1. Introduction
Bisphenols (BPs), such as Bisphenol A (BPA) and Bisphenol S (BPS), are extensively used in industrial products, posing significant environmental and health risks. BPA, due to its higher solubility, is the most frequently detected bisphenol in aquatic environments, while other bisphenols are less soluble. The widespread usage of bisphenols has resulted in their occurrence in both surface water and drinking water, raising concerns about potential health risks. BPA and BPS, in particular, are known for their bioaccumulation potential and endocrine-disrupting properties, which necessitate accurate detection and monitoring [1-3].
One of the major challenges in detecting bisphenols in aquatic products is the matrix effect, which refers to interference caused by other substances present in the sample matrix, potentially affecting the detection accuracy of LC-MS. In aquatic products, matrix effects are primarily attributed to the high content of phospholipids, which may co-elute with bisphenols during chromatographic separation, resulting in signal suppression or enhancement during ionization. Addressing matrix effects is essential to improve the reliability and accuracy of bisphenol detection, especially in complex matrices like aquatic products.
This study aims to investigate the sources of matrix effects and identify effective mitigation strategies. The findings will contribute to enhancing the detection accuracy of bisphenols, providing valuable insights for environmental monitoring and public health protection. The paper is organized as follows: Section 2 describes the materials and methods used, including chromatographic conditions and reagents. Section 3 presents the results and discusses the matrix effects observed, as well as the impact of phospholipids. Finally, Section 4 concludes the study by summarizing key findings and future directions.
2. Materials and Methods
2.1. Chemicals and Reagents
Bisphenol A (BPA) and Bisphenol S (BPS) standards were obtained from Sigma-Aldrich (USA). Methanol and acetonitrile were of HPLC grade, and ultrapure water was obtained from a purification system (Exceed-AD-16, China).
2.2. Chromatographic Conditions
An UltiMate 3000 HPLC system (ThermoFisher, USA) was used with a Symmetry C18 column (4.6 mm ×250 mm, 5 µm, Waters, USA). The mobile phase consisted of methanol (70%) and water (30%), with a flow rate of 1.0 mL/min. The injection volume was 20 µL, and detection was performed at 224 nm.
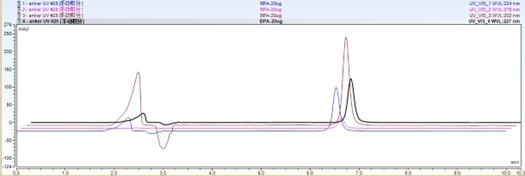
Figure 1: Chromatogram of BPA and BPS standard solutions under optimized chromatographic conditions.
3. Results and Discussion
3.1. Matrix Effect in LC-MS Analysis
The matrix effect is a significant factor influencing the accuracy of LC-MS analysis, particularly for polar compounds such as BPA and BPS. In this study, it was observed that BPA could be reliably detected without interference, whereas BPS co-eluted with substances in the matrix, likely phospholipids, leading to signal suppression. The chromatogram of BPA and BPS standard solutions under optimized chromatographic conditions is shown in Figure 1, illustrating the separation of these compounds and the retention times observed under the established method.
Phospholipids are amphipathic molecules that are prone to cause matrix effects during electrospray ionization (ESI), leading to ion suppression or enhancement of co-eluting analytes [4, 5]. The presence of matrix effects indicates that careful method development is essential to obtain reliable results for bisphenols in aquatic products.
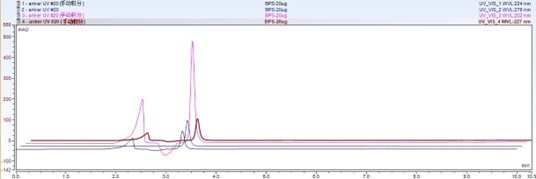
Figure 2: Comparison of BPA chromatograms under different wavelengths: 224 nm, 278 nm, 202 nm, 227 nm.
Figure 2 shows the chromatograms of BPA at different detection wavelengths. The results indicate that the choice of detection wavelength can have a significant impact on sensitivity and accuracy.
3.2. Phospholipids as a Source of Matrix Effect
To confirm the presence of phospholipids and their contribution to the matrix effect, samples of fish, crabs, and shellfish were analyzed. Phospholipids were detected in all three types of matrices, with fish samples having the highest phospholipid content. The use of evaporative light scattering detection confirmed the presence of phospholipids, and their co-elution with BPS demonstrated their role in causing matrix effects during LC-MS analysis [6, 7]. This observation is consistent with previous studies that indicate phospholipids are a major contributor to ion suppression in ESI-MS, primarily due to their amphipathic nature, which allows them to compete with analytes for ionization [8, 9].
