1. Introduction
Retrosynthesis is an important method for solving organic synthesis routes, and is certainly the most basic and fundamental way to design organic synthesis. Its essence lies in analyzing the structure of the target molecule and then disassembling it step by step into simpler, more easily synthesized precursors and raw materials. Retrosynthesis is often found in organic chemistry, chemical engineering and biochemistry. Its role includes the synthesis of natural products, the synthesis of new drugs, and the reduction of products. It relies on the rules of chemical reactions to break down or split the target structure into precursors and then evaluate the availability and suitability of the product. This usually involves a number of steps and a range of potential retrosynthetic routes, where it can be determined which routes will be more practical, efficient and cost-effective than others.
In the subject of organic chemistry, it is usually found in the various reactions of complex molecules[1], starting with the starting molecule. The various intermediate reactions require the chemist to make his own predictions and guesses. Therefore, today, in order to improve the feasibility and efficiency of retrosynthesis, "synthesis plans"[2] have emerged, which are usually performed through retrosynthesis analysis, in which the desired compound is repeatedly broken down into intermediates or smaller precursors, directly finding the smallest known or purchasable structural units. "synthetic programs" have led to rapid changes in the field of retrosynthesis, with sophisticated and automated algorithms having the potential to provide retrosynthesis analysis with a wider range of applications and greater accuracy. But no matter how much it evolves, retrosynthesis always acts to proceed among the concepts it contains. Like the figure 1, it is an example of complex molecules. Therefore, we will proceed with the introduction of the concepts [3].
|
Figure 1. Complex protein molecules containing bovine serum. |
2. Concepts included in the Retrosynthesis
2.1. Retrosynthesis analysis terms
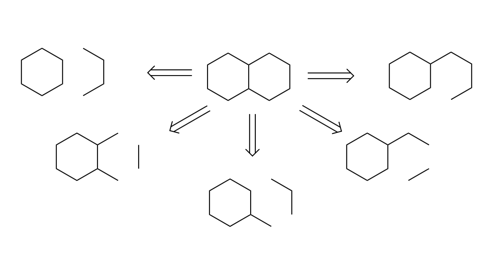
2.1.1. Disconnection. Disconnection is the most important part of the retrosynthesis, which acts in the course of a hypothetical chemical reaction, speculating on possible broken bonds and generating synthons up to the desired target molecule. Now, if we consider the substance in Figure 2 below as cyclic ethane, when considering only bond-breaking, we can break the bond at a tertiary carbon, only one benzene ring and one functional group is retained in the form. Optionally, two tertiary carbons can be broken and one of the benzene rings will be completely opened to form a ring opening reaction. When a ring-opening reaction occurs, the disconnection can act on any position of the benzene ring, and when more than two chemical bonds are severed, for example, in the Figure of 2 below left and below right, ethane is formed directly when two chemical bonds are disconnected [4].
|
Figure 2. The example substance. |
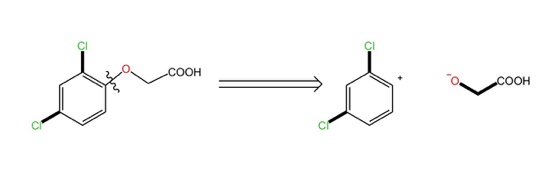
Disconnection also requires some guidelines and should not be done blindly. Like, the cut-off must be located in a reliable and known chemical reaction. At the bond of phenyl ether, we can know that the ether bond is very active according to the common sense of tasting, so we can choose to cut at the ether bond. But when choosing to disconnect at the phenyl, as Figure 3 It will produce "phenyl cation" is an unrealistic product.
|
Figure 3. Disconnect at the phenyl. |
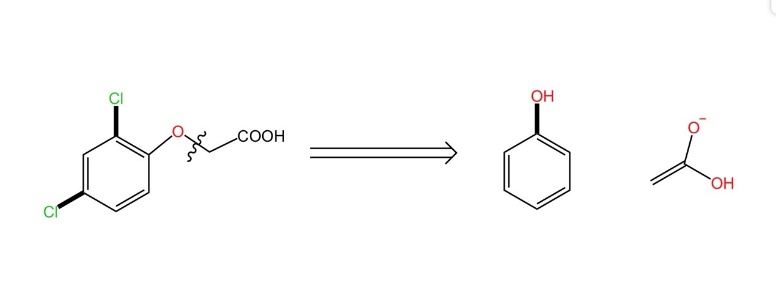
Now we need to focus on the version on the ether key, the right at this oxygen bond. The products at this point become phenols and enols. This is consistent with the fact that the disconnection must act in a reliable and known chemical reaction (Figure 4).
|
Figure 4. The second way of disconnection. |
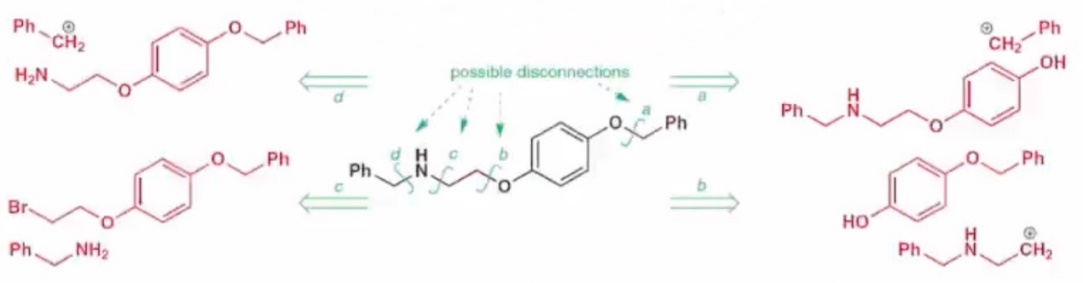
In addition to conforming to the rules of chemical reactions, alternative disconnection can be considered to avoid the condition of chemical selectivity. This usually means that when there are multiple disconnection options available. Among the complex molecules containing phenyl groups as in Figure 5, there are four ways of cutting, but only one of them is actually feasible.
|
Figure 5. Four ways of disconnection. |
First of all, the eyes were directed to route a, and the disconnection from a benzyloxy bond was chosen. But the amine on the left is also a nucleophile and will react with benzyl. Therefore, it will cause the hydroxyl and amino groups to react with benzyl at the same time, this leads to the problem of chemical selectivity. Next is route b, which also produces complex molecules with amino groups after the disconnection. The problem that arises is similar to route a, the amino group and the hydroxyl group will compete to react with the benzyl group. Next, looking at route c, which follow the rule by the preferential disconnection of the active group mentioned previously. Cut directly at the amine, at this point, the hydroxyl group becomes an ether, it is no longer possible to be competitive with amino. So all in all route c is the only correct one. Finally, route d, just look at this step, it is the same with route c, follow the rule that disconnect the active group, avoids the condition of chemical selectivity. But when considering the next step (usually disconnect the benzyloxy bond), once again, there is a problem similar to route a, b. Amino and carbonyl groups react with benzyl groups by a competitiveness. Therefore it is also unrealistic.
2.1.2. Retrosynthesis arrow. In disconnection section during the picture in the introduction, there appeared and ordinary chemical reaction (E.g. equal sign, single arrow, two-way arrow). Figure 6 shows the correct arrow. The different arrows indicate the reaction process, which is the unique "double arrow" of the retrosynthesis reaction. The two-way double arrow similar to its length does not represent a reaction of Retrosynthesis and therefore needs to be distinguished. During some way, it will also indicate the word Retrosynthesis above the arrow.
|
Figure 6. Retrosynthesis arrow. |
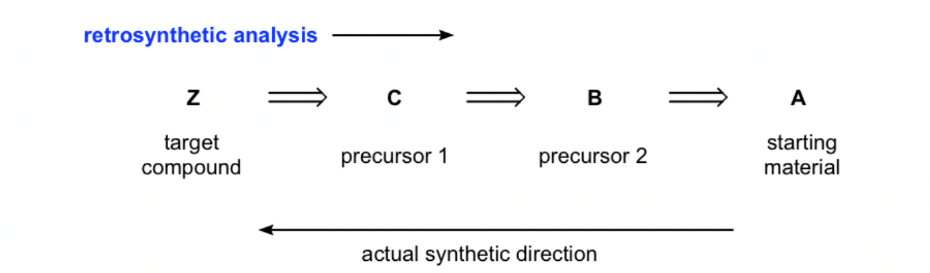
2.1.3. Target molecule. The synthesis of the desired compound usually requires from a few steps to 20 even more steps [5]. Therefore it will be a challenge to visualize all the necessary steps from the beginning. A common strategy for designing synthesis is to work backwards; that is, instead of looking at the starting material and deciding how to proceed to the first step, we look at the product and decide how to proceed to the final step. Therefore, in the synthetic route, the first molecule is the target molecule described by the retrosynthesis analysis, and we first identify the precursor that can be reacted in one step to produce the target compound, then determine the next available reaction to generate the precursor and repeat the process until we have the original material. We should notice that; the analysis is done in this way to show the "idea" of solving the problem. Therefore, the reagents/conditions required for each step are usually not specified. In Figure 7, until the synthetic route is written in a forward direction. In addition, the synthesis process is likely to propose multiple routes using different precursors, which can then be evaluated by assessing the possible advantages and disadvantages of each route to determine the most effective synthetic route.
