1. Introduction
Water pollution is a critical environmental issue that has garnered increasing attention in recent decades. A way to purify water is now needed in urgent to solve water pollution more efficiently. So here comes up with this concept: Semiconductor photocatalyst. By constructing heterojunctions, such as p-n, Z-type, and S-type heterojunctions, photogenerated carriers (electrons and holes) can be effectively separated and photocatalytic efficiency can be improved. These heterojunctions show excellent performance in reducing recombination losses and extending the optical response range. However, in terms of environmental purification, how to control the intermediates and end products generated during the photocatalysis process to make them safer and harmless still needs further research. The article is studied in photonic crystal heterostructure with anatase thin film between silica layers by way of integrating previous manufacturing processes to develop new devices to achieve better performance. During the study, a green and harmless water treatment method is provided. Compared with traditional physical or chemical methods, photocatalysis can completely mineralize organic pollutants into carbon dioxide and water, avoiding secondary pollution. Also, this paper provides a reference for the scholars who later study semiconductor photocatalysis.
2. Structure of different TiO2
Fujishima and Honda made a groundbreaking discovery of the photocatalytic splitting of water on TiO2 electrodes in 1972, heralding the dawn of a new age in heterogeneous photocatalysis [1]. So far, three crystal structures of titanium dioxide have been discovered in the nature: Anatase, rutile, and brookite, These three substances have different structures, which leads to them having different properties, especially in photocatalysis. [2]. The anatase of TiO2 shows a higher photocatalytic activity [3]. The structures of rutile and anatase are characterized by chains of TiO2 octahedra, which differ in terms of the distortion of individual octahedra and the arrangement of these chains [1]. (See Fig.1)
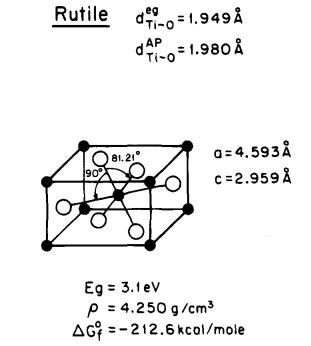
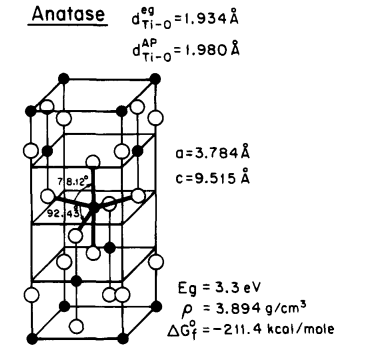
Figure 1: Structure of rutile and anatase TiO2. [1]
3. Principle of photocatalysis to transform energy
The principle of photocatalysis is the redox capabilities of photocatalysts in light irradiation. This process uses light energy to excite compound semiconductors, such as TiO2, and to harness the generated electrons and holes to facilitate oxidation-reduction reactions. When light shines on nanomaterials with an energy level greater than the semiconductor's bandgap energy, electrons jump from valence band to conduction band, creating holes in valence band. This makes electron-hole pairs. Nanomaterials, due to their high number of dangling bonds, can easily trap these electrons or holes, preventing their recombination and enhancing their time to exert power. Then, the electrons and holes would move to the surface of the particle and exhibit strong redox potentials. Semiconductor photocatalysis, which is a sustainable technology, has been widely applied to solve various environmental problems more than water and air purification. It has proved the efficiency in destroying microorganisms like bacteria, kill cancer cells, controlling odors, photosplitting water to generate hydrogen gas, fixing nitrogen, and mitigating oil spills [4].
During a photocatalyzed reaction, five main steps are included: i) Semiconductors absorb light from outside, ii) Electrons and hole pairs are generated in the materials, iii) Electrons and holes move through the material and combine with each other to annihilate, iv) The reaction product detaches from the surface of the material, and v) A redox reaction took place on the semiconductor (see Fig. 2).During such reaction, Electron and hole polymerization and annihilation will have a bad effect on the performance of the device because this produces unwanted energy, it does not help the redox reaction. The movement of electrons and holes within the device to promote material conduction is the most important reason to promote the redox reaction [5].