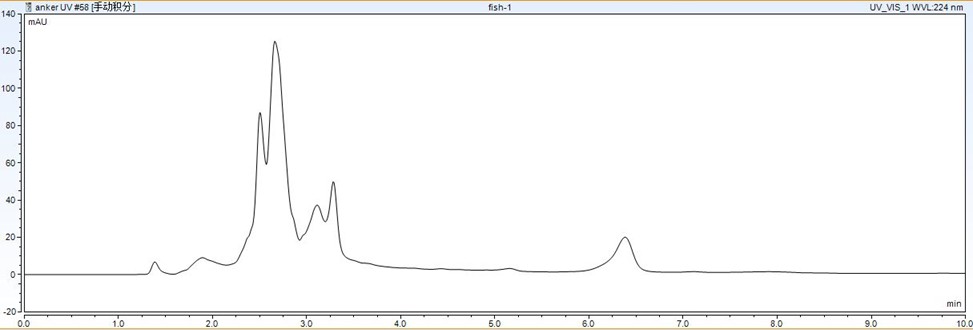
Figure 3: Phospholipid content in different aquatic matrices analyzed by evaporative light scattering detection.
Figure 3 presents the phospholipid content in different aquatic matrices. The highest content was found in fish, which correlated with the highest matrix effect observed during analysis.
3.3. Ionization Mechanisms: IEM and CEM
Two ionization models, the ion evaporation model (IEM) and the chain ejection model (CEM), were introduced to explain the matrix effect in the ionization process. In the IEM, the strong polarity of BPS hinders its migration to the droplet surface, which reduces ionization efficiency. Phospholipids, on the other hand, are readily ionized due to their ability to access the droplet surface efficiently, as explained by the CEM. This disparity in ionization efficiency between BPS and phospholipids accounts for the observed signal suppression during mass spectrometric detection [9, 10].
3.4. Mitigation Strategies
To mitigate the effects of the matrix, several strategies were evaluated. Modifying chromato- graphic conditions, such as adjusting the mobile phase composition and pH, proved effective in reducing the matrix effect. The use of solid-phase extraction (SPE) was also investigated as a sample cleanup step to remove phospholipids before LC-MS analysis. SPE significantly reduced the phospholipid content in the sample, which in turn reduced the matrix effect. Additionally, the use of isotopic internal standards was found to be an effective strategy for compensating for matrix-induced signal suppression or enhancement [3, 11].
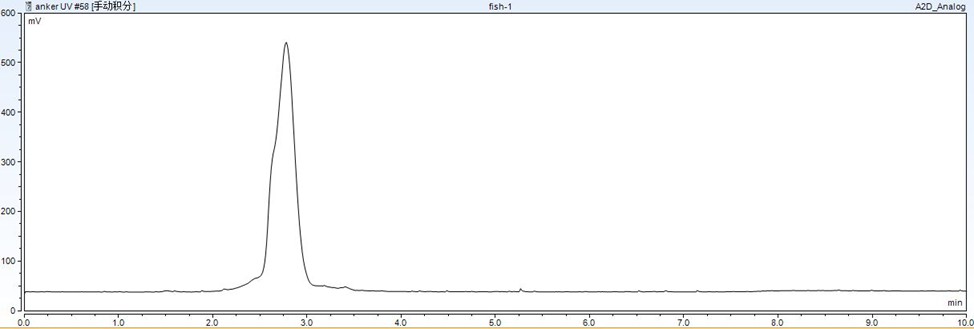
Figure 4: Chromatogram showing the effect of solid-phase extraction (SPE) on reducing matrix interference for BPS.
Figure 4 shows the effect of solid-phase extraction on reducing matrix interference for BPS. The results clearly demonstrate that SPE can effectively remove interfering substances, thereby improving the accuracy of LC-MS analysis.
Isotopic internal standards can help correct for variability in the ionization process by co- eluting with the target analyte and undergoing the same ionization conditions. This approach has been widely applied in LC-MS/MS analysis to improve the accuracy and precision of quan- titative results, especially for challenging matrices such as aquatic products [8, 12].
4. Conclusion
A rapid chromatographic method was established for the detection of bisphenol compounds in aquatic products. The study confirmed that phospholipids are a significant contributor to the matrix effect, particularly in the detection of BPS. Mitigation strategies such as modify- ing chromatographic conditions, using solid-phase extraction, and employing isotopic internal standards were found to be effective in reducing matrix effects. These findings highlight the importance of addressing matrix effects in the analysis of bisphenols to ensure accurate and reliable results.
References
[1]. Song, Z., Qiu, Y., Zhang, H., & et al. (2020). The occurrence and research progress of bisphenol analogues in aquatic environment. Environmental Chemistry, 39(6), 1496–1503.
[2]. Huang, Y., Zhang, W., Wang, R. G., & et al. (2022). Advances on pollution status and endocrine disrupting effects of bisphenols. Asian Journal of Ecotoxicology, 17(1), 1–10.
[3]. Regueiro, J., & Wenzl, T. (2015). Advances in bisphenol analysis by chromatography. Journal of Chromatography A, 1422, 230–238. https://doi.org/10.1016/j.chroma.2015.09.028
[4]. Meng, S. U., & Ai, L. F. (2014). Matrix effects and elimination methods in LC-MS/MS. Journal of Food Safety and Quality, 5(2), 320–329.