|
Figure 7. The direction of retrosynthetic analysis. |
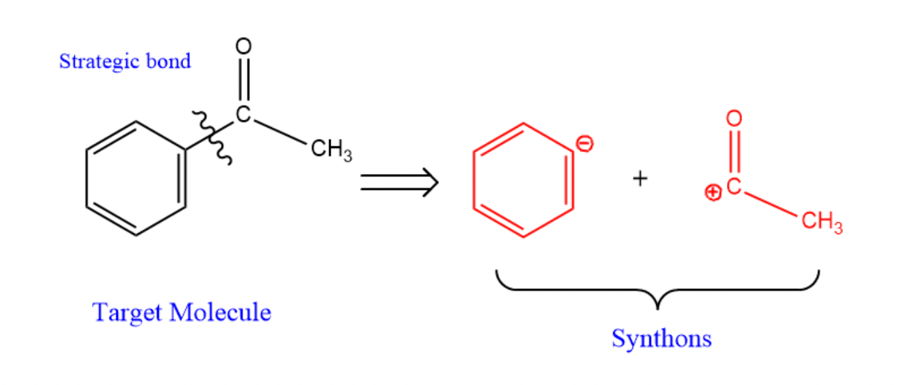
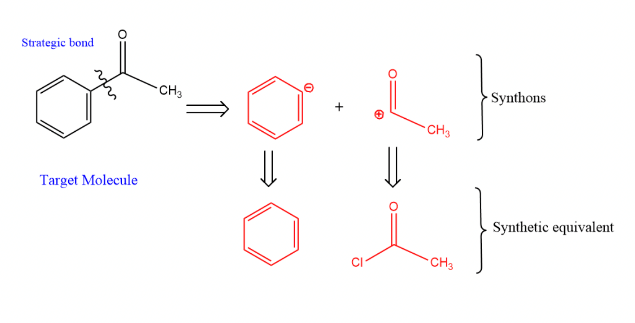
2.1.4. Synthon. The concept of a synthon was introduced by Elias James Corey in 1967. It is a hypothetical unit in the target compound that represents the potential reagents experienced by the target molecule in the retrosynthesis. When some key chemical bonds in the target molecule are broken, two charged compunds are sometimes formed, which are synthons. However, it is worth noting that synthon can be as large as a complex molecule or as small as a hydrogen molecule. The first step is to identify the chemical bonds that have been disconnected in the molecule to form one type of negatively charged and another type of positively charged materials, all collectively referred to as synthons. After predicting as well as understanding the smallest fragment of the target molecule as well as the building blocks, the synthons are combined to form the target molecule, thus synthons are very important substances in the process of retrosynthesis and can lead to the synthetic route that generates the target molecule.
As in Figure 8 of acetophenone (phenyl compound):
|
Figure 8. Synthons of acetophenone. |
There is a disconnection on the carbon which contain a carbon-oxygen double bond with the benzene ring. The acetyl group with positively charged, benzene ring with the negatively charged were obtained, respectively, and their three-dimensional structures are shown in the following Figures:
|
Figure 9. The acetyl group with positively charged. |
|
Figure 10. Benzene ring with the negatively charged. |
During the figure 9 and 10, these two diagrams resemble molecules because they are not labeled with electrons like the Lewis structure diagram, so it is worth noting that; the synthon cannot be obtained on a realistic basis, it only exists in the reaction process. To solve this puzzle, a new concept is introduced at this point, the synthesis equivalent.[6]
2.1.5. Synthetic equivalents. It has already been shown that synthons have a positive/negative charge and therefore cannot be obtained in cases other than reactions, hence the synthetic equivalents as alternatives. In short explanation, synthetic equivalents are reagents that perform the functions contained in the synthetics and, since they can exist directly, do not carry a positive/negative charge.
Let's take phenylacetic acid as an example, regarding its synthesis, we were able to find two suitable synthons in designing as well as planning the synthetic route, which can be carboxyl (COOH) and benzyl. With a synthetic substrate, we need to find the corresponding synthetic equivalent to replace it. Usually, the effective synthetic equivalents for the carboxyl group are cyanide anions and benzyl bromide is a suitable synthetic equivalent for the benzyl group. Their chemical reactions proceed as follows:
\( PhC{H_{2}}Br+NaCN →PhC{H_{2}}CN+NaBr \)
\( PhC{H_{2}}CN +2{H_{2}}O →PhC{H_{2}}COOH+N{H_{3}} \)
With synthetic equivalents, the problem that synthons can only appear in reactions is directly solved. It can also be said that the synthon is the charged product conceived during the reaction. We return our attention to the acetophenone mentioned in Section 4, acetyl group with positive charge and benzene ring with negative charge, both of them can be found synthetic equivalents as acetyl chloride and benzene rings, as shown in Figure 11.
* Regarding the distinction between synthons as well as synthetic equivalents.
The synthons and the synthetic equivalents belong to the branch of retrosynthetic analysis.
|
Figure 11. Synthetic equivalent of acetophenone. |
A synthon is a part of a compound that can be formed by a known synthetic process. By definition, a synthon is a compound produced during a retrosynthesis reaction, and a synthetic equivalent is a chemical reagent that can be used in place of a synthon. In terms of role, the synthetics are part of the substrate molecule, its own structure will be changed to re-form into the target molecule, and the synthetic equivalent is required to react with it to produce the target molecule.[7]
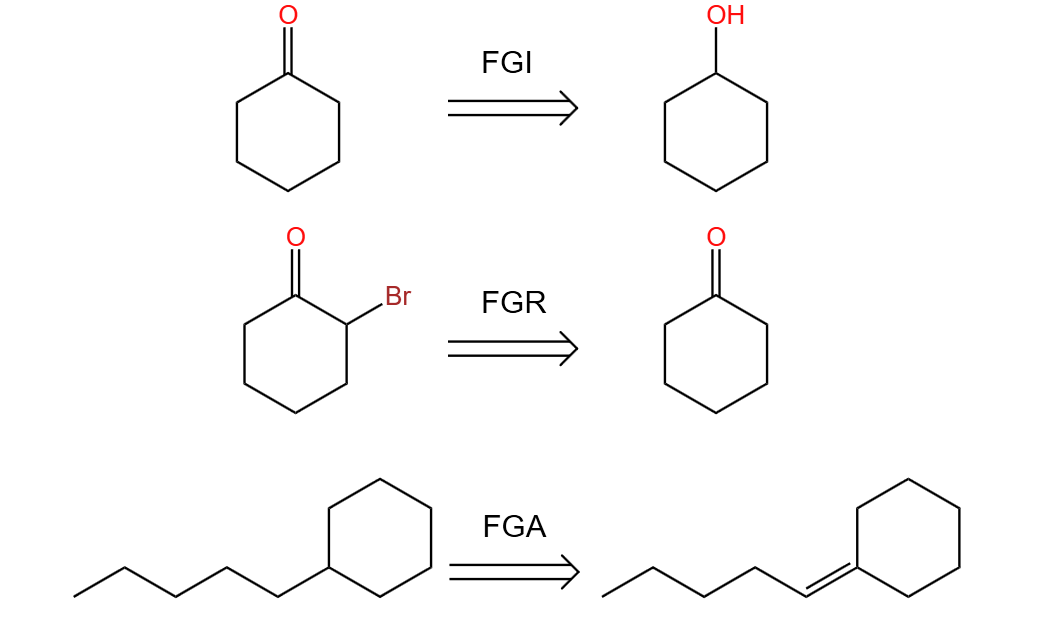
2.1.6. Fine tuning. In the retrosynthesis reactions, the conversion of one functional group to another by substitution, addition, elimination, oxidation or reduction usually occurs. This way called fine tuning. Small changes are made to the original compound. Usually there are three ways of fine tuning: FGI(Functional Group Interconversion) FGR(Functional Group Remove) [8]FGA(Functional Group Addition). Like figure 12, it shows sample tunings.
|
Figure 12. Fine tuning. |
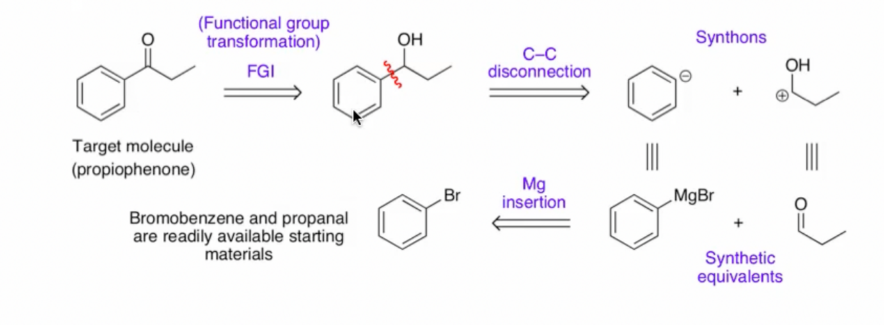
Sometimes it is useful to remove functional groups from a molecule, for example, decarbonylation is a way to remove the formyl group from a steroid molecule. Although there are no large-scale commercial applications for this type of reaction, a number of laboratory-scale syntheses have done it.[9] In the example below, we will try to isolate ketones from the propiophenone in the containing alcoholic compound, which eventually forms brominated benzene.
|
Figure 13. FGI. |
By the process of figure 13. The first step in the reaction is Function group interconversion, we can see that the ketone is replaced with a hydroxyl group (alcohol), after that is the disconnection of Carbon-Carbon single bond. A negatively charged phenyl group and a positively charged ethoxymethyldecyl group will be formed (obtained by the loss of one electron from n-propanol) The next step is to find suitable synthetic equivalents and finally insert magnesium to form brominated benzene and propionaldehyde. But at this point we focus our attention only on the first step, where the ketone is replaced with an alcohol, which is “FGI”.
