1. Introduction
Nanotechnology is the technology that can research nano-meter-sized substances. Nano-level particles which are usually ranging from 1 to 100 nanometers play a significant role in modern medicine such as improvement in drug delivery, enhanced diagnostics, cancer therapy, vaccines, and immunotherapy, and so on [1]. Silver nanoparticles (AgNPs) are one of the important nano-substances that have fantastic antibacterial properties. Its antibacterial properties make it valuable in healthcare applications like antimicrobial agents, coatings for medical devices, and wound dressings. Also, it has common properties with silver, such as thermal conductivity, high electrical conductivity, and biological properties.
Considering the historical development of silver, the ancient Phoenicians, Greeks, Romans, and Egyptians used it to preserve food and water due to its antibacterial effects until World War II ended. Hippocrates described the use of silver for wound healing around 400 BC. In medieval medicine, silver nitrate was used as a cauterizing agent to prevent infection. Also, surgeons used silver sutures for wound closure to lower infection rates in the 19th century. In the late 19th and early 20th centuries, the antimicrobial properties of silver were formally recognized. However, scientists began exploring silver at the nanoscale after the advent of nanotechnology in the late 20th century [2]. They also discovered that the nanoscale size of silver particles greatly enhanced the antimicrobial efficacy of silver. By the 2000s, silver nanoparticles were incorporated into wound dressings, antimicrobial sprays, and coatings for medical devices [3].
Both the physical and chemical properties of AgNPs are strongly related to their size, shape, surface area, and environment. According to different degrees of effects on them, they can be widely applied in such as electrical, optical, and medical manufacturing. Due to AgNPs' strong antimicrobial properties, they play an important role in facing a wide range of pathogens, including bacteria, fungi, and viruses effectively. Therefore, they can used in combating multi-drug-resistant bacteria, wound dressings, coatings for medical devices, drug delivery, cancer therapy, imaging, and diagnostics. However, AgNPs can be cytotoxic and may hurt the human body, wildlife, and environment.
2. Properties of AgNPs
2.1. Physical properties
2.1.1. Size and shape
AgNPs typically have a range from 1 to 100 nanometers in size. According to geometrical principles, the smaller particles have a higher surface area-to-volume ratio which enhances their reactivity. Their tiny scale has significant influences on their biological, physical, and chemical properties. For example, silver nanoparticles that are below 20 nm show greater bactericidal ability due to their better penetration into bacterial cells [4].
AgNPs with the same surface area but different shapes illustrate different bactericidal activity. Their shapes include rods, cubes, spheres, and triangular platelets, which affect their functionality in applications. The most common shape is spheres which is often used in antimicrobial and optical applications [5]. The AgNPs with rod-shaped offers unique optical properties for imaging and photo-thermal therapy. Also, the triangular or plate-like provides improvement in catalytic activity and interaction with light due to anisotropic structures. The less common shapes are cubic and hexagonal. Because they have higher surface energy and beneficial active sites, they can be used in catalysis and pollutant degradation [6].
2.1.2. Optical properties
The optical properties of AgNPs are related to the phenomenon called Localized Surface Plasmon Resonance (LSPR). When surface electrons resonate with light, causing strong absorption and scattering. LSPR is highly affected by size, shape, and the surrounding medium. These features contribute to AgNPs ideal for bio-sensing and imaging applications. Spherical AgNPs typically absorb light in the range of 400–450 nm. The absorption peak shifts with aggregation and particle size, so they can used for diagnostics and colorimetric sensing [7]. AgNPs amplify Raman signals to molecular detection and it is useful in environmental monitoring and medical diagnostics. AgNPs can increase or decrease fluorescence and this depends on their proximity to fluorophores. AgNPs can absorb light and convert it into heat. So they may apply in photothermal treatments and cancer therapy [8].
2.1.3. Thermal and electrical properties
AgNPs have a higher thermal conductivity due to the effective transmission of phonons and this is similar to bulk silver. The thermal conductivity of AgNPs depends on size. The smaller sizes of AgNPs show a lower conductivity because of the increased scattering of phonons on their surface. However, AgNPs retain excellent thermal transport properties, which is important in the applications of efficient heat transfer. AgNPs are generally stable at higher temperatures compared to other metal nanoparticles, but their stability can decrease with increasing temperature due to particle aggregation [9]. For instance, prolonged exposure to high temperatures can lead to the coalescence of AgNPs. This can reduce their effective surface area and their properties [7].
