1. Introduction
Supramolecular catalysis stands at the forefront of catalysis innovation, emphasizing the use of non-covalent interactions to govern reactivity and selectivity in catalytic transformations. In this overview, I succinctly present the historical trajectory and evolution of this burgeoning field. The origins of supramolecular catalysis starts in the latter half of the 20th century, aligning with the ascent of supramolecular chemistry. Coined by Jean-Marie Lehn in the 1970s [1], “supramolecular chemistry” denotes the investigation of systems formulated through the bonding of two or more chemical species connected with non-covalent intermolecular forces. The 1980s and 1990s witnessed the inaugural instances of supramolecular catalysts. The synthesis of crown ethers [2], cyclodextrins [3], and calixarenes [4] laid the foundational groundwork for encapsulating substrates through non-covalent interactions.
In the late 1990s and early 2000s, supramolecular catalysis began to significantly impact asymmetric synthesis [5]. Scientists were able to achieve enantioselective transformations by controlling the spatial arrangement of substrates within supramolecular structures Subsequently, metal-containing supramolecular catalysts gained attention. These catalytic systems have been designed to mimic the active sites of enzymes, and their modular nature enables fine-tuning of catalytic properties. In recent years, research in supramolecular catalysis has proliferated, exploring new frontiers such as responsive catalysis, where catalysts can adapt to changing conditions, and the integration of supramolecular catalysts into materials and devices. The history of supramolecular catalysis encapsulates a fascinating journey from the conceptual understanding of non-covalent interactions to the development of sophisticated catalytic systems that are both efficient and selective. Through a multidisciplinary approach that combines principles of organic, inorganic, and physical chemistry, supramolecular catalysis continues to evolve, offering promising solutions to some of the most pressing challenges in modern synthetic chemistry. In this paper I will discuss current advancement in supramolecular catalyst, which will be separated to three major components: responsive supramolecular catalysts, metal-organic supramolecular catalysts, and their future implications.
2. Responsive Supramolecular Catalysts
Responsive supramolecular catalysts encompass a highly specialized field of catalysis that integrates the principles of supramolecular chemistry with responsive materials. These catalysts are characterized by their ability to undergo controlled, reversible changes in structure or function in response to specific external stimuli. The responsiveness to stimuli such as light, pH, temperature, or specific chemicals offers an innovative way to control catalytic activity and selectivity. The development of responsive supramolecular catalysts draws from two intersecting scientific paradigms: supramolecular chemistry and stimuli-responsive materials. The convergence of these fields has only recently led to the advent of responsive supramolecular catalysts, marking an innovative direction in modern catalytic science [6]. One of the stimuli-responsive catalysts that has been researched extensively in recent years is the photo responsive catalyst.
Photosensitive supramolecular catalysts have emerged as a promising field of research in recent years[7]. The use of non-covalent interactions to create supramolecular assemblies has opened up new avenues for the design of catalysts with enhanced selectivity, activity, and stability. One of the most exciting developments in this field is the use of photo responsive supramolecular switches, which can be controlled by light stimuli to achieve reversible binding affinity changes for guest molecules.
The foundation of photosensitive supramolecular catalysis rests on exploiting non-covalent interactions to engineer catalysts with remarkable selectivity and efficiency. The strategic design of supramolecular assemblies capable of selectively binding to specific substrates enables the creation of catalysts that can execute reactions with precision and high efficiency. Notably, photosensitive supramolecular catalysts offer the advantage of increased stability and reusability compared to conventional catalysts, as they can be easily separated from the reaction mixture and employed multiple times.
A noteworthy advancement in the realm of photosensitive supramolecular catalysis involves the integration of photoresponsive supramolecular switches. These switches are tailored to respond to light stimuli, providing a method to regulate the binding strength between the host and guest [7]. This feature facilitates the controlled, reversible capture, and release of guest molecules, a characteristic particularly advantageous for applications such as chemical sensing and drug delivery.
Application of photoresponsive supramolecular switches in catalysis has been substantiated through various recent studies. Notably, the Ballester group has undertaken extensive investigations into photoresponsive calix[4]arene and calix[4]pyrrole. In 2015, they devised and synthesized a series of tetraureacalix[4]arenes featuring four appended azobenzene groups terminally. The modulation of host molecules in response to light stimuli induced reversible changes in binding affinity for guest molecules, ultimately enabling controlled release and capture of guests [7].