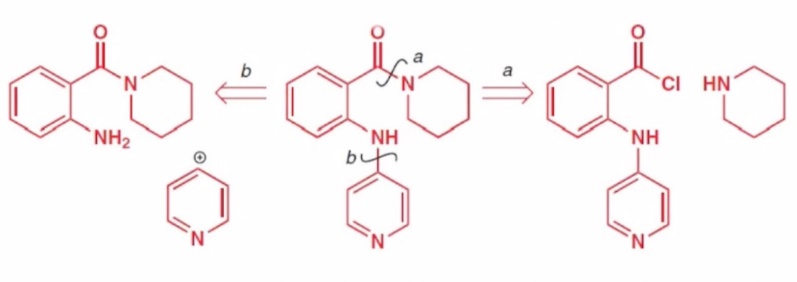
We can see in the chemical structure formula of Ofornine in figure 14, there are two types of disconnection, when we recall the guidelines for the priority phase of the active group.
|
Figure 14. FGI of Ofornine. |
As described in the first "disconnection" article. We can deduce that the more reliable is route a, first cutting off the amide. The amine produced by route B continues to exist, and the functional group remains attached to it.
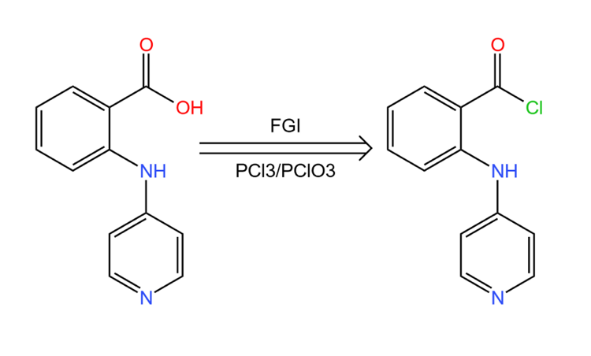
But in general, route a is only more reliable compared to route b. Therefore route a also has some problems we can see the presence of chloride in its raw material, so we need to consider the effect of chloride activation at every step, which will be very troublesome. To solve this problem, like Figure 15, just like finding synthetic equivalents for synthons, we will displace it and turn it into carboxylic acids.
|
Figure 15. Step of FGI. |
The reaction between the two relies only on phosphorus trichloride or phosphorus trichloride reagents. This is a very common as well as the easiest functional group interconversion to achieve.
2.1.7. Function group protection. In synthetic or retrosynthetic reactions, a compound will usually include more than two groups containing different chemical properties, sometimes we only need to react on one of the groups, so there is step of protecting the group. Used in synthesis/Retrosynthesis to temporarily mask the characteristic chemistry of a functional group. Because it interferes with another reaction. A good protection set should be easy to put on and easy to remove. In high-yield reactions, the conditions required for the reaction are inert. We identified the response in a study from the University of Calgary, as shown in Figure 16 below.[10]
|
Figure 16. Reaction way. |
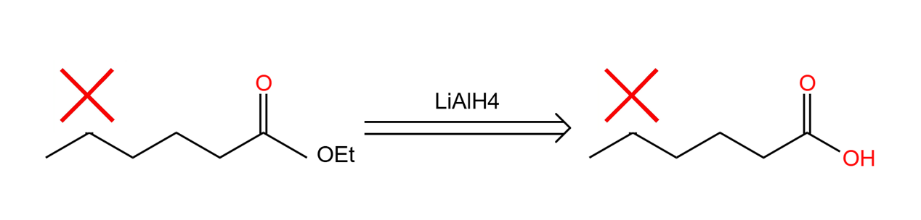
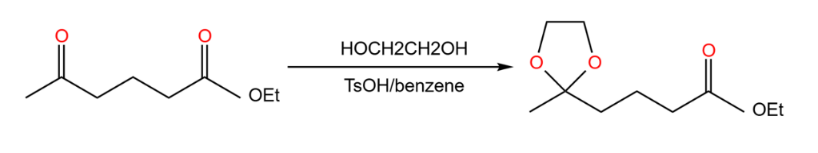
Assuming that the whole conversion process is a reduction reaction of the ester to the primary alcohol, so the agent is LiAlH4, one of the problems faced at this point is that LiAlH4 will also react with the ketone in a reduction reaction. If we want to avoid this condition, you need to change the ketone to a different functional group. Shield the ketone in the reaction as shown in Figure 17.
|
Figure 17. Cover one functional group. |
By this way, the two red forks are "molecular overlays". That means the protection of functional groups. To keep it from reacting with LiAlH4, we turn it into acetal (An ether) Like the Figure 18.
|
Figure 18. Change the functional group. |
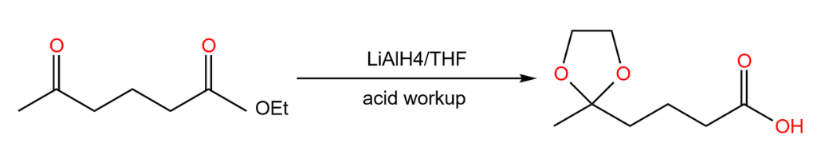
Next, let the ester alone undergo the reduction reaction, as shown in Figure 19.
|
Figure 19. Start to react. |
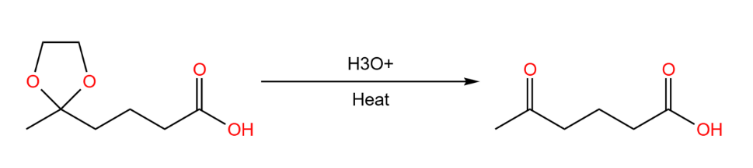
After completing the reduction reaction, we can just remove the condensed alcohol and change back to ketone. As shown in Figure 20.
|
Figure 20. Recover the used functional group. |
In the above reaction, we need to step the reduction reaction of esters, instead of ketones, but the process of ketones will also participate in the reaction, therefore replacing it with acetal. After the reaction is completed, just replace with ketone. It looks like the FGI, but the difference is that functional group protection does not change the substances participating in the reaction, just try to avoid the chemical selectivity. Functional group interchanges, on the other hand, require changes in the target molecule, and both of them play different roles in the synthetic route.
2.2. Reaction selectivity
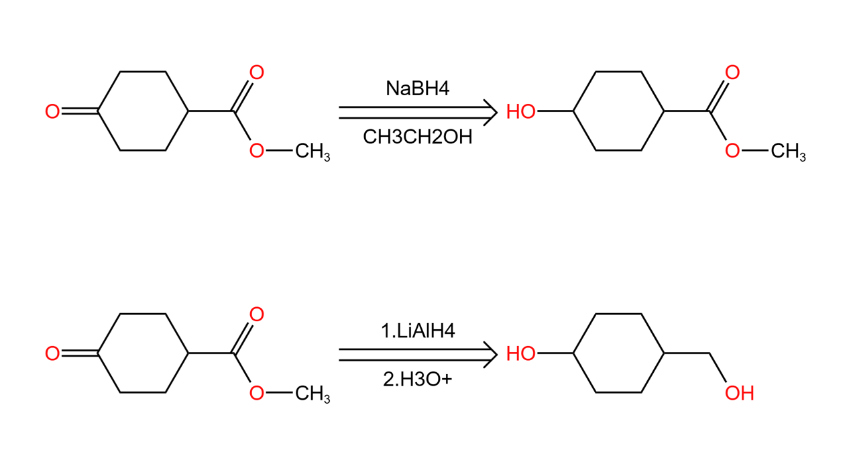
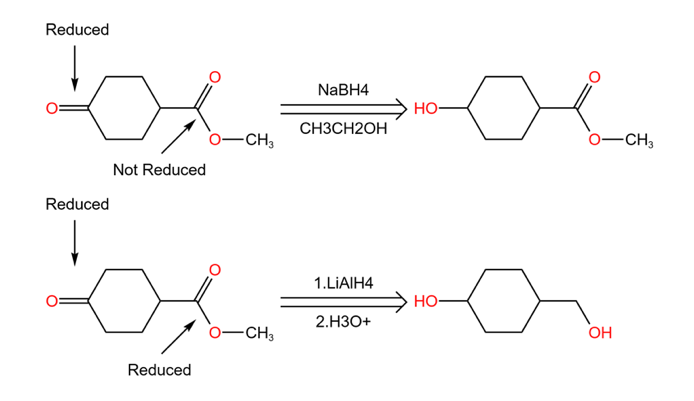
2.2.1. Chemical selectivity (Chemoselectivity)[11]. We have mentioned the chemical selectivity in the section of “Disconnection” even functional group protection. So actually, it is refers to the ability of a reagent or intermediate to react with one group or atom in a molecule rather than another group or atom present in the same molecule. Of course, one of the functional groups reacts in preference to the other one or more functional groups. In Figure 21, as in the two chemical reactions of methyl 1,4-cyclohexanone carboxylate;
|
Figure 21. Chemoselectivity. |
We focus on the ketone to the left of the cyclohexane while both reactions reduce it, the only difference is that the ester on the right side of the compound does not undergo any change when NaBH4 is the reagent, but when the reagent was LiAlH4, the ester was also reduced (as in Figure 22).
|
Figure 22. Chemical selectivity. |
In this case, according to the definition of chemo selectivity, a reaction that acts on only one functional group in the presence of other functional groups. Therefore it is chemo selective when the reagent is NaBH4. Because the ester is retained. the reaction of LiAlH4 doesn’t have chemo selective because both functional groups ketone, ester are reduced.