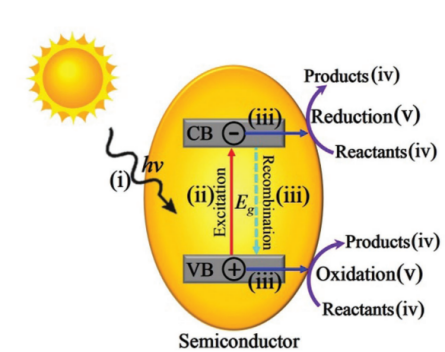
Figure 2: Schematic diagram of semiconductor photocatalysis.[5]
However, we are now lacking an appropriate catalyst. Many oxides consist in metal cations of d0 and d10 configurations, metal sulfide and nitride photocatalysts have been reported in the latest decade [6]. and we are now paying attention to TiO2. The biggest disadvantage of photocatalyst application is the high recombination rate of electrons and holes that will reduce the efficiency of the quantum and decrease the activity of photocatalytic. One way to overcome this problem is to use multiphase TiO2 because it shows higher photocatalytic activity than single-phase because of more and more charge transfer generated between different TiO2 polymorphs (have different levels of bandgaps), result in the improvement of the efficiency in separation of charge carriers and then prevent the recombination of electrons and holes [2].
Fujishima's research group found that when TiO2 film is in exposure to ultraviolet (UV) light, the contact Angle of water gradually decreases to almost zero [5-6]. We call this phenomenon superhydrophilicity. Then, the thin film composed only of TiO2 shows the contact Angle of the water becomes almost zero under the exposure of UV [7]. Superhydrophilic surfaces with water contact angles (WCAs) lower than 5° are very useful in antifogging, self-cleaning and drug delivery systems. Superhydrophilicity is of great importance in the self-cleaning of TiO2 and photocatalytic films. If the film is composed only of TiO2, when stopping the light irradiation, the moisture angle of water on the surface of the TiO2 film would gradually increase and return back to the original state. The latest research found that the composite film prepared by TiO2 and SiO2 mixed sol on the surface of the ceramic tile has superhydrophilicity under the exposure of light induction, and the superhydrophilicity is also maintained well [7-8].
4. Experimental procedures by self-assembly
There are three classical methods for preparing titanium dioxide films: sputtering, chemical vapor deposition(CVD), and pulsed laser deposition(PLD) [9-11]. However, these methods mostly need the equipment which is specialized and complicated processing and causing the limitation of their widespread utilization. Now the sol-gel method to build film is more efficient for the fabrication of TiO2 thin film because it is a more simple way to create the high purity films. The precursor solutions are made of polymer and sol, then use annealing and spin coating to build the films. Many technologic applications are in low temperature of processing because of the feature that preventing the film from reacting with the substrate [12]. However the sol-gel method needs high temperature for over ~400 ℃ in the case of precursor solutions of metal-organic solutions to remove the crystallization and organic impurities. [13]. Also, the film is prepared in lower refractive index because of the low density of the TiO2 film structure made in such method [14].
Ethyl orthosilicate (TEOS) and butyl titanate were used as materials for the synthesis of precursor solution, and taking action in two-step hydrolysis method [15]. The steps were as follows: according to the molar ratio of TEOS: C2H5OH: H2O: HCI= 1:4:2:0.03, mixing solution, heated up to 70℃, reflux for 2h, and then homace-tylacetone: Butyl titanate, anhydrous ethanol =1:1:4 solution mixed and stirred, and then added anhydrous ethanol (the same volume of the mixed solution), H2O and HCI solution, wherein: "TEOS+ butyl titanate) : HaO:HCI= 1:2:0.3, the molar percentage of SiO2 is: 5, 10, 15, 20, 33 and 50. After placing the solution at room temperature for 24h, it can be used for coating. TiO2/SiO composite film is using clean ordinary glass slide as the substrate from the above sol precursor by diffuse stain lifting method, and the speed of pulling is 2mm. Then drying at 100℃ for 5min, putting wet film into the Muffle furnace, kept at 500℃ for 1h, removed the Muffle furnace, and cooled to room temperature to obtain TiO2/SiO [8]. TiO2 films can be prepared in such method described in reference [10].