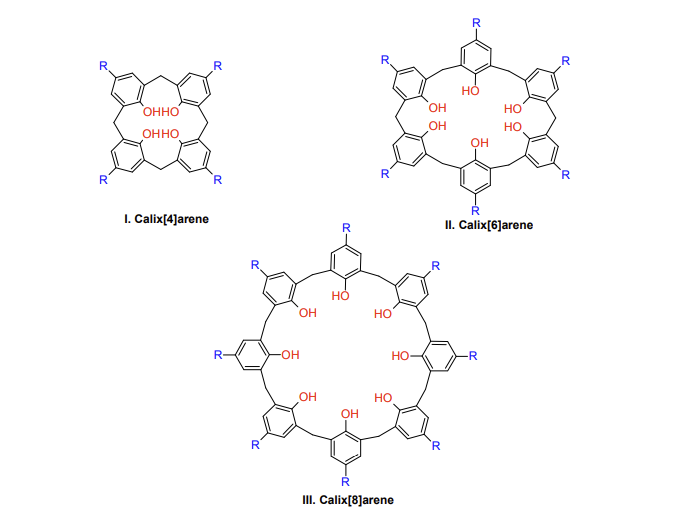
Figure 1. Structure of Different Calix[n]arene
Another example of the use of photoresponsive supramolecular switches in catalysis is the work of the group of Stoddart, who have developed a range of mechanically interlocked molecules (MIMs) that can be controlled by light stimuli. In one study, they designed a rotaxane-based supramolecular catalyst that has two possible conformations switched by light stimuli. The catalyst was found to be highly selective for the oxidation of alcohols, and the reversible switching of the catalyst allowed for the selective oxidation of different alcohols in a controlled manner.
In addition to their use in catalysis, photosensitive supramolecular switches have also been applied in other areas such as drug delivery and sensing. For example, the group of Li developed a photosensitive supramolecular system using cucurbituril [7] and azobenzene, which can be used for the controlled release of drugs. The system was found to be highly efficient in releasing the drug in response to light stimuli, and the reversible switching of the system allowed for the repeated release and capture of the drug.
Another responsive catalyst that has gained extensive attention in the recent years is the pH-responsive supramolecular catalysts [6]. The process of supramolecular self-assembly serves as a versatile pathway for the creation of precisely defined and distinct materials on the nanoscopic scale. The encapsulation of Keggin-type polyoxometalate with a COOH-functionalized surface active ionic liquid results in a material with pH-sensible and reusable catalyst properties for dye. The pH-responsive nature of the hybrid nanomaterials is due to the protonation and deprotonation of the surface active ionic liquid, which leads to changes in the structure and properties of the material.
The renewable catalyst properties of the hybrid nanomaterials are due to the encapsulation of the polyoxometalate, which acts as a catalyst for the methyl orange degradation. The polyoxometalate is known for its catalytic properties, and the encapsulation process enhances its stability and reusability. The COOH-functionalized surface acts as a stabilizer and pH-responsive component, which enhances the catalytic activity of the polyoxometalate. The pH-responsive nanomaterials have several potential applications in various fields. The reversible pH-response of the material makes it suitable for use in pH-sensitive drug delivery systems. The renewable catalyst properties of the material make it suitable for use in wastewater treatment and other catalytic applications. The encapsulation process can also be extended to other polyoxometalates and surface active ionic liquids, which can lead to the development of new supramolecular catalysts with unique properties and applications. In conclusion, the pH-responsive supramolecular hybrid catalysts discussed in this paper are a promising class of supramolecular catalysts with unique properties and potential applications in various fields[6]. The pH-responsive nature of the material makes it suitable for use in pH-sensitive drug delivery systems, while the renewable catalyst properties making it possible for wastewater treatment and other catalytic applications. The encapsulation process can also be extended to other polyoxometalates and surface active ionic liquids, which can lead to the development of new supramolecular catalysts with unique properties and applications.