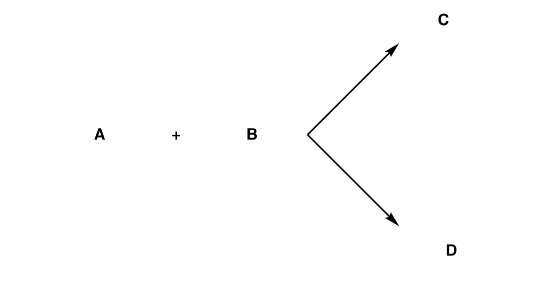
2.2.2. Stereoselectivity. Stereoselectivity, if more than one reaction may occur between a group of reactants under the same conditions, producing stereoisomeric products, and if one product is formed in greater amounts than the others, the whole reaction is said to be stereoselective. [12]Suppose two reactions are possible between the hypothetical reactants A and B under the same conditions to give stereoisomeric products C and D (Figure 23)
|
Figure 23. The condition of products. |
Two cases occur at this point.
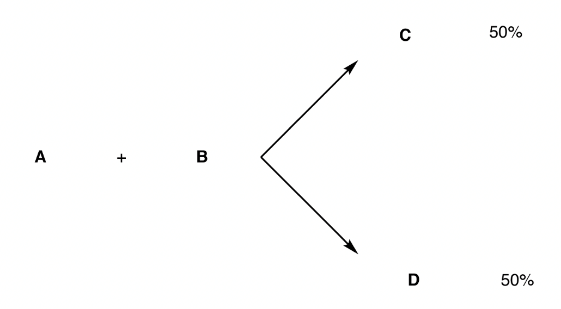
Case 1; the relative yields of the two products are 50% (i.e. half.), Figure 24
|
Figure 24. Same yield of products. |
When the yields are each 50%, it is not stereoselective, the overall reaction between A and B is not stereoselective.
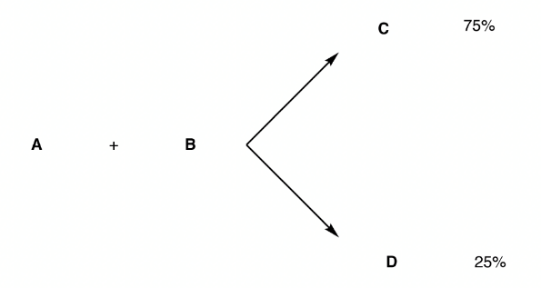
Case 2: The amount of one product formed is greater than the other. For example, C is 75% and D is 25%. When the products are in different proportions, it is consistent with stereoselectivity, Figure 25
|
Figure 25. Different yield of products. |
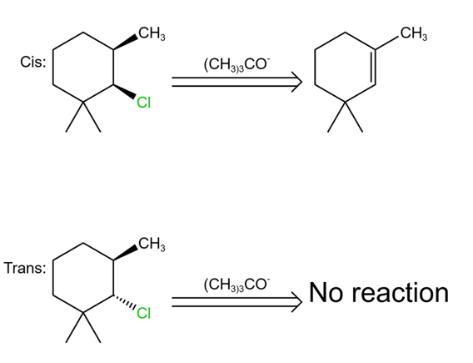
Of course, we can also determine stereoselectivity from the cis as well as trans stereoisomers. As shown in Figures 26.
|
Figure 26. cis/trans steroismers. |
We can see that in such an elimination reaction, only the cis diastereoisomer reacts so it consistent with the definition of stereoselectivity.
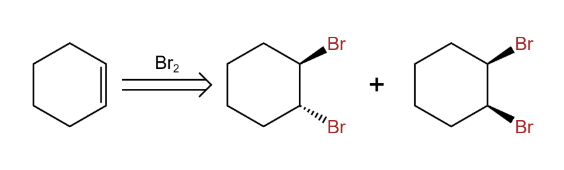
In addition to the reactant being cis or trans, it can also be viewed from the perspective of the product, as in Figure 27 an example of adding molecular bromine to cyclohexene.
|
Figure 27. cis/tran. |
We can clearly see that only the trans dibromide is formed and the cis 1,2-dibromide cyclohexane is not formed. Therefore this is also consistent with stereoselectivity.[13]
2.2.3. Regioselectivity. Regioselectivity refers to reactions formed by the selection of an enantiomer, a diastereomer or a double bond isomer over other isomers. Any process that facilitates the formation of bonds on specific atoms rather than on other possible atoms.
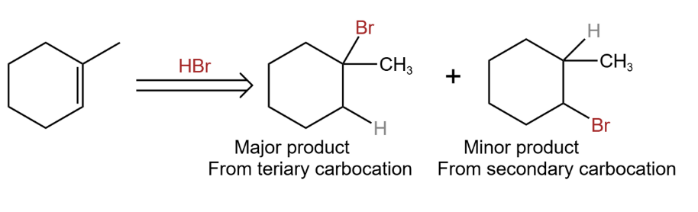
In the example of the addition of hydrogen bromide with 1-methylcyclohexene as in Figure 28.
|
Figure 28. Regioselectivity. |
Because it facilitates the formation of bonds between the olefin tertiary carbon and the bromine atom, rather than between the olefin secondary carbon and the bromine atom, thus it is consistent with the concept of regioselectivity, which is of course one of the underlying regioselectivities of Markovnikov's rule.
3. Mechanism of reaction
3.1. Aldol condensation
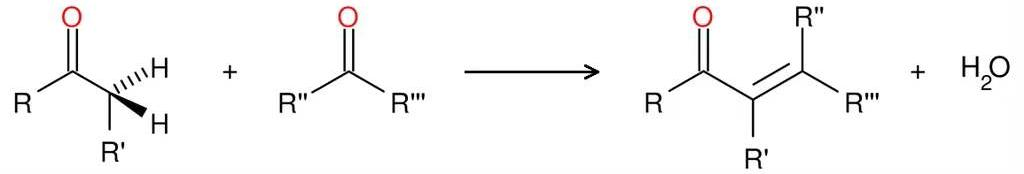
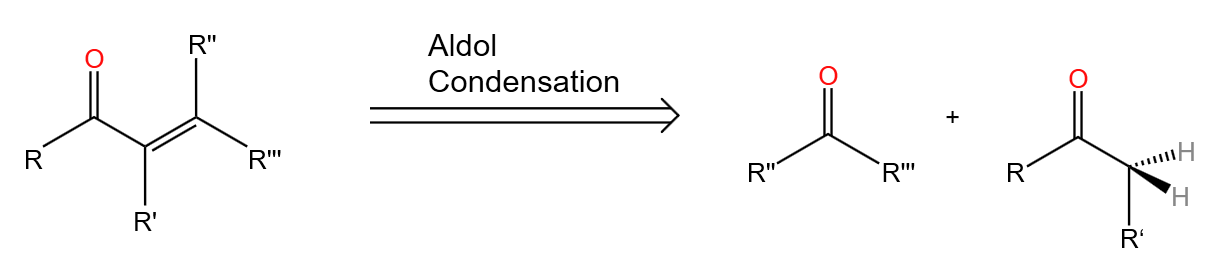
3.1.1. Acid (Base)-catalyzed condensation. In Aldol Condensation reaction enol or enol ion reacts with carbonyl compound to form β-hydroxy aldehyde or β-hydroxy ketone.[14] And the product can sometimes lose a water molecule to form α,β-unsaturated carbonyl compounds.(Figure 29)
|
Figure 29. Aldol condensation. |
In the retrosynthesis analysis, aldol condensation can be written in the form of the following diagram, double arrows is used to indicate the retrosynthesis.
|
Figure 30. Aldol condensation in retrosynthesis. |
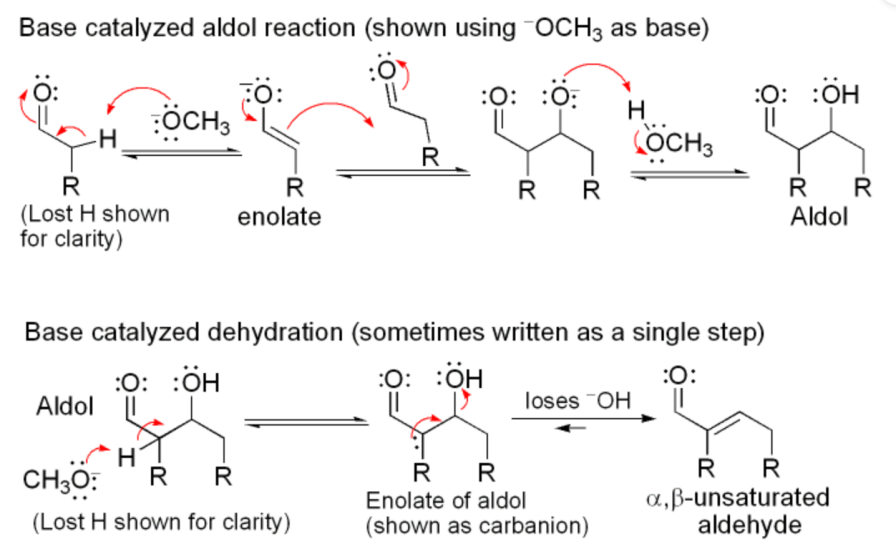
In Aldol Condensation, the condensation is divided into alkali-catalyzed condensation and acid-catalyzed condensation. Like Figure 31.
|
Figure 31. Base catalyzed aldol reaction. |
In the above diagram the reaction is divided into two parts: aldol reaction (aldehyde+alcohol) and dehydration or elimination reaction. In the first part, methoxy deprotonates the aldehyde to produce enolate ion, which is added to the unreacted aldehyde to react with it, then it reacts with methanol to form aldol. In the second part, the lone pair of electrons on the methoxy attack aldol to form enolate of aldol, followed by the loss of the hydroxide ion from enolate of aldol to form α,β-unsaturated aldehydes and water.