Using dipcoating can deposite the thin films of TiO2 and TiO2–SiO2 on the substrates. But adhesion of TiO2 film show worse on the substrate .The reason is that the stress is accumulated in the interface, also coating two times result in the separation of the thin film and the substrate. One wayt to make stronger adhersion is a high temperature. So the silica is introduced in as a material to bind gaps. Fig. 3 is the image of transmission electron microscope about cross-section at the intersection of titanium dioxide and silica thin films which are created by the dip coating method and then heating to 100℃ for 1 h. And in the inset of Fig. 3, TiO2 exist as the form of anatase by the way of technique that selected area diffraction,also it can be seen that in (110) plane of Debye-Scherrer ring in anatase TiO2 [16].
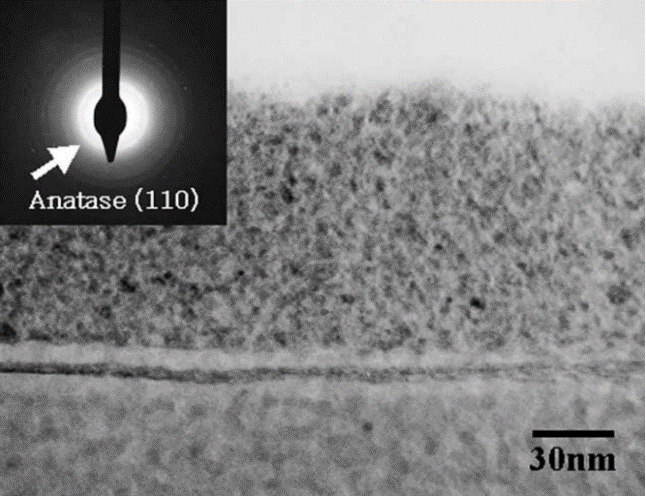
Figure 3: transmission electron microscope about cross-section at the intersection of titanium dioxide and silica thin films which are created by the dip coating method and then heating to 100°C for 1 h,and in (110) plane of Debye-Scherrer ring in anatase TiO2 (inset) [8]
5. Results and discussion
As shown in Fig. 4, atomic force microscopy(AFM) shows surface of TiO2 films in the nano-scale of different amounts of SiO2(0%,30%,50%,70%) and then heated to 100°C for 1 h. The AFM indicated that with increasing SiO2 content, the nano-composite film surface became curvier compared to the TiO2 films which were not dopped in silica. The crystal anatase TiO2 formed dendrites in glass with height of crystals of the TiO2–SiO2 glassy phase increasing as the temperature increasing in the report of Karthikeyan et,al [17]. Changing the amount of SiO2 can customize the surface structure and the physical and chemical properties [16].
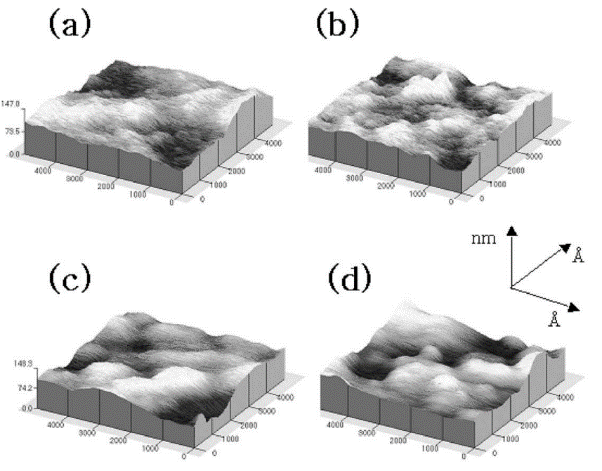
Figure 4: thin films of images in atomic force microscopy (a) 0% SiO2 +100% TiO2 (b) 30% SiO2 +70% TiO2 (c) 50% SiO2 +50% TiO2 and (d) 70%SiO2+30% TiO2 heated to 100°C for 1 h on the glass substrate. [8]
6. Improvements
It is generally believed that nano-sized semiconductors differ in physical and chemical properties from bulk semiconductors, are primarily determined by the particle surface, So in this small scale, surface area is becoming more and more important. Therefore, controlling the diameter of the particle of the titanium dioxide material can significantly change its response to photocatalysis and one way is synthesizing nano-scale TiO2 particles. Moreover, in order to expand its applicability to various substrates, it is necessary to improve the adhesive force and hardness by introducing binder materials such as silica [16].
