]. US EPA. “Global Greenhouse Gas Emissions Data.” epa.gov. https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed Nov. 12, 2022)
[2]. IEA. “Energy Statistics Data Browser.” iea.org. https://www.iea.org/data-and-statistics/data-tools/energy-statistics-data-browser?country=WORLD&fuel=CO2%20emissions&indicator=TotCO2 (accessed Nov. 12, 2022)
[3]. US EIA. “Use of energy explained.” eia.gov. https://www.eia.gov/energyexplained/use-of-energy/transportation-in-depth.php (accessed Nov. 12, 2022)
[4]. M. K. Gupta and V. Singhal, ‘Review on materials for making lightweight vehicles’, Mater. Today Proc., vol. 56, pp. 868–872, Jan. 2022, doi: 10.1016/j.matpr.2022.02.517
[5]. Z. Gao, Y. Liu C. Wang H. Yang, L, Xu, and L. Qiao,” The study on the influence of aluminum on the CO2 corrosion resistance of 3%Cr steel,”,anti-corrosion methods and materials, vol.69 no.2, pp.869-888, Feb. 2022,doi:10.1108/ACMM-11-2021-2565
[6]. S. Lou G. Zhao R. Wang and X. Wu, "Numerical simulation of steady and unsteady aluminum profile extrusion processes using finite volume method,", Engineering Computations, vol.25, no.6, pp. 589-605, Aug.,2008doi:10.1108/02644400810891562
[7]. J. R. Handforth, "Modern Aluminum Alloys," Aircraft Engineering and Aerospace Technology, vol. 11, no. 3, pp. 101-106, 1939, doi: 10.1108/eb030452.
[8]. M. Patel, B. Pardhi, S. Chopara, and M. Pal, "Lightweight Composite Materials for Automotive -A Review," vol. 5, pp. 41-47, 11/01 2018.
[9]. A. A. Luo and A. K. Sachdev, "12 - Applications of magnesium alloys in automotive engineering," in Advances in Wrought Magnesium Alloys, C. Bettles and M. Barnett Eds.: Woodhead Publishing, 2012, pp. 393-426.
[10]. https://en.wikipedia.org/wiki/Carbon_fibers in the synthesis 2022.
[11]. Bhatt, Pooja (2017). Carbon Fibres: Production, Properties and Potential Use (Thesis). Archived from the original on 2021-04-30. Retrieved 2021-07-25.
[12]. Bhatt, Pooja (2017). Carbon Fibres: Production, Properties and Potential Use (Thesis). Archived from the original on 2021-04-30. Retrieved 2021-07-25.
[13]. http://www.tanxw.com/news/xgzx/2708.html P1 Via New Materials,an article to understand the overall performance of carbon fiber and rigid comparison, what is the difference.
[14]. R. Singh, ‘6 - Classification of steels’, in Applied Welding Engineering (Third Edition), R. Singh, Ed. Butterworth-Heinemann, 2020, pp. 53–60. doi: 10.1016/B978-0-12-821348-3.00014-8.
[15]. C.-H. Seo et al., ‘Deformation behavior of ferrite–austenite duplex lightweight Fe–Mn–Al–C steel’, Scr. Mater., vol. 66, no. 8, pp. 519–522, Apr. 2012, doi: 10.1016/j.scriptamat.2011.12.026.
[16]. D. Haber, Lightweight Materials for Automotive Applications: A Review. 2015.
[17]. H. Dieringa and K. Kainer, "“Magnesium – Der Zukunftswerkstoff für Die Automobilindustrie?”," Materialwissenschaft und Werkstofftechnik, vol. 38, pp. 91-96, 02/01 2007, doi: 10.1002/mawe.200600114.
[18]. W. H. Sillekens and N. Hort, "Magnesium and magnesium alloys," 2013, pp. 113-150.
[19]. B. Tang, X.-S. Wang, S.-S. Li, D. B. Zeng, and R. Wu, "Effects of Ca combined with Sr additions on microstructure and mechanical properties of AZ91D magnesium alloy," Materials Science and Technology, vol. 21, pp. 574-578, 05/01 2005, doi: 10.1179/174328405X43180.
[20]. E. Ghassemieh, "Materials in Automotive Application, State of the Art and Prospects," 2011.
[21]. “Online Materials Information Resource - MatWeb.” https://matweb.com/index.aspx (accessed Nov. 30, 2022).
[22]. L. Liu, "4 - Welding materials for magnesium alloys," in Welding and Joining of Magnesium Alloys, L. Liu Ed.: Woodhead Publishing, 2010, pp. 23-37.
[23]. ‘Lightweight, stable, and high-tech – all there is to know about carbon fiber | BMW.com’. https://www.bmw.com/en/performance/carbon-fiber-in-a-car.html (accessed Dec. 04, 2022).