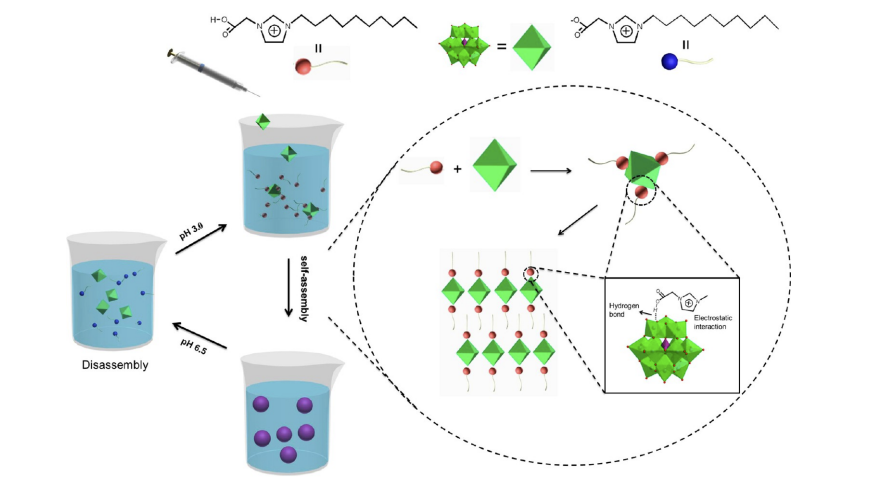
Figure 2. Illustration of the Formation of COOH Supramolecular Host
However, there are still some challenges that need to be addressed in the development of pH-responsive polyoxometalate-based supramolecular hybrid nanomaterials. One of the challenges is the optimization of the encapsulation process to achieve maximum stability and catalytic activity. The encapsulation process is sensitive to various factors such as temperature, pH, and concentration, which can affect the stability and catalytic effectiveness of the material[6].
Another challenge is the scalability of the synthesis process. The synthesis process of the supramolecular hybrid nanomaterials is complex and time-consuming, which can limit its scalability. Therefore, further studies are needed to develop a scalable synthesis process that can produce the material in large quantities.
Despite these challenges, these materials have the potential to revolutionize the field of supramolecular catalysis. The unique properties of the material make it suitable for various applications, and the encapsulation process can be extended to other polyoxometalates and surface active ionic liquids, which can lead to the development of new supramolecular catalysts with unique properties and applications [6].
In recent times, an increasing focus has emerged on the development of thermoresponsive supramolecular catalysts designed to react to temperature variations and manifest heightened catalytic efficacy. In a study, researchers utilized a guest molecule forming a homoternary complex with the cucurbituril macrocycle, attaching it to the terminals of Pluronic F-127 polymers [8]. The temperature-responsive formation of the polymer facilitates physical cross-linking through CB[n], engendering a percolated hydrogel network by leveraging the interaction between host and guest along with the appended reactants. The specific guests involved in homoternary complex formation can undergo photo-dimerization, thereby substituting the physical interaction with a covalent bond. Despite resulting in reductions approximately two-orders-of-magnitude in hydrogel dynamics, the temperature-sensitive gelation and network structure remain seemingly unaltered.
These resultant hydrogels, comprising micelles cross-linked by both supramolecular and photo-dimerized reactions, showcase heightened catalytic activity owing to the dynamic characteristic of the supramolecular interactions. The authors demonstrated potential applications of hydrogels in injecting and encapsulating cells, as well as incorporating and releasing macromolecular payloads both in vitro and in vivo. This innovative approach represents a method for integrating outer stimuli into supramolecular structures through the amalgamation of light-responsive supramolecular structures and polymers.
The utilization of homoternary complex formation and photo-cross-linking to modify network dynamics in supramolecular gels represents a notable stride in the realm of thermoresponsive supramolecular catalysts. The capacity to regulate hydrogel network dynamics through temperature and light variations offers a potent tool for crafting catalysts with enhanced activity and selectivity. Moreover, the reversible binding of substrates facilitated by the dynamic nature of supramolecular interactions holds promise for bolstering catalytic efficiency and minimizing waste [8].
3. Metallo-Supramolecular Catalysts
Metallosupramolecular hosts are complex structures that are formed through the self-assembly of metal ions and ligands. These hosts have a wide range of potential applications in fields such as catalysis, materials science, and drug delivery. The design of metallosupramolecular hosts is a complex and multifaceted process that involves careful consideration of a variety of factors, including the choice of metal ion and ligand, the overall topology of the host structure, the stability of the resulting host, and the intended application of the host [9]. Advances in synthetic chemistry and computational modeling have greatly expanded the range of possible metallosupramolecular hosts, and have enabled researchers to design hosts with increasingly complex and sophisticated structures. In this section of the paper, I will discuss metallosupramolecular catalysts in two different aspects, their design and synthetic process.