The contact Angle is found to rise and recover relatively early in dark places. It is best to raise the contact Angle slowly in a dark place and keep the contact Angle low for a long time, as it is not always exposed to ultraviolet light (such as sunlight) if considering actual use. Therefore, With the addition of silica, the contact angle of water becomes much lower, it also remained hydrophilic well in the dark. As for TiO2-SiO2 system materials, many studies have been carried out in the past in glass, humidity sensors, etc. For example, Kamiya and Sakka used IR-spectra to measure the amount of water about TiO2-SiO2 glasses which are made up of 0–15 wt % TiO2 and heated to 1173 K. Surface hydrophilicity was tested by Hosaka and Meguro, using metal alkoxide to produce the TiO2-SiO2 pellet with 0–100 mol % SiO2 in 773 K [18-20].
It is generally believed that when ultraviolet light cast on TiO2 film, the redox reaction takes place to produce reactive oxygen species on the film. This is very conducive to the occurrence of hydrolysis reactions and produces the superhydrophilicity of the substance in the process. However, due to the wide band gap of TiO2, only energy greater than or equal to the amount of energy provided by ultraviolet light can excite the electrons in the device. This will be an obstacle in the applications of TiO2. One way to change the wettability of TiO2 film is to adjust its surface roughness. According to studies of Quéré and Wenzel, the roughness of surface coefficient becomes very large for porous films so the hydrophilic material is easily wetted [21]. Therefore, superhydrophilicity can be achieved by introducing holes in the hydrophilic film.
For the practical application of superdroplet TiO2 films, it is of great importance in high transmittance. But TiO2 can reflect most incident light due to its large index of refraction. Introducing a SiO2 layer between the substrate and the TiO2 film can improve the transmission rate in J. J. Wang's research group [21].
7. Conclusion
In this paper, a self-assembly method is designed to prepare titanium dioxide film and place it in two layers of silica, and introduce the concept and principle of photocatalytic water purification from sewage treatment. In terms of the material itself, the advantages of superhydrophilicity, the advantages of sol-gel film preparation and the necessity of placing silica layer are described, and the contact Angle and transmittance also play a great role in the application of the film. The film prepared by sol-gel method used in this paper usually has pores and cracks, resulting in poor compactness. Such films may have insufficient durability and corrosion resistance in some applications (such as anti-corrosion coatings, protective films, etc.). In the process of drying and heat treatment, due to solvent volatilization, sol shrinkage and thermal stress, the surface of the film is easy to crack and non-uniform, which affects the performance of the film. Possible future research directions will focus on improving the visible light response performance, inhibiting the recombination of photogenerated carriers, and enhancing the stability and recycling ability of photocatalysts.
References
[1]. L. L. Amy, G. Q. Lu, T. Y. John, Jr.*” Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results”, Chem. Rev. ,(1995), 95, 735-758
[2]. D. R. Eddy , M. D. Permana , L. K. Sakti , G. A. N. Sheha , Solihudin , S. Hidayat , T. Takei , N. Kumada ,I.Rahayu,” Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity”, Nanomaterials ,(2023), 13, 704.
[3]. Augustynski, J. Electrochim.,” The role of the surface intermediates in the photoelectrochemical behaviour of anatase and rutile TiO2”, Acta, (1993), 38, 43.