AgNPs are excellent conductors of electricity, thanks to the high electrical conductivity of bulk silver which is one of the best among metals. When silver is reduced to nanoparticles, AgNPs retain their conductive properties that make it possible to use in electronic devices, flexible electronics, and conductive links [9]. However, the conductivity of AgNPs may be affected by their size and aggregation. Smaller nanoparticles or aggregated particles may exhibit lower electrical conductivity due to increased electron scattering at the surface and grain boundaries. This phenomenon is particularly evident in AgNP embedded in polymer substrates or films [10]. At the nanoscale, quantum effects on AgNPs (e. g. quantum tunneling and size-dependent conductivity) become significant. These effects are attributed to the phenomenon of trapping electrons in small volumes and the quantization effects arising from the small size of the nanoparticles. This causes a size-dependent change in conductivity, and environmental conditions, like temperature and the surrounding medium, can also play a role [10]. It is well known that AgNPs exhibit high electron mobility and thus present favorable charge transport characteristics. They are employed in a wide range of applications such as transparent conductive films, biosensors, and solar cells. Due to their high electron mobility, they can be electrically responsive very quickly, making them important for use in sensors and diagnostic tools [11].
2.2. Chemical properties
AgNPs have a high chemical reactivity due to their large surface-area-to-volume ratio and active surface atoms. In oxidation and reduction processes, they can be effective catalyst. The efficiency of catalyst depends on the shape and size of the nanoparticles. So the smaller size and strange shape of nanoparticles show better catalytic properties [12]. It is easy for AgNPs to react with oxygen resulting in a thin layer of silver oxide (Ag₂O) on its surface. The rate of oxidation depends on factors such as nanoparticle size, shape, and environmental conditions. Moreover, the oxide layer can protect the nanoparticle core from further corrosion and enhance its stability [13]. Because of the affinity of silver for sulfur, nitrogen, and oxygen-containing groups, AgNPs can be easily combined with polymers, various ligands, or biomolecules. The surface combination enhances their stability, solubility, and biocompatibility. This kind of AgNPs is more resistant to aggregation and oxidation. AgNPs can interact chemically with lipids, nucleic acids, and proteins through electrostatic interactions, hydrophobic interactions, and thiol binding. Such interactions often involve the formation of a protein corona on their surface, which can alter their chemical properties and biological interactions [14].
2.3. Antimicrobial mechanisms
AgNPs show strong antimicrobial properties through several mechanisms which act on different cellular structures and processes in microbes. These mechanisms are effective in the face of a wide range of pathogens, including bacteria, fungi, and viruses.
AgNPs interact with the microbial cell membrane. This leads to increased permeability and damage to the membrane structure. AgNPs attach to membrane proteins and phospholipids. They disrupt the lipid bilayer. As a result, cellular contents, such as ions, proteins, and metabolites, leak out into the surrounding environment. This leakage contributes to cell death. The small size and large surface area of AgNPs help them bind to and cross the cell membrane [5].
AgNPs help produce reactive oxygen species (ROS) like hydrogen peroxide (H₂O₂), superoxide anions (O₂⁻), and hydroxyl radicals (OH·). These ROS cause oxidative stress. This stress damages important biomolecules. For example, lipid peroxidation weakens cell membranes. Oxidation can also affect enzymes and their function. ROS can break DNA or RNA strands. They can also change bases, which stops proper replication and transcription. AgNPs interact with nitrogen bases and phosphate groups in DNA. They disrupt the DNA's helical structure and replication process. AgNPs also bind to sulfur-containing and thiol-rich proteins. This prevents the enzymes needed for microbial survival from working [15].
AgNPs release Ag⁺ ions, which boost their antimicrobial activity. These Ag⁺ ions bind to microbial cell components and affect different areas. They interact with thiol groups and cause protein denaturation. Ag⁺ ions also target cellular respiration pathways, disrupting ATP production. Even at very low concentrations, Ag⁺ ions are toxic to microbes. This makes AgNPs highly effective [16].
AgNPs can stop biofilm formation and remove existing biofilms. Biofilms are protective layers created by microbial colonies. These layers make the microbes resistant to regular antibiotics. AgNPs can enter the biofilm matrix and break it apart. They also kill the microorganisms trapped inside [17].
AgNPs can act synergistically with antibiotics to enhance antimicrobial efficacy. They can increase bacterial cell permeability and allow antibiotics to penetrate more effectively. This reduces the required antibiotic dose and helps combat antibiotic resistance [15].
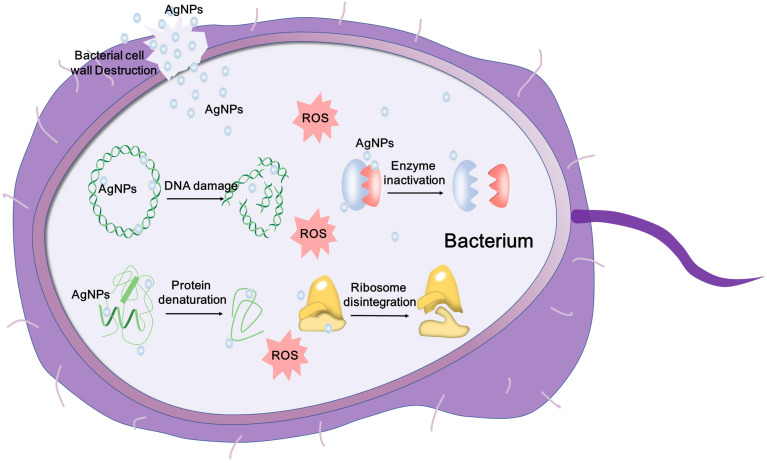
Figure 1: Antimicrobial Mechanisms of AgNPs [16].