|
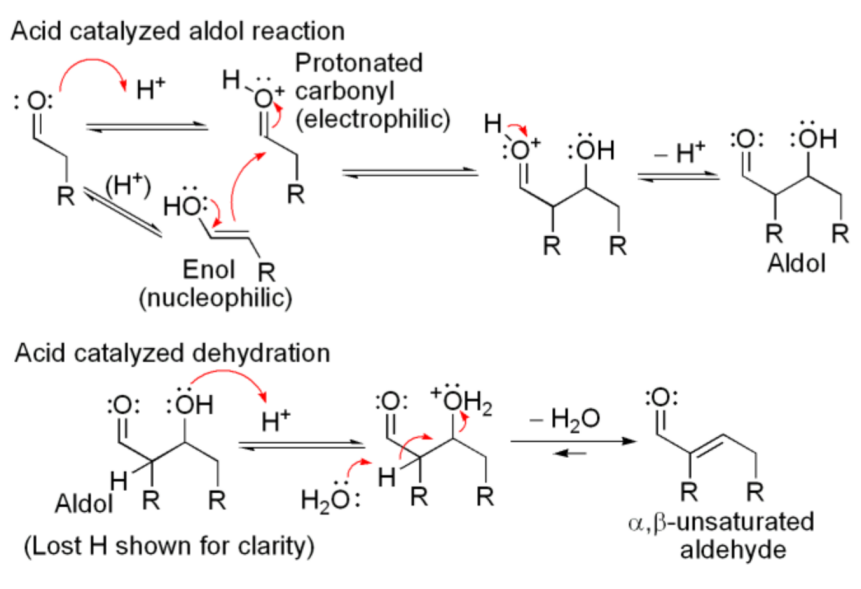
Figure 32. Acid catalyzed aldol reaction. |
The Figure above is an example of an acid-catalyzed condensation (Figure 32). In the acid-catalyzed aldol reaction, acid activates carbonyl carbon by donating a proton while the conjugate base reacts with it to form enol. As shown in the Figure, protonated carbonyl reacts with enol as an electrophilic reagent to produce aldol after the removal of a hydrogen ion. aldol undergoes a dehydration reaction in the second part to form α,β-unsaturated aldehydes.
3.1.2. Intramolecular aldol condensation. The term "intramolecular" refers to "within the same molecule". In this case, the nucleophilic and electrophilic reagents appear within the same molecule, as shown in the Figure below. (Figure 33). The reaction mechanism is identical to aldol condensation, the only difference is that the two ketones are joined to form a ring and the final product is a five-membered ring.
|
Figure 33. Inside the molecular. |
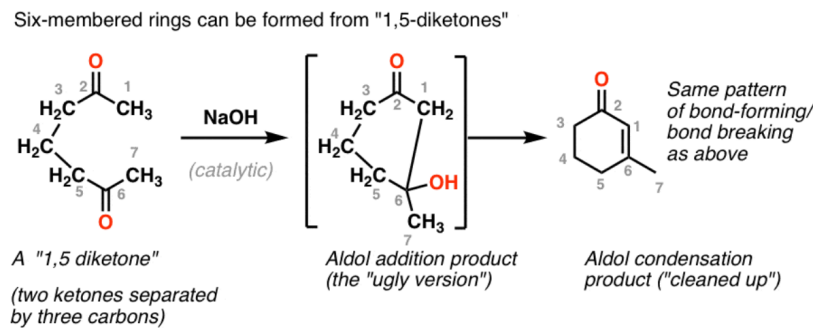
We all know that ternary and quaternary rings are relatively unstable because they are more susceptible to ring strain, while heptad rings and higher rings are relatively slow to form. Considering the low ring strain and reasonable reaction rates, the best options for ring closure tend to be five- and six-membered rings.
|
Figure 34. Five-/Six-mermbered rings. |
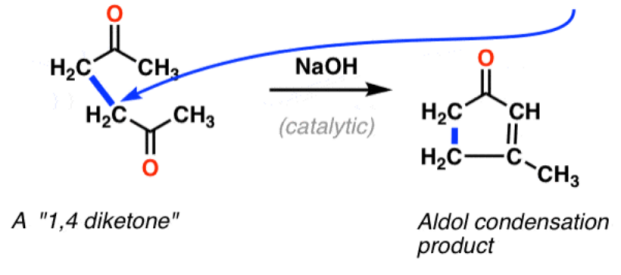
The above Figure shows an example (Figure 34) of the formation of a six-membered ring by an intramolecular reaction of 1,5 diketones with the same mechanism as aldol condensation.
3.2. Michael addition
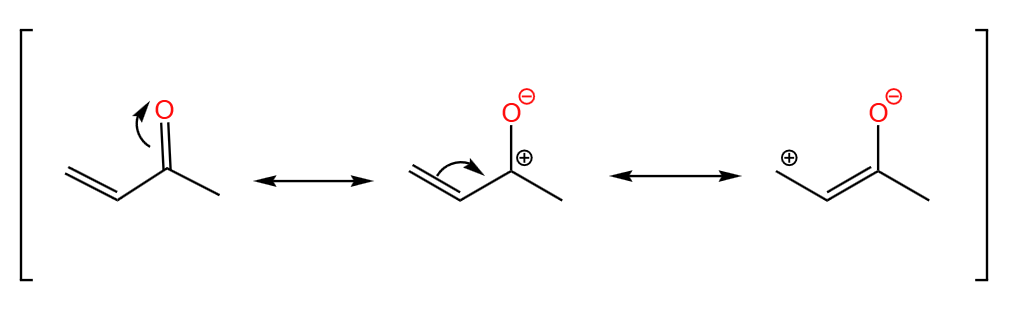
Michael addition is also known as 1,4-addition or conjugate addition. [15]This reaction was first discovered by Arthur Michael in 1887 and is one of the common methods for growing carbon chains in organic synthesis. As shown in the Figure 35, we can see from the resonance structure of the α,β-unsaturated carbonyl compound that it has two electrophilic positions, that is, it carries a partial positive charge at its positions 2 and 4. The nucleophilic reagent is usually used as a Michael donor to attack the position 4 of α,β-unsaturated carbonyl compound in Michael addition, and the 1,4 addition reaction is carried out to it under basic conditions.
|
Figure 35. Michael addition. |
3.2.1. Reaction mechanisms. Figure 36 shows an example from Michael Addition, from which we will develop the reaction mechanism about this reaction.
|
Figure 36. Michael addition. |
The Michael addition reaction consists of three steps, all of which are carried out under basic conditions.
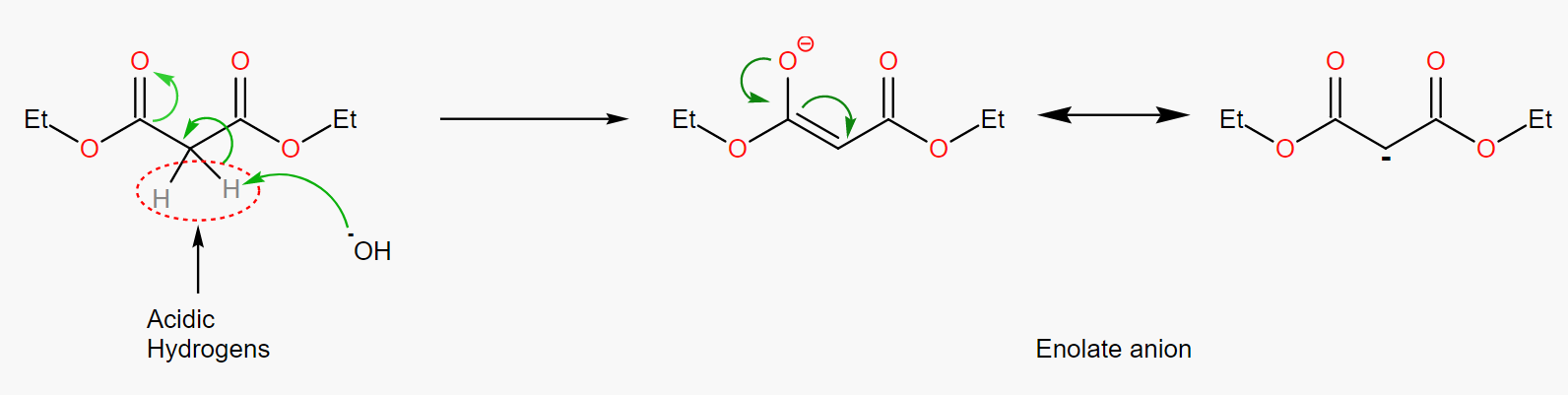
Step 1. Deprotonation:
Hydroxide ions in alkaline solutions attack acidic hydrogens, giving rise to carbon negative ions also known as Enolate anion in the Figure. enolate anion in the Figure 37, has a resonance structure to maintain stability.