The design of metallosupramolecular hosts is a complex and multifaceted process that involves careful consideration of a variety of factors. One of the most important considerations in host design is the choice of metal ion and ligand. The metal ion serves as the central coordinating atom around which the ligands are arranged, and the choice of metal ion can have a significant impact on the properties of the resulting host. For example, metal ions with a high coordination number can lead to the formation of larger, more complex host structures, while metal ions with a lower coordination number may result in smaller, more compact hosts. Another important consideration in host design is the choice of ligand. The ligand serves as the building block for the host structure, and the choice of ligand can have a significant impact on the stability, size, and shape of the resulting host. For example, ligands with a high degree of symmetry can lead to the formation of highly symmetric host structures, while ligands with a lower degree of symmetry may result in more irregularly shaped hosts. In addition to the choice of metal ion and ligand, the design of metallosupramolecular hosts also involves consideration of the overall topology of the host structure [9]. The topology of the host structure refers to the way in which the ligands are arranged around the central metal ion, and can have a significant impact on the properties of the resulting host. For example, hosts with a cage-like topology can provide a large internal cavity that is capable of encapsulating guest molecules, while hosts with a more open topology may be better suited for catalytic applications. The design of metallosupramolecular hosts also involves consideration of the stability of the resulting host structure. Hosts that are too unstable and may not be suitable for practical applications, while hosts that are too stable may be difficult to modify or functionalize. Achieving the right balance of stability is therefore an important consideration in host design. One promising area of research in the design of metallosupramolecular hosts is the use of dynamic covalent chemistry. Dynamic covalent chemistry refers to the reversible formation and breaking of covalent bonds, and can be used to create hosts that are capable of adapting to changes in their environment. For example, hosts that are capable of undergoing conformational changes in response to changes in temperature or pH may be useful for applications in drug delivery or sensing . Another promising area of research in the design of metallosupramolecular hosts is the use of supramolecular polymers. Supramolecular polymers are linear or branched structures that are formed through the self-assembly of multiple monomers, and can be used to create hosts with a high degree of structural complexity. For example, supramolecular polymers that are capable of forming helical or branched structures may be useful for applications in catalysis or materials science [9].
One approach to the synthesis of metallosupramolecular hosts is the use of self-assembly techniques. Self-assembly involves the spontaneous formation of a host structure from its constituent parts, and can be used to create hosts with a high degree of structural complexity. For example, self-assembly of metal ions and ligands can be used to create cage-like structures that are capable of encapsulating guest molecules. The self-assembly of metal ions and ligands can be achieved through a variety of different mechanisms. One common mechanism is the use of coordination bonds between the metal ion and the ligand. Coordination bonds are formed through the donation of electron pairs from the ligand to the metal ion, and can be used to create a variety of different host structures, including cages, helices, and sheets. Another mechanism for self-assembly is the use of hydrogen bonding between the ligands. Hydrogen bonding involves the formation of a weak electrostatic interaction between a hydrogen atom and a lone pair of electrons on a nearby atom [5]. This mechanism can be used to create a variety of different host structures, including helices, sheets, and capsules. In addition to self-assembly, the synthesis of metallosupramolecular hosts can also be achieved through the use of template-directed synthesis. Template-directed synthesis involves the use of a pre-existing template molecule to guide the formation of the host structure. This approach can be used to create hosts with a high degree of structural complexity, and can be particularly useful for the synthesis of hosts with specific shapes or sizes. The choice of synthetic method for the synthesis of metallosupramolecular hosts depends on a variety of factors, including the desired size and shape of the host, the choice of metal ion and ligand, and the intended application of the host. For example, self-assembly may be a more suitable approach for the synthesis of cage-like structures, while template-directed synthesis may be more suitable for the synthesis of hosts with specific shapes or sizes. One of the challenges in the synthesis of metallosupramolecular hosts is achieving the desired level of structural complexity. Metallosupramolecular hosts can be highly complex structures, and achieving the desired level of complexity requires careful control over a variety of factors, including the stoichiometry of the metal ion and ligand, the geometry of the ligand, and the conditions under which the self-assembly or template-directed synthesis is carried out. For example, the use of ligands with a high degree of symmetry can lead to the formation of highly symmetric host structures, while the use of ligands with a lower degree of symmetry may result in more irregularly shaped hosts [5].