Cite this article
Fan,Q.;Guo,Y.;Zhou,Y.;Ji,Y. (2024). A review of new lightweight materials for the automotive industry. Applied and Computational Engineering,85,203-211.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. US EPA. “Global Greenhouse Gas Emissions Data.” epa.gov. https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed Nov. 12, 2022)
[2]. IEA. “Energy Statistics Data Browser.” iea.org. https://www.iea.org/data-and-statistics/data-tools/energy-statistics-data-browser?country=WORLD&fuel=CO2%20emissions&indicator=TotCO2 (accessed Nov. 12, 2022)
[3]. US EIA. “Use of energy explained.” eia.gov. https://www.eia.gov/energyexplained/use-of-energy/transportation-in-depth.php (accessed Nov. 12, 2022)
[4]. M. K. Gupta and V. Singhal, ‘Review on materials for making lightweight vehicles’, Mater. Today Proc., vol. 56, pp. 868–872, Jan. 2022, doi: 10.1016/j.matpr.2022.02.517
[5]. Z. Gao, Y. Liu C. Wang H. Yang, L, Xu, and L. Qiao,” The study on the influence of aluminum on the CO2 corrosion resistance of 3%Cr steel,”,anti-corrosion methods and materials, vol.69 no.2, pp.869-888, Feb. 2022,doi:10.1108/ACMM-11-2021-2565
[6]. S. Lou G. Zhao R. Wang and X. Wu, "Numerical simulation of steady and unsteady aluminum profile extrusion processes using finite volume method,", Engineering Computations, vol.25, no.6, pp. 589-605, Aug.,2008doi:10.1108/02644400810891562
[7]. J. R. Handforth, "Modern Aluminum Alloys," Aircraft Engineering and Aerospace Technology, vol. 11, no. 3, pp. 101-106, 1939, doi: 10.1108/eb030452.
[8]. M. Patel, B. Pardhi, S. Chopara, and M. Pal, "Lightweight Composite Materials for Automotive -A Review," vol. 5, pp. 41-47, 11/01 2018.
[9]. A. A. Luo and A. K. Sachdev, "12 - Applications of magnesium alloys in automotive engineering," in Advances in Wrought Magnesium Alloys, C. Bettles and M. Barnett Eds.: Woodhead Publishing, 2012, pp. 393-426.
[10]. https://en.wikipedia.org/wiki/Carbon_fibers in the synthesis 2022.
[11]. Bhatt, Pooja (2017). Carbon Fibres: Production, Properties and Potential Use (Thesis). Archived from the original on 2021-04-30. Retrieved 2021-07-25.
[12]. Bhatt, Pooja (2017). Carbon Fibres: Production, Properties and Potential Use (Thesis). Archived from the original on 2021-04-30. Retrieved 2021-07-25.
[13]. http://www.tanxw.com/news/xgzx/2708.html P1 Via New Materials,an article to understand the overall performance of carbon fiber and rigid comparison, what is the difference.
[14]. R. Singh, ‘6 - Classification of steels’, in Applied Welding Engineering (Third Edition), R. Singh, Ed. Butterworth-Heinemann, 2020, pp. 53–60. doi: 10.1016/B978-0-12-821348-3.00014-8.
[15]. C.-H. Seo et al., ‘Deformation behavior of ferrite–austenite duplex lightweight Fe–Mn–Al–C steel’, Scr. Mater., vol. 66, no. 8, pp. 519–522, Apr. 2012, doi: 10.1016/j.scriptamat.2011.12.026.
[16]. D. Haber, Lightweight Materials for Automotive Applications: A Review. 2015.
[17]. H. Dieringa and K. Kainer, "“Magnesium – Der Zukunftswerkstoff für Die Automobilindustrie?”," Materialwissenschaft und Werkstofftechnik, vol. 38, pp. 91-96, 02/01 2007, doi: 10.1002/mawe.200600114.
[18]. W. H. Sillekens and N. Hort, "Magnesium and magnesium alloys," 2013, pp. 113-150.
[19]. B. Tang, X.-S. Wang, S.-S. Li, D. B. Zeng, and R. Wu, "Effects of Ca combined with Sr additions on microstructure and mechanical properties of AZ91D magnesium alloy," Materials Science and Technology, vol. 21, pp. 574-578, 05/01 2005, doi: 10.1179/174328405X43180.
[20]. E. Ghassemieh, "Materials in Automotive Application, State of the Art and Prospects," 2011.
[21]. “Online Materials Information Resource - MatWeb.” https://matweb.com/index.aspx (accessed Nov. 30, 2022).
[22]. L. Liu, "4 - Welding materials for magnesium alloys," in Welding and Joining of Magnesium Alloys, L. Liu Ed.: Woodhead Publishing, 2010, pp. 23-37.
[23]. ‘Lightweight, stable, and high-tech – all there is to know about carbon fiber | BMW.com’. https://www.bmw.com/en/performance/carbon-fiber-in-a-car.html (accessed Dec. 04, 2022).