|
Figure 37. Resonance structure to maintain stability. |
Step 2. Attack of nucleophile
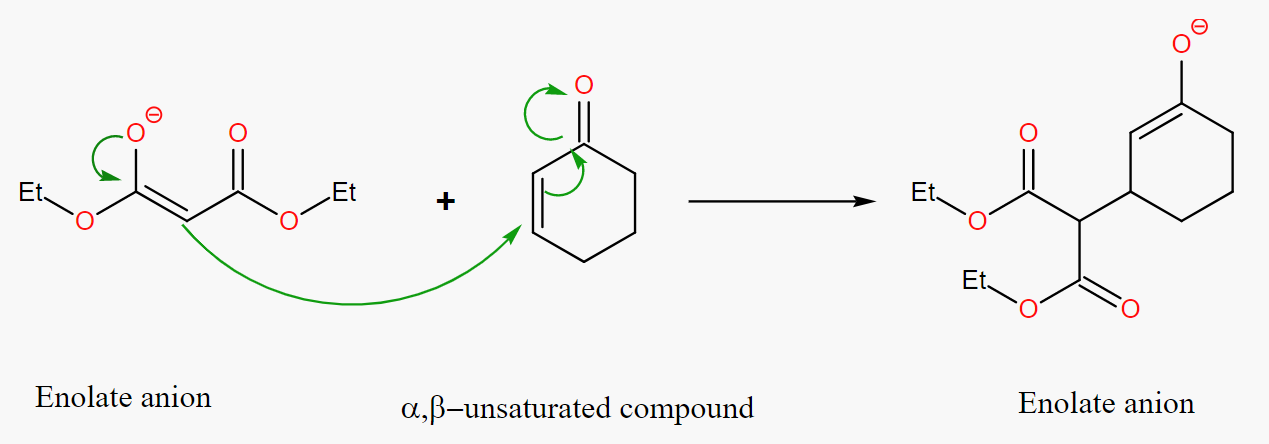
In the second step shown in Figure 38, the carbon anion acts as a Michael donor, which is electron-rich, providing electrons to the Michael acceptor, which is electron-deficient. in the Figure below, the enolate anion obtained from the first step acts as a Michael donor, attacking the α,β-unsaturated carbonyl compound of the β- carbon, resulting in the formation of enolate anion.
|
Figure 38. Enolate anion. |
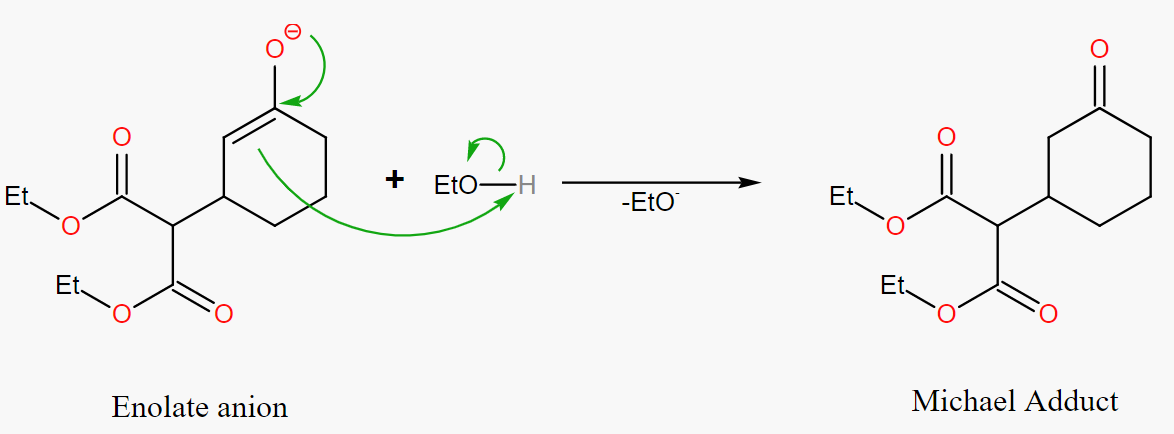
Step 3. Protonation
In Figure 39, the carbonyl compound is protonated in the third step by accepting electrons from the solvent or Michael donor and forming the final product called "Michael Adduct".
|
Figure 39. Michael Adduct. |
3.2.2. Michael addition reaction and retrosynthesis.
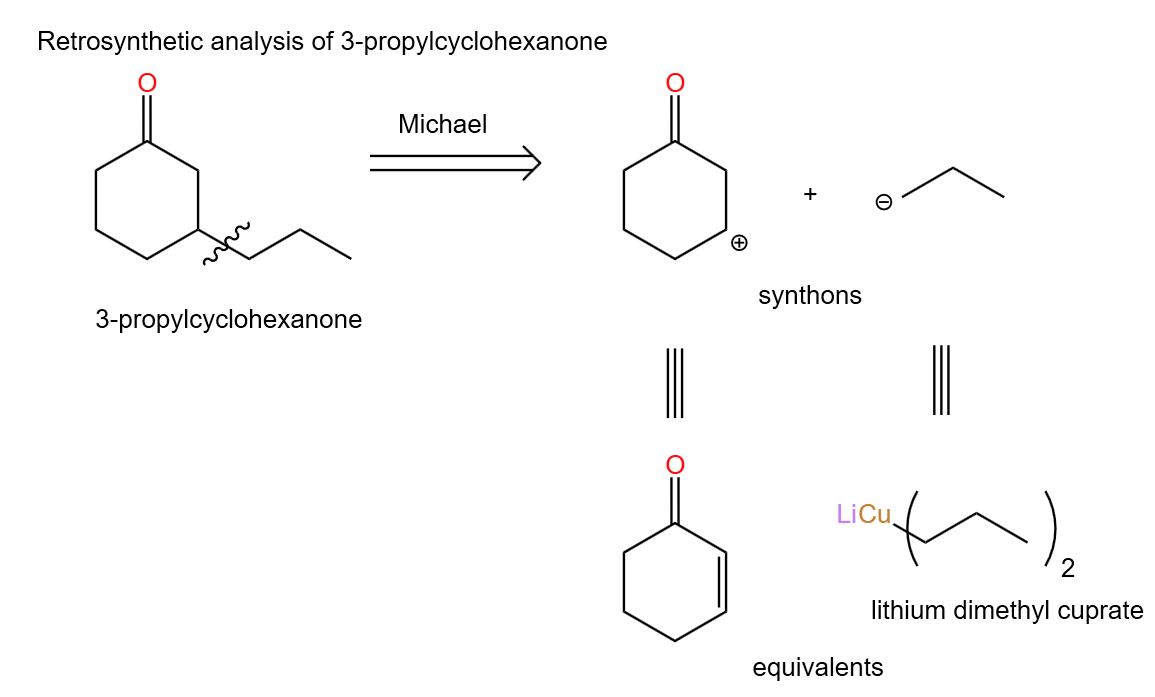
(1). Analysis of retrosynthesis of 3-propylcyclohexanone:
The following Figure shows a simple analysis about the retrosynthesis of 3-propylcyclohexanone. Based on what we have learned about Michael addition reactions, we can follow the bond-breaking method. In the Figure 40, we can introduce two synthetic substrates and obtain their corresponding synthetic equivalents.
|
Figure 40. Retrosynthetic analysis of 3-propylcyclohexanone. |
Among them, lithium dimethyl cuprate also known as Gilman Reagents can be prepared by organolithium compounds. And the two equivalent compounds obtained from the retrosynthesis analysis can be used as reactants to obtain the target molecule which is 3-propylcyclohexanone by forward synthesis.
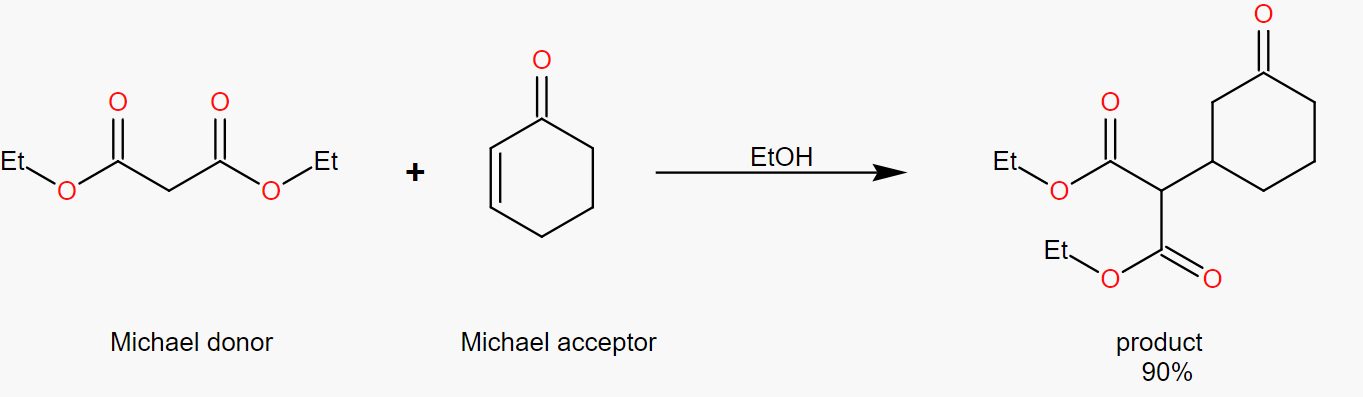
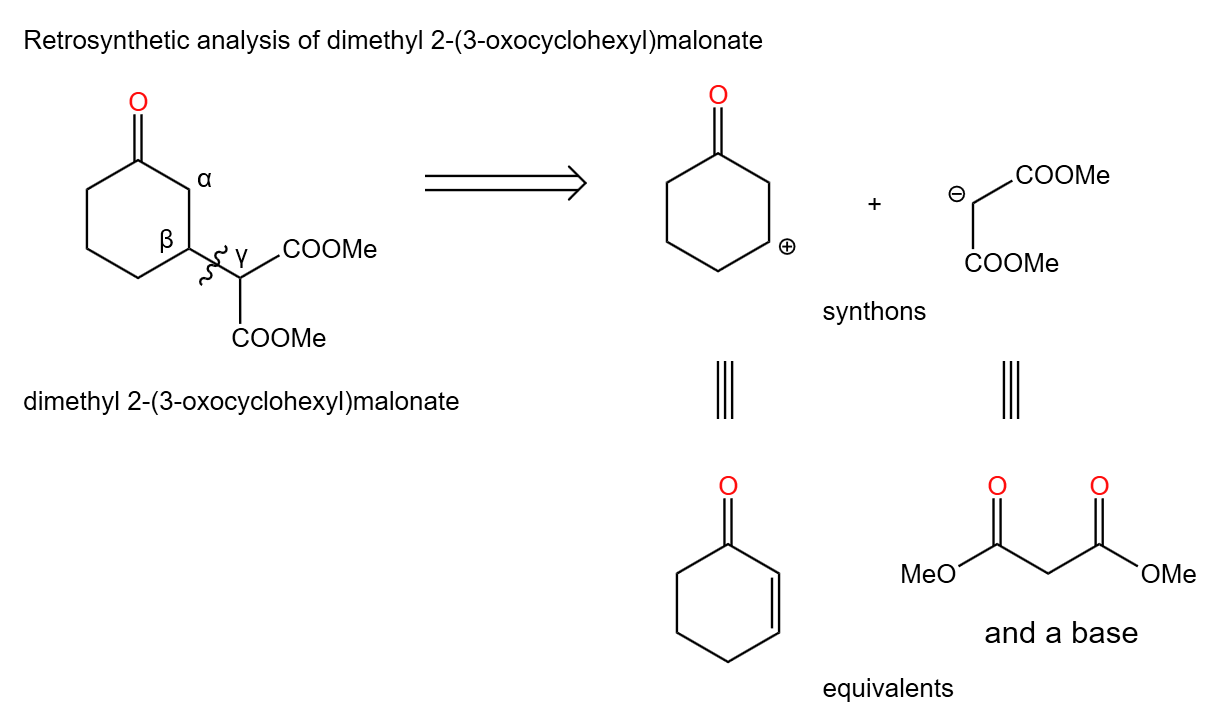
(2). Analysis of the retrosynthesis of dimethyl 2-(3-oxocyclohexyl) malonate
In the Figure 41, dimethyl 2-(3-oxocyclohexyl) malonate is labeled with pairs of α,β and γ carbons. In the Michael addition reaction we know that the reactants in the forward reaction contain α,β-unsaturated carbonyl compounds, so in the retrosynthesis we should choose to break the carbon-carbon bond between the β and γ carbons.