Another challenge in the synthesis of metallosupramolecular hosts is achieving the desired level of stability. Hosts that are too unstable may not be suitable for practical applications, while hosts that are too stable may be difficult to modify or functionalize. Achieving the right balance of stability is therefore an important consideration in host design. One approach to achieving the desired level of stability is the use of dynamic covalent chemistry. Dynamic covalent chemistry refers to the reversible formation and breaking of covalent bonds, and can be used to create hosts that are capable of adapting to changes in their environment. For example, hosts that are capable of undergoing conformational changes in response to changes in temperature or pH may be useful for applications in drug delivery or sensing. The synthesis of metallosupramolecular hosts can also be influenced by the choice of metal ion and ligand. The metal ion serves as the central coordinating atom around which the ligands are arranged, and the choice of metal ion can have a significant impact on the properties of the resulting host. For example, metal ions with a high coordination number can lead to the formation of larger, more complex host structures, while metal ions with a lower coordination number may result in smaller, more compact hosts [10]. The choice of ligand can also have a significant impact on the stability, size, and shape of the resulting host. For example, ligands with a high degree of symmetry can lead to the formation of highly symmetric host structures, while ligands with a lower degree of symmetry may result in more irregularly shaped hosts. In recent years, advances in synthetic chemistry and computational modeling have greatly expanded the range of possible metallosupramolecular hosts. Computational modeling can be used to predict the properties of a host structure before it is synthesized, allowing researchers to design hosts with specific properties or functions in mind. For example, computational modeling can be used to predict the binding affinity of a host for a specific guest molecule or to optimize the geometry of a host structure for a specific catalytic reaction [10].
4. Application of Supramolecular Catalysts
Supramolecular catalysts possess great potential for future applications, in this section I will elaborate upon their practices in asymmetric synthesis and green chemistry.
Supramolecular hosts, such as crown ethers, have been extensively used in asymmetric catalysis to regulate the activity and selectivity of chiral catalysts. This review systematically introduces the design of supramolecular catalysts, along with their wide applications in asymmetric catalysis and influencing catalytic stereoselectivity. One example of the use of crown ethers in asymmetric catalysis is the construction of pseudorotaxanes and rotaxanes derivatives. These compounds consist of a macrocycle (the crown ether) and a linear molecule (the axle), which can be threaded through the macrocycle to form a mechanically interlocked molecule. The resulting supramolecular structure can be used as a chiral catalyst, with the crown ether acting as a recognition site for the substrate and the axle providing the chiral environment for the reaction. For example, a pseudorotaxane consisting of a crown ether and a chiral diamine was used as a catalyst for the enantioselective Michael addition of nitroalkanes to enones, while achieving high enantioselectivity. Another example of the use of crown ethers in asymmetric catalysis is the construction of metal-organic frameworks (MOFs) containing crown ether units [11]. MOFs are porous materials consisting organic ligands connecting together with metal ions or clusters, making them extremely porous. By incorporating crown ether units into the organic ligands, the resulting MOFs can act as chiral catalysts for various reactions. For example, a MOF containing a crown ether unit and a chiral diamine was used as a catalyst for the enantioselective Henry reaction, with high enantioselectivity achieved. In addition to crown ethers, other supramolecular motifs can also be used in asymmetric catalysis [11]. For example, cucurbiturils, which are cyclic molecules consisting of glycoluril units, can act as recognition sites for various substrates. By incorporating chiral groups into the cucurbituril structure, chiral recognition can be achieved, and the resulting supramolecular complex can be used as a chiral catalyst. For example, a cucurbituril derivative containing a chiral amine was used as a catalyst for the enantioselective Michael addition of nitroalkanes to enones [12]. The synergism of multiple supramolecular interactions can also be utilized in asymmetric catalysis. For example, a supramolecular catalyst consisting of a chiral phosphoric acid and a chiral crown ether was used for the enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes. The chiral phosphoric acid provided the chiral environment for the reaction, while the crown ether acted as a recognition site for the substrate. The resulting supramolecular complex exhibited high enantioselectivity and good yields [12].
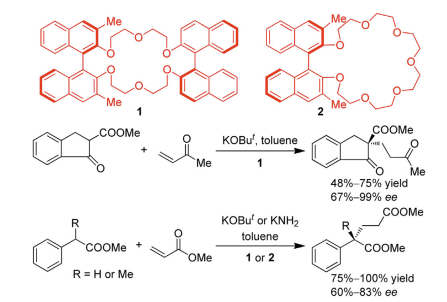
Figure 3. Michael Addition Catalysized by Crown Ether
For future implications, development of simpler yet more effective supramolecular asymmetric catalysis possesses great research potential. One example of such a catalyst is a chiral squaramide-based supramolecular catalyst, which consists of a squaramide unit and a chiral amine. The squaramide unit acted as a hydrogen bond donor, while the chiral amine provided the chiral environment for the reaction. This simple and effective catalyst has potential for practical applications in industry. Utilizing supramolecular asymmetric catalysis can contribute to the development of more cost-effective and practical catalytic systems. For example, the creation of efficient asymmetric catalysts using crown ethers could significantly decrease the required quantities, thereby reducing costs and enhancing practicality. Furthermore, the dynamic and reversible characteristics of supramolecular interactions enable the adjustment of electron density and spatial structures within chiral catalytic centers. which can lead to improved generality and economy of the catalytic systems. In conclusion, the use of supramolecular hosts in asymmetric catalysis has led to the development of a wide range of chiral catalysts with high activity and selectivity. The designing and construction process of supramolecular catalysts, along with their potential catalytic reactions, have been systematically introduced in this review. Specific examples of the use of crown ethers, cucurbiturils, and other supramolecular motifs in asymmetric catalysis have been discussed, highlighting the potential of these systems for practical applications in industry. The development of simple, but effective, supramolecular asymmetric catalysts is an attractive direction for future research, which could lead to more economical and practical catalytic systems [12].