[5]. Bergeron, A., & Garofolo, F. (2013). Importance of matrix effects in LC-MS/MS bioanalysis. Bioanalysis, 5(19), 2489–2490. https://doi.org/10.4155/bio.13.237
[6]. Li, M., Si, D., Fu, Z., & et al. (2019). Identification of phospholipids in aquatic matrices. Journal of Chromatography B, 1109, 99–111. https://doi.org/10.1016/j.jchromb.2019.02.001
[7]. Ciric, J., Verberne, M., Bouwman, L., & Baltussen, E. (2019). Development of UPLC-MS/MS methods for environmental toxicology. Journal of Chromatography B, 1109, 128–131. https://doi.org/10.1016/j.jchromb.2019.02.003
[8]. Gallart-Ayala, H., Nunez, O., & Lucci, P. (2013). Matrix effects in LC-MS: A review. Trends in Analytical Chemistry, 42, 99–124. https://doi.org/10.1016/j.trac.2012.09.006
[9]. Konermann, E., Ahadi, E., & Rodriguez, D. (2013). Mechanism of electrospray ionization. Analytical Chemistry, 85(1), 2–9. https://doi.org/10.1021/ac302789c
[10]. King, B., Vance, J., Wall, G. M., & Shoup, R. (2019). Quantitation of bisphenols in plasma. Journal of Chromatography B, 1109, 25–36. https://doi.org/10.1016/j.jchromb.2019.01.013
[11]. García-Córcoles, M., Cipa, M., Rodríguez-Gómez, R., & et al. (2018). Detection of bisphenols in aquatic environments. Talanta, 178, 441–448. https://doi.org/10.1016/j.talanta.2018.03.076
[12]. Xu, Y. Y., Li, S., Zhang, Q., & et al. (2017). Matrix effects in liquid chromatography-tandem mass spectrometry. Pesticides, 56(3), 162–167.
Cite this article
Abduhabar,A. (2025). Investigation of Matrix Effect in the Determination of Bisphenols in Aquatic Products by Liquid Chromatography-Mass Spectrometry. Applied and Computational Engineering,124,15-19.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Song, Z., Qiu, Y., Zhang, H., & et al. (2020). The occurrence and research progress of bisphenol analogues in aquatic environment. Environmental Chemistry, 39(6), 1496–1503.
[2]. Huang, Y., Zhang, W., Wang, R. G., & et al. (2022). Advances on pollution status and endocrine disrupting effects of bisphenols. Asian Journal of Ecotoxicology, 17(1), 1–10.
[3]. Regueiro, J., & Wenzl, T. (2015). Advances in bisphenol analysis by chromatography. Journal of Chromatography A, 1422, 230–238. https://doi.org/10.1016/j.chroma.2015.09.028
[4]. Meng, S. U., & Ai, L. F. (2014). Matrix effects and elimination methods in LC-MS/MS. Journal of Food Safety and Quality, 5(2), 320–329.
[5]. Bergeron, A., & Garofolo, F. (2013). Importance of matrix effects in LC-MS/MS bioanalysis. Bioanalysis, 5(19), 2489–2490. https://doi.org/10.4155/bio.13.237
[6]. Li, M., Si, D., Fu, Z., & et al. (2019). Identification of phospholipids in aquatic matrices. Journal of Chromatography B, 1109, 99–111. https://doi.org/10.1016/j.jchromb.2019.02.001
[7]. Ciric, J., Verberne, M., Bouwman, L., & Baltussen, E. (2019). Development of UPLC-MS/MS methods for environmental toxicology. Journal of Chromatography B, 1109, 128–131. https://doi.org/10.1016/j.jchromb.2019.02.003
[8]. Gallart-Ayala, H., Nunez, O., & Lucci, P. (2013). Matrix effects in LC-MS: A review. Trends in Analytical Chemistry, 42, 99–124. https://doi.org/10.1016/j.trac.2012.09.006
[9]. Konermann, E., Ahadi, E., & Rodriguez, D. (2013). Mechanism of electrospray ionization. Analytical Chemistry, 85(1), 2–9. https://doi.org/10.1021/ac302789c
[10]. King, B., Vance, J., Wall, G. M., & Shoup, R. (2019). Quantitation of bisphenols in plasma. Journal of Chromatography B, 1109, 25–36. https://doi.org/10.1016/j.jchromb.2019.01.013
[11]. García-Córcoles, M., Cipa, M., Rodríguez-Gómez, R., & et al. (2018). Detection of bisphenols in aquatic environments. Talanta, 178, 441–448. https://doi.org/10.1016/j.talanta.2018.03.076
[12]. Xu, Y. Y., Li, S., Zhang, Q., & et al. (2017). Matrix effects in liquid chromatography-tandem mass spectrometry. Pesticides, 56(3), 162–167.