|
Figure 41. Dimethyl 2-(3-oxocyclohexyl) malonate in retrosynthesis. |
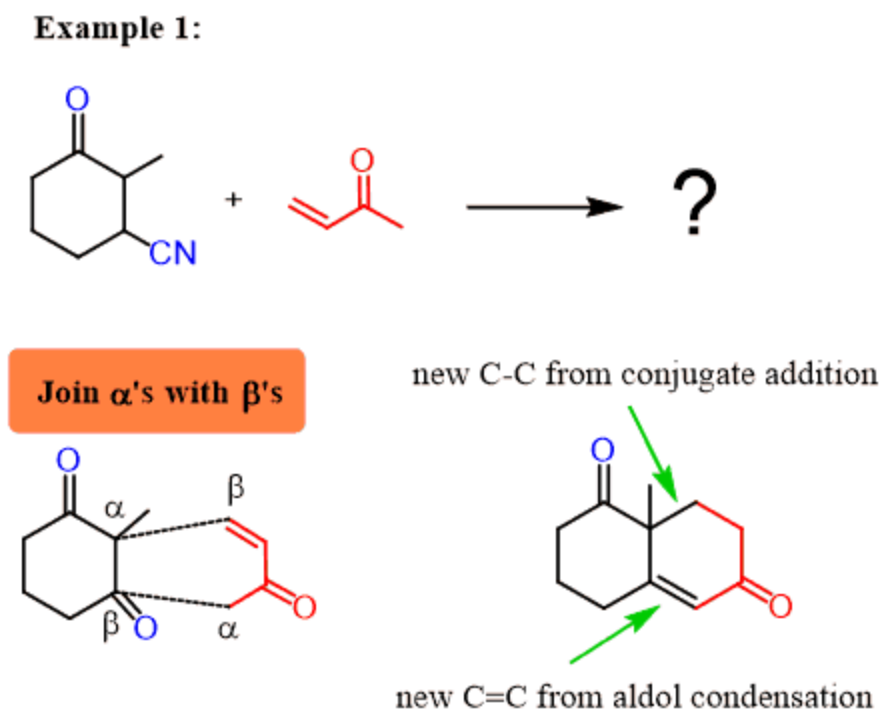
3.3. The Robinson Annulation
3.3.1. Introduction[16]. Michael addition reactions combined with intramolecular hydroxyl aldol condensation reactions to construct rings are called Robinson cyclization reactions. When we are predicting the product of this reaction, we can simply place the β-carbon of the Michael acceptor next to the ɑ-position of the Michael donor and place the ɑ-carbon of the Michael acceptor next to the β-position of the Michael donor, as shown below. Then a new bond is formed between the corresponding ɑ-carbon β-carbon. (Figure 42)
|
Figure 42. ɑ-carbon β-carbon. |
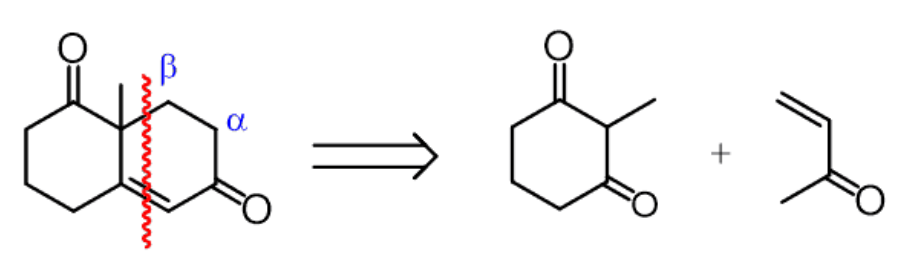
3.3.2. Robinson Annulation Retrosynthesis. However, after studying the Michael addition and intramolecular hydroxyl aldol condensation reactions and understanding the Robinson cyclization reaction, we can try to think in the reverse direction. (i.e., to analyze the retrosynthesis.)[17]
|
Figure 43. In retrosynthesis. |
The Figure 43 shows the results of the retrosynthesis analysis of the target molecule, and then we will add a more complete retrosynthesis pathway for this target molecule.
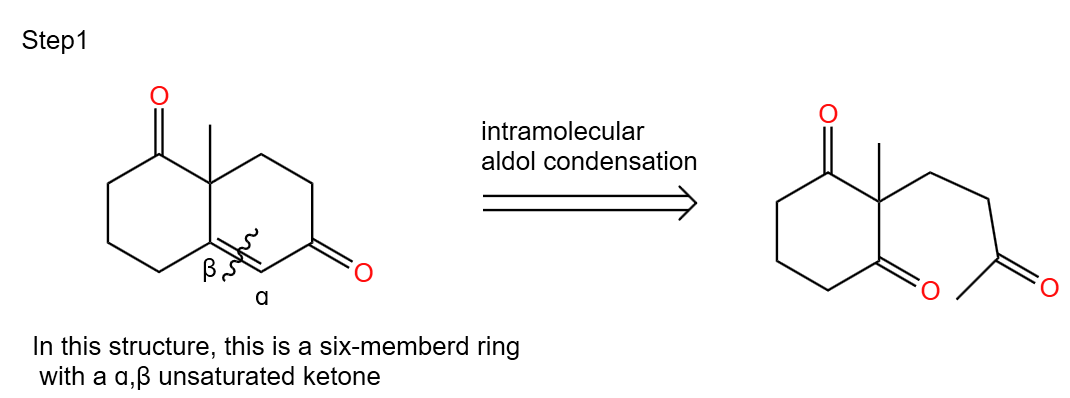
Step 1. work backwards from intramolecular aldol condensation (Figure 44)
|
Figure 44. Step 1. |
In the Robinsonian cyclization reaction, the first step is the Michael addition reaction and the second step is the intramolecular hydroxyl aldol condensation reaction. Therefore, the first step in our retrosynthetic analysis should be to explore its intramolecular hydroxyl-aldol condensation reaction.
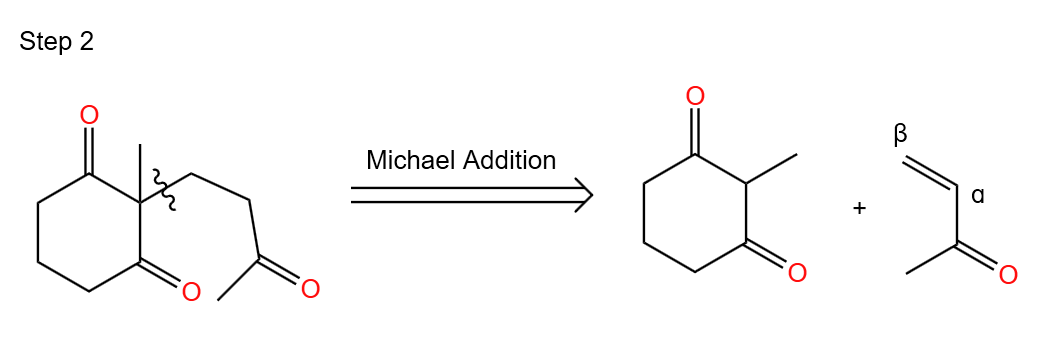
Step 2. Working backwards from Michael Addition
In the second step, we can obtain the results of the retrosynthesis based on our knowledge of the Michael addition reaction. (i.e., inferring the starting materials of the target molecule.) As shown in Figure 45.
|
Figure 45. Step 2. |
Based on the analysis of the target molecule in the two Figures above, we can conclude that a ketone and an α,β-unsaturated ketone can be obtained from the target molecule by the Robinsonization ring reaction (Michael addition as well as intramolecular hydroxyl aldol condensation reaction).