Supramolecular chemistry has emerged as a promising field in green chemistry, offering a range of applications in organic transformations. In particular, supramolecular hosts such as cyclodextrins have been extensively studied for their ability to catalyze organic reactions in a green and sustainable manner. Current research focuses on the application of b-Cyclodextrin (b-CD) as a green and efficient supramolecular catalyst for organic transformations (Español & Villamil, 2019). One of the key advantages of b-CD is its ability to form inclusion complexes with a wide range of organic compounds, leading to increased reactivity and selectivity in organic transformations. For example, b-CD has been shown to catalyze the acylation of alcohols and amines with acetic anhydride, leading to high yields and selectivities in a variety of solvents . In addition, b-CD has been used as a catalyst for the synthesis of chalcones, a class of biologically active compounds, via the Claisen-Schmidt condensation reaction . The use of b-CD as a catalyst in this reaction led to high yields and selectivities, as well as reduced reaction times and improved reaction conditions compared to traditional methods (Español & Villamil, 2019). Another important application of b-CD in green chemistry is its ability to catalyze cycloaddition reactions in both aqueous and non-aqueous media. For example, b-CD has been used as a catalyst for the synthesis of 1,2,3-triazoles via the Huisgen cycloaddition reaction . The use of b-CD as a catalyst in this reaction led to high yields and selectivities, as well as reduced reaction times and improved reaction conditions compared to traditional methods. In addition, b-CD has been used as a catalyst for the synthesis of pyrazoles via the 1,3-dipolar cycloaddition reaction . The use of b-CD as a catalyst in this reaction led to high yields and selectivities, as well as reduced reaction times and improved reaction conditions compared to traditional methods. The use of b-CD as a catalyst in organic transformations also offers several advantages in terms of sustainability and environmental impact. For example, b-CD is a biodegradable and non-toxic catalyst, making it an attractive alternative to traditional catalysts that may be hazardous or difficult to dispose of . In addition, b-CD can be easily recovered and reused in subsequent reactions, leading to reduced waste generation and improved efficiency in the overall synthetic process. Furthermore, the use of b-CD as a catalyst often allows for the use of water as a solvent, which is a highly eco-friendly and benign solvent compared to traditional organic solvents that may be hazardous or difficult to dispose of. The application of b-CD in organic transformations also highlights the importance of adopting the principles of green chemistry, particularly in terms of reducing waste and avoiding the use of hazardous solvents and toxic reagents. For example, the use of b-CD as a catalyst in the synthesis of chalcones via the Claisen-Schmidt condensation reaction led to reduced waste generation and improved atom economy compared to traditional methods [13]. Similarly, the use of b-CD as a catalyst in the synthesis of pyrazoles via the 1,3-dipolar cycloaddition reaction led to improved atom economy and reduced waste generation compared to traditional methods. Overall, the application of b-CD as a green and efficient supramolecular catalyst for organic transformations offers several advantages in terms of improved efficiency, reduced waste generation, and improved sustainability. The ability of b-CD to form inclusion complexes with a wide range of organic compounds, as well as its biodegradability, non-toxicity, and ability to be easily recovered and reused, make it an attractive alternative to traditional catalysts in organic synthesis. Furthermore, the use of b-CD as a catalyst highlights the importance of adopting the principles of green chemistry in organic transformations, particularly in terms of reducing waste and avoiding the use of hazardous solvents and toxic reagents [13].
5. Conclusion
Despite the significant progress made in the field of supramolecular catalysis, there are still several challenges that need to be addressed. One of the major challenges is the optimization of the encapsulation process to achieve maximum stability and catalytic activity. This requires a better understanding of the factors that affect the stability and catalytic activity of these materials.