[4]. M.R. Hoffmann, S. T. Martin, W. Y.Choi, and D. W. Bahnemann,” Environmental Applications of Semiconductor Photocatalysis”, Chem. Rev. (1995), 95, 69-96
[5]. J. X. Low, J. G Yu, M. Jaroniec, S. Wageh, A. A. Al-Ghamdi, “Heterojunction Photocatalysts”, Adv. Mater, (2017), 29, 1601694
[6]. A. Kudo, Y. Miseki ,” Heterogeneous photocatalyst materials for water splitting”, Chem. Soc. Rev., (2009), 38, 253–278
[7]. K. sunada, Y. kikuchi, K. hashimoto, A. fujishima,“Bactericidal and Detoxification Effects of TiO2 Thin Film Photocatalysts”, environmental science & technology/vol. ,(1998), 32, no. 5,
[8]. J. G. Yu, X. J. Zhao, L.Lin, J.J. Han, Q. N. Zhao, “Preparation and Characterization of Superhydrophilic TiO2-SiO2 composite film”, Journal of Inorganic Materials, (2001), 16, No. 3
[9]. C. Byun, J. W. Jang, I. T. Kim, K. S. Hong, B. W. Lee, “Anatase-torutile transition of titania films prepared by MOCVD”, Mater. Res. Bull., (1997), 32 (4), 431
[10]. L. Escobar-Alarcon, E. Haro-Poniatowski, M.A. Chamacho-Lopez, “Structural characterization of TiO2 thin films obtained by pulsed laser deposition”, Appl. Surf. Sci., (1999), 137 ,38.
[11]. G. H. Li, L. Yang, Y. X. Jin, L. D. Zhang, “Structural and optical properties of TiO2 thin films and TiO2+2wt% ZnFe2O4 composite film prepared by r.f. sputtering”, Thin Solid Films , (2000), 368, 163.
[12]. K. M. S. Khalil, M. I. Zaki, Synthesis of high surface area titania powders via basic hydrolysis of titanium (IV) isopropoxide, Powder Technol. 92 (1997) 233.
[13]. V.M. Gun Ko, F. Villieras, R. Leboda, M. Marciniak, B. Charmas, J. Skubiszewska-Zieba, Characterization of titania/ silica gel by means of low-pressure nitrogen adsorption, J. Col-loid Interface Sci. 230 (2000) 320.
[14]. R. Leboda, V.M. Gun Ko, M. Marciniak, A. Malygin, A.A. Malkin, W. Grzegorczyk, et al., Structure of chemical vapor deposition titania/ silica gel, J. Colloid Interface Sci. 218 (1999) 23.
[15]. R. Burch, Z. Paal, P.G. Menon, Hydrogen effects in catalysis, Marcel Dekker, New York, 1988, p. 345.
[16]. C. H. Kwon, J. H. Kim, I. S. Jung, H. Shin, K. H. Yoon,“Preparation and characterization of TiO2–SiO2 nano-composite thin films”, Ceramics International, (2003), 29, 851–856
[17]. A. Karthikeyan, R. M. Almeida, “Crystallization of TiO2–SiO2 glassy films studied by atomic force microscopy”, J. Non-Cryst. Solids (2000), 274, 169
[18]. K. KAMIYA and S . SAKKA, “Thermal expansion of TiO2–SiO2 and TiO2-GeO2 glasses”, Chem. Soc. Jpn. (1981), 52, 1571–1576.
[19]. H. HOSAKA and K. MEGURO, “Estimation of surface properties of some metal oxides and pigments by investigation of charge-transfer phenomena at their surfaces”, Chem. Soc. Jpn., (1971), 44, 1252–1256.
[20]. M. Machida, K. Norimoto, T. Watanabe,“The effect of SiO2 addition in super-hydrophilic property of TiO2 photocatalyst”, Journal of Materials Science, (1999), 34 ,2569 – 2574.
[21]. J. J. Wang, D. S. Wang, J. Wang, W. L. Zhao, C. W. Wang,“ High transmittance and superhydrophilicity of porous TiO2/SiO2 bilayer films without UV irradiation”, Surface & Coatings Technology, (2011), 205, 3596–3599.
Cite this article
Wang,Y. (2025). A Self-assembly-prepared TiO2-SiO2 Heterostructure Film with Enhanced Photocatalysis Activity. Applied and Computational Engineering,123,59-65.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. L. L. Amy, G. Q. Lu, T. Y. John, Jr.*” Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results”, Chem. Rev. ,(1995), 95, 735-758
[2]. D. R. Eddy , M. D. Permana , L. K. Sakti , G. A. N. Sheha , Solihudin , S. Hidayat , T. Takei , N. Kumada ,I.Rahayu,” Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity”, Nanomaterials ,(2023), 13, 704.
[3]. Augustynski, J. Electrochim.,” The role of the surface intermediates in the photoelectrochemical behaviour of anatase and rutile TiO2”, Acta, (1993), 38, 43.