4. Conclusion
After the introduction of the basic concepts of retrosynthesis, the reaction mechanism and the reaction terms, we can already grasp the basic application of retrosynthesis, but this, like other contents, requires a lot of experience to apply it to organic chemistry or even to a wider field. Therefore, it is not enough to focus only on the content of the article, but we still need to apply it to the real world to gain experience.
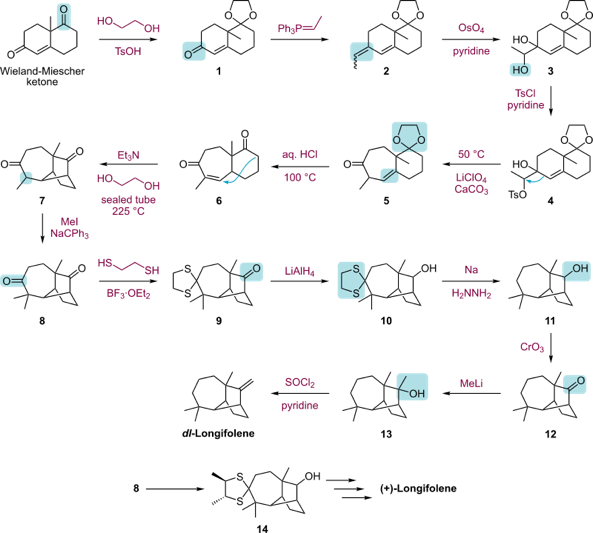
Arguably the most important thing needed for retrosynthesis is experience and how to use what you have learned to innovate. Of course, the reaction mechanisms are not only the three mentioned in our article, and the reaction selectivity is not only those we have mentioned. For example, in the case of very complex substances such as Longifolen, Figure 46, we usually need to solve it across disciplines, just as it can be synthesized by multiple routes, even including biosynthesis. And the final verification that the method works is confirmed using modern quantum mechanical calculations.
|
Figure 46. Longifolen. |
References
[1]. Complex Organic Molecules(Authors and Affiliations Yokohama National University, 240-8501, Tokiwadai, Hodogaya-ku Yokohama, Japan K. Kobayashihttps://link.springer.com/referenceworkentry/10.1007/978-3-642-11274-4_337
[2]. AiZynthFinder:a fast, robust and flexible open-source software for retrosynthetic planning https://jcheminf-biomedcentral-com.translate.goog/articles/10.1186/s13321-020-00472-1?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=sc
[3]. A Simple Approach to Retrosynthesis in Organic Chemistry https://leah4sci-com.translate.goog/organic-chemistryretrosynthesis/?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=sc
[4]. disconnection:retrosynthetic analysis (https://youtu.be/Jr9OYlE8q9g) equivalent/?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[5]. Synthesis of Target Molecules: Introduction to Retrosynthetic Analysishttps://kpu pressbooks-pub.translate.goog/organicchemistry/chapter/9-6-synthesis-of-target-molecules-introduction-of-retrosynthetic-analysis/?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=sc
[6]. Synthon and synthetic equivalent: Definition, easy examples https://chemistnotes-com.translate.goog/organic/synthon-and-synthetic
[7]. Difference Between Synthon and Synthetic Equivalent https://www-differencebetween-com.translate.goog/difference-between-synthon-and synthetic-equivalent/?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[8]. FGR:https://link-springer-com.translate.goog/chapter/10.1007/978-94-009-5267-6_6?error=cookies_not_supported&code=8c8fca34-51fd-4623-9040-94af8107d67e&_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=sc
[9]. Functional Group Removal https://link-springer-com.translate.goog/chapter/10.1007/978-94-009-5267-6_6?error=cookies_not_supported&error=cookies_not_supported&code=8c8fca34-51fd-4623-9040-94af8107d67e&code=1b257d5a-e63c-4b39-877a-d781f8c4bb8e&_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[10]. protecting Groups https://www.chem.ucalgary.ca/courses/351/Carey5th/Ch17/ch17-3-4-3.html
[11]. Chemoselectivity; https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Radical_Reactions_of_Carbohydrates_(Binkley)/I%3A_Structure_and_Reactivity_of_Carbohydrate__Radicals/09%3A_Chemoselectivity
[12]. Illustrated Glossary of Organic Chemistry https://www-chem-ucla-edu.translate.goog/~harding/IGOC/D/diastereoselective.html?_x_tr_sch=http&_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[13]. Stereoselective:https://chem-libretexts-org.translate.goog/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Stereoselective?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[14]. Organic Reactions Wiki. (2020, September 13). Aldol condensation – chemistry libretexts. Chemistry LibreTexts. Retrieved September 10, 2022, from https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Organic_Reactions/Aldol_Condensation
[15]. Akhter, J. (2022, September 2). Michael addition reaction or 1,4-conjugate addition -psiberg. PSIBERG. Retrieved September 10, 2022, from https://psiberg.com/michael-addition/
[16]. Ashenhurst, J. (2022, August 17). The Robinson annulation – master organic chemistry. Master Organic Chemistry. Retrieved September 10, 2022, from https://www.masterorganicchemistry.com/2018/12/10/the-robinson-annulation/
[17]. Gevorg, D. S. (2020, August 16). Robinson annulation-mechanism and Shortcut. Chemistry Steps. Retrieved September 11, 2022, from https://www.chemistrysteps.com/robinson-annulation-shortcut-and-retrosynthesis/
Cite this article
Liu,L.;Zheng,J. (2023). Retrosynthesis-Introduction to the Analysis and Mechanism. Applied and Computational Engineering,3,143-160.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023)
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Complex Organic Molecules(Authors and Affiliations Yokohama National University, 240-8501, Tokiwadai, Hodogaya-ku Yokohama, Japan K. Kobayashihttps://link.springer.com/referenceworkentry/10.1007/978-3-642-11274-4_337
[2]. AiZynthFinder:a fast, robust and flexible open-source software for retrosynthetic planning https://jcheminf-biomedcentral-com.translate.goog/articles/10.1186/s13321-020-00472-1?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=sc
[3]. A Simple Approach to Retrosynthesis in Organic Chemistry https://leah4sci-com.translate.goog/organic-chemistryretrosynthesis/?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=sc
[4]. disconnection:retrosynthetic analysis (https://youtu.be/Jr9OYlE8q9g) equivalent/?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[5]. Synthesis of Target Molecules: Introduction to Retrosynthetic Analysishttps://kpu pressbooks-pub.translate.goog/organicchemistry/chapter/9-6-synthesis-of-target-molecules-introduction-of-retrosynthetic-analysis/?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=sc
[6]. Synthon and synthetic equivalent: Definition, easy examples https://chemistnotes-com.translate.goog/organic/synthon-and-synthetic
[7]. Difference Between Synthon and Synthetic Equivalent https://www-differencebetween-com.translate.goog/difference-between-synthon-and synthetic-equivalent/?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[8]. FGR:https://link-springer-com.translate.goog/chapter/10.1007/978-94-009-5267-6_6?error=cookies_not_supported&code=8c8fca34-51fd-4623-9040-94af8107d67e&_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=sc
[9]. Functional Group Removal https://link-springer-com.translate.goog/chapter/10.1007/978-94-009-5267-6_6?error=cookies_not_supported&error=cookies_not_supported&code=8c8fca34-51fd-4623-9040-94af8107d67e&code=1b257d5a-e63c-4b39-877a-d781f8c4bb8e&_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[10]. protecting Groups https://www.chem.ucalgary.ca/courses/351/Carey5th/Ch17/ch17-3-4-3.html
[11]. Chemoselectivity; https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Radical_Reactions_of_Carbohydrates_(Binkley)/I%3A_Structure_and_Reactivity_of_Carbohydrate__Radicals/09%3A_Chemoselectivity
[12]. Illustrated Glossary of Organic Chemistry https://www-chem-ucla-edu.translate.goog/~harding/IGOC/D/diastereoselective.html?_x_tr_sch=http&_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[13]. Stereoselective:https://chem-libretexts-org.translate.goog/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Stereoselective?_x_tr_sl=en&_x_tr_tl=zh-CN&_x_tr_hl=zh-CN&_x_tr_pto=op,sc
[14]. Organic Reactions Wiki. (2020, September 13). Aldol condensation – chemistry libretexts. Chemistry LibreTexts. Retrieved September 10, 2022, from https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Reactions/Organic_Reactions/Aldol_Condensation
[15]. Akhter, J. (2022, September 2). Michael addition reaction or 1,4-conjugate addition -psiberg. PSIBERG. Retrieved September 10, 2022, from https://psiberg.com/michael-addition/
[16]. Ashenhurst, J. (2022, August 17). The Robinson annulation – master organic chemistry. Master Organic Chemistry. Retrieved September 10, 2022, from https://www.masterorganicchemistry.com/2018/12/10/the-robinson-annulation/
[17]. Gevorg, D. S. (2020, August 16). Robinson annulation-mechanism and Shortcut. Chemistry Steps. Retrieved September 11, 2022, from https://www.chemistrysteps.com/robinson-annulation-shortcut-and-retrosynthesis/