Another challenge is the development of new supramolecular catalysts with unique properties and applications. This requires a multidisciplinary approach that combines principles of organic, inorganic, and physical chemistry.
However, despite these challenges, the future of supramolecular catalysis looks bright. There is a growing interest in this field, and we can expect to see continued growth and innovation. Supramolecular catalysis offers promising solutions to some of the most pressing challenges in modern synthetic chemistry. With the integration of supramolecular catalysts into materials and devices, we can expect to see new and exciting applications in the future.
In conclusion, supramolecular catalysis has emerged as a fascinating and innovative frontier in catalysis, offering a versatile and adaptable approach to controlling reactivity and selectivity in catalytic transformations. Responsive supramolecular catalysts, in particular, have shown great promise in various fields, including drug delivery, wastewater treatment, and asymmetric synthesis. The integration of supramolecular chemistry with responsive materials has enabled the development of catalysts that can undergo controlled, reversible changes in structure or function in response to specific external stimuli, offering a new way to control catalytic activity and selectivity [7]. However, there are still challenges that need to be addressed in the development of responsive supramolecular catalysts, such as optimizing the encapsulation process to achieve maximum stability and catalytic activity. Further studies are needed to understand the factors that affect the stability and catalytic activity of these materials and to explore new supramolecular catalysts with unique properties and applications. Overall, the future of supramolecular catalysis looks bright, and we can expect to see continued growth and innovation in this exciting field.
References
[1]. Lehn, J.-M. (1988). Supramolecular chemistry—scope and perspectives molecules, Supermolecules, and molecular Devices(nobel lecture). Angewandte Chemie International Edition in English, 27(1), 89–112. https://doi.org/10.1002/anie.198800891
[2]. Pedersen, C. J. The Discovery of Crown Ethers. The Pedersen Memorial Issue 1992, 7–10. DOI:10.1007/978-94-011-2532-1_1.
[3]. Challa, R.; Ahuja, A.; Ali, J.; Khar, R. K. Cyclodextrins in Drug Delivery: An Updated Review. AAPS PharmSciTech 2005, 6 (2). DOI:10.1208/pt060243.
[4]. Español, E.; Villamil, M. Calixarenes: Generalities and Their Role in Improving the Solubility, Biocompatibility, Stability, Bioavailability, Detection, and Transport of Biomolecules. Biomolecules 2019, 9 (3), 90. DOI:10.3390/biom9030090.
[5]. Ballester, P.; Scarso, A. Editorial: Supramolecular Aspects in Catalysis. Frontiers in Chemistry 2019, 7. DOI:10.3389/fchem.2019.00174.
[6]. Gong, Y.; Guo, Y.; Hu, Q.; Wang, C.; Zang, L.; Yu, L. PH-Responsive Polyoxometalate-Based Supramolecular Hybrid Nanomaterials and Application as Renewable Catalyst for Dyes. ACS Sustainable Chemistry & Engineering 2017, 5 (5), 3650–3658. DOI:10.1021/acssuschemeng.6b02791.
[7]. Yang, Z.; Liu, Z.; Yuan, L. Recent Advances of Photoresponsive Supramolecular Switches. Asian Journal of Organic Chemistry 2020, 10 (1), 74–90.
[8]. Zou, L.; Su, B.; Addonizio, C. J.; Pramudya, I.; Webber, M. J. Temperature-Responsive Supramolecular Hydrogels by Ternary Complex Formation with Subsequent Photo-Cross-Linking to Alter Network Dynamics. Biomacromolecules 2019, 20 (12), 4512–4521. DOI:10.1021/acs.biomac.9b01267.
[9]. Brown, C. J.; Toste, F. D.; Bergman, R. G.; Raymond, K. N. Supramolecular Catalysis in Metal–Ligand Cluster Hosts. Chemical Reviews 2015, 115 (9), 3012–3035. DOI:10.1021/cr4001226.
[10]. Hong, C. M.; Morimoto, M.; Kapustin, E. A.; Alzakhem, N.; Bergman, R. G.; Raymond, K. N.; Toste, F. D. Deconvoluting the Role of Charge in a Supramolecular Catalyst. Journal of the American Chemical Society 2018, 140 (21), 6591–6595. DOI:10.1021/jacs.8b01701.
[11]. Meeuwissen, J.; Reek, J. N. Supramolecular Catalysis beyond Enzyme Mimics. Nature Chemistry 2010, 2 (8), 615–621. DOI:10.1038/nchem.744. DOI:10.1002/ajoc.202000501.