[4]. M.R. Hoffmann, S. T. Martin, W. Y.Choi, and D. W. Bahnemann,” Environmental Applications of Semiconductor Photocatalysis”, Chem. Rev. (1995), 95, 69-96
[5]. J. X. Low, J. G Yu, M. Jaroniec, S. Wageh, A. A. Al-Ghamdi, “Heterojunction Photocatalysts”, Adv. Mater, (2017), 29, 1601694
[6]. A. Kudo, Y. Miseki ,” Heterogeneous photocatalyst materials for water splitting”, Chem. Soc. Rev., (2009), 38, 253–278
[7]. K. sunada, Y. kikuchi, K. hashimoto, A. fujishima,“Bactericidal and Detoxification Effects of TiO2 Thin Film Photocatalysts”, environmental science & technology/vol. ,(1998), 32, no. 5,
[8]. J. G. Yu, X. J. Zhao, L.Lin, J.J. Han, Q. N. Zhao, “Preparation and Characterization of Superhydrophilic TiO2-SiO2 composite film”, Journal of Inorganic Materials, (2001), 16, No. 3
[9]. C. Byun, J. W. Jang, I. T. Kim, K. S. Hong, B. W. Lee, “Anatase-torutile transition of titania films prepared by MOCVD”, Mater. Res. Bull., (1997), 32 (4), 431
[10]. L. Escobar-Alarcon, E. Haro-Poniatowski, M.A. Chamacho-Lopez, “Structural characterization of TiO2 thin films obtained by pulsed laser deposition”, Appl. Surf. Sci., (1999), 137 ,38.
[11]. G. H. Li, L. Yang, Y. X. Jin, L. D. Zhang, “Structural and optical properties of TiO2 thin films and TiO2+2wt% ZnFe2O4 composite film prepared by r.f. sputtering”, Thin Solid Films , (2000), 368, 163.
[12]. K. M. S. Khalil, M. I. Zaki, Synthesis of high surface area titania powders via basic hydrolysis of titanium (IV) isopropoxide, Powder Technol. 92 (1997) 233.
[13]. V.M. Gun Ko, F. Villieras, R. Leboda, M. Marciniak, B. Charmas, J. Skubiszewska-Zieba, Characterization of titania/ silica gel by means of low-pressure nitrogen adsorption, J. Col-loid Interface Sci. 230 (2000) 320.
[14]. R. Leboda, V.M. Gun Ko, M. Marciniak, A. Malygin, A.A. Malkin, W. Grzegorczyk, et al., Structure of chemical vapor deposition titania/ silica gel, J. Colloid Interface Sci. 218 (1999) 23.
[15]. R. Burch, Z. Paal, P.G. Menon, Hydrogen effects in catalysis, Marcel Dekker, New York, 1988, p. 345.
[16]. C. H. Kwon, J. H. Kim, I. S. Jung, H. Shin, K. H. Yoon,“Preparation and characterization of TiO2–SiO2 nano-composite thin films”, Ceramics International, (2003), 29, 851–856
[17]. A. Karthikeyan, R. M. Almeida, “Crystallization of TiO2–SiO2 glassy films studied by atomic force microscopy”, J. Non-Cryst. Solids (2000), 274, 169
[18]. K. KAMIYA and S . SAKKA, “Thermal expansion of TiO2–SiO2 and TiO2-GeO2 glasses”, Chem. Soc. Jpn. (1981), 52, 1571–1576.
[19]. H. HOSAKA and K. MEGURO, “Estimation of surface properties of some metal oxides and pigments by investigation of charge-transfer phenomena at their surfaces”, Chem. Soc. Jpn., (1971), 44, 1252–1256.
[20]. M. Machida, K. Norimoto, T. Watanabe,“The effect of SiO2 addition in super-hydrophilic property of TiO2 photocatalyst”, Journal of Materials Science, (1999), 34 ,2569 – 2574.
[21]. J. J. Wang, D. S. Wang, J. Wang, W. L. Zhao, C. W. Wang,“ High transmittance and superhydrophilicity of porous TiO2/SiO2 bilayer films without UV irradiation”, Surface & Coatings Technology, (2011), 205, 3596–3599.