[12]. Zhang, Z.; Shao, Y.; Tang, J.; Jiang, J.; Wang, L.; Li, S. Supramolecular Asymmetric Catalysis Mediated by Crown Ethers and Related Recognition Systems. Green Synthesis and Catalysis 2021, 2 (2), 156–164. DOI:10.1016/j.gresc.2021.03.007.
[13]. Dalal, D. S.; Patil, D. R.; Tayade, Y. A. Β‐cyclodextrin: A Green and Efficient Supramolecular Catalyst for Organic Transformations. The Chemical Record 2018, 18 (11), 1560–1582. DOI:10.1002/tcr.201800016.
Cite this article
Yan,H. (2024). Supramolecular catalysts: A critical review. Applied and Computational Engineering,84,124-133.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Lehn, J.-M. (1988). Supramolecular chemistry—scope and perspectives molecules, Supermolecules, and molecular Devices(nobel lecture). Angewandte Chemie International Edition in English, 27(1), 89–112. https://doi.org/10.1002/anie.198800891
[2]. Pedersen, C. J. The Discovery of Crown Ethers. The Pedersen Memorial Issue 1992, 7–10. DOI:10.1007/978-94-011-2532-1_1.
[3]. Challa, R.; Ahuja, A.; Ali, J.; Khar, R. K. Cyclodextrins in Drug Delivery: An Updated Review. AAPS PharmSciTech 2005, 6 (2). DOI:10.1208/pt060243.
[4]. Español, E.; Villamil, M. Calixarenes: Generalities and Their Role in Improving the Solubility, Biocompatibility, Stability, Bioavailability, Detection, and Transport of Biomolecules. Biomolecules 2019, 9 (3), 90. DOI:10.3390/biom9030090.
[5]. Ballester, P.; Scarso, A. Editorial: Supramolecular Aspects in Catalysis. Frontiers in Chemistry 2019, 7. DOI:10.3389/fchem.2019.00174.
[6]. Gong, Y.; Guo, Y.; Hu, Q.; Wang, C.; Zang, L.; Yu, L. PH-Responsive Polyoxometalate-Based Supramolecular Hybrid Nanomaterials and Application as Renewable Catalyst for Dyes. ACS Sustainable Chemistry & Engineering 2017, 5 (5), 3650–3658. DOI:10.1021/acssuschemeng.6b02791.
[7]. Yang, Z.; Liu, Z.; Yuan, L. Recent Advances of Photoresponsive Supramolecular Switches. Asian Journal of Organic Chemistry 2020, 10 (1), 74–90.
[8]. Zou, L.; Su, B.; Addonizio, C. J.; Pramudya, I.; Webber, M. J. Temperature-Responsive Supramolecular Hydrogels by Ternary Complex Formation with Subsequent Photo-Cross-Linking to Alter Network Dynamics. Biomacromolecules 2019, 20 (12), 4512–4521. DOI:10.1021/acs.biomac.9b01267.
[9]. Brown, C. J.; Toste, F. D.; Bergman, R. G.; Raymond, K. N. Supramolecular Catalysis in Metal–Ligand Cluster Hosts. Chemical Reviews 2015, 115 (9), 3012–3035. DOI:10.1021/cr4001226.
[10]. Hong, C. M.; Morimoto, M.; Kapustin, E. A.; Alzakhem, N.; Bergman, R. G.; Raymond, K. N.; Toste, F. D. Deconvoluting the Role of Charge in a Supramolecular Catalyst. Journal of the American Chemical Society 2018, 140 (21), 6591–6595. DOI:10.1021/jacs.8b01701.
[11]. Meeuwissen, J.; Reek, J. N. Supramolecular Catalysis beyond Enzyme Mimics. Nature Chemistry 2010, 2 (8), 615–621. DOI:10.1038/nchem.744. DOI:10.1002/ajoc.202000501.
[12]. Zhang, Z.; Shao, Y.; Tang, J.; Jiang, J.; Wang, L.; Li, S. Supramolecular Asymmetric Catalysis Mediated by Crown Ethers and Related Recognition Systems. Green Synthesis and Catalysis 2021, 2 (2), 156–164. DOI:10.1016/j.gresc.2021.03.007.
[13]. Dalal, D. S.; Patil, D. R.; Tayade, Y. A. Β‐cyclodextrin: A Green and Efficient Supramolecular Catalyst for Organic Transformations. The Chemical Record 2018, 18 (11), 1560–1582. DOI:10.1002/tcr.201800016.