Schematic representation of the mechanisms of AgNPs against bacteria, DNA damage, protein denaturation, depicting ROS-dependent pathway, and enzyme inactivation for the antibacterial action of AgNPs (Figure 1).
3. Synthesis of silver nanoparticles
3.1. Chemical synthesis
The most common chemical method is the reduction of silver salts (e.g., silver nitrate, AgNO₃) to produce AgNPs using reducing agents and stabilizers. Firstly, a silver precursor (e.g., AgNO₃) is dissolved in a solvent. Secondly, use reducing agents like sodium borohydride (NaBH₄) or ascorbic acid to reduce the oxidation number of silver ions from +1 to 0. And then, use stabilizers such as polyvinyl alcohol (PVA) or citrate to prevent aggregation [18].
3.2. Physical synthesis
Physical methods produce nanoparticles from bulk silver through high-energy processes. The physical methods include Laser ablation and evaporation-condensation method.
Laser ablation uses a high-intensity laser beam to target a silver piece in a liquid, like water or ethanol. The focused laser energy makes the silver vaporize. This creates a plasma of silver ions and atoms. These particles then nucleate and condense in the surrounding liquid. As a result, nanoparticles form. Laser ablation is a versatile method. It produces nanoparticles with high purity. It does not require chemical reagents, making it more environmentally friendly [19].
In the evaporation-condensation method, heat silver until it becomes vapor. This vapor is then cooled down in a controlled chamber. As the vapor cools, it condenses into tiny silver nanoparticles. By controlling the temperature and pressure in the chamber, this can change the size and quality of the nanoparticles. This method is effective for producing large amounts of silver nanoparticles that have a uniform size [20].
3.3. Green synthesis
Green synthesis involves the use of biological entities such as plant extracts, microorganisms, or enzymes. In this process, biological reducing agents present in plant extracts or microorganisms reduce silver ions (Ag⁺) to silver nanoparticles (Ag⁰) under mild reaction conditions (often at room temperature and without the need for toxic chemicals). This method is considered environmentally friendly because it does not use harsh chemicals and high energy. This method can reduce energy consumption and produce biocompatible nanoparticles which are less toxic to humans and the environment. The reduction of Ag⁺ ions is promoted by natural compounds such as polyphenols, flavonoids, or proteins present in the plant extracts, or by the metabolic activities of microorganisms [21]. Table 1 shows the comparison of chemical synthesis, physical synthesis, and green synthesis [22].
Table 1: Comparison of Chemical synthesis, Physical synthesis, and Green synthesis.
Aspect | Chemical Synthesis | Physical Synthesis | Green Synthesis |
Cost | Moderate | High | Low |
Environmental Impact | High (toxic chemicals) | Low (no chemicals used) | Very low (eco-friendly methods) |
Scalability | High | Low | Moderate |
Purity | Moderate (depends on reagents) | High | Moderate (due to capping agents) |
Energy Requirement | Low | High | Low |
Reproducibility | High | High | Moderate (biological variability) |
4. Applications of AgNPs in healthcare
4.1. Antibacterial and anti-fungal applications
AgNPs show broad-spectrum antimicrobial activity against MDR pathogens, including Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. They can effectively against antibiotic- resistant strains through membrane disruption, ROS generation, and DNA damage [23]. Use in wound dressings and coatings for medical devices. AgNP-based dressings promote wound healing faster by relieving microbial load and inflammation. They are beneficial in treating chronic wounds like burns and diabetic ulcers. This way can reduce infection risks associated with indwelling devices and prevent biofilm formation by coating AgNPs on implants, catheters, and prosthetics [24].
4.2. Drug delivery systems
AgNPs can be combined with ligands for targeted drug delivery and they can enhance drug accumulation in diseased tissues through the Enhanced Permeability and Retention (EPR) effect. Also, AgNPs enhance antibiotic efficacy by increasing bacterial membrane permeability and reducing the required dosage [23]. Shrivastava et al. (2007) found that AgNPs synergy with antibiotics like ampicillin and gentamicin to combat resistant pathogens [25].
4.3. Cancer therapy
AgNPs cause oxidative stress, which leads to mitochondrial dysfunction and DNA fragmentation in cancer cells. Normal cells are not affected in the same way. Gurunathan et al. (2013) found that AgNPs improve anticancer effectiveness by triggering ROS-mediated apoptosis in breast cancer models. When AgNPs are used with chemotherapeutic drugs, they increase drug delivery and help overcome drug resistance [26].
4.4. Imaging and diagnostics
AgNPs are employed in biosensors due to their high conductivity and sensitivity, and they can enhance pathogen detection and biomarker identification. Jain et al. (2008) described their role in glucose monitoring and pathogen detection. AgNPs improve contrast in imaging techniques like Surface-Enhanced Raman Scattering (SERS) [27].
5. Challenges and concerns
5.1. Toxicity
AgNPs can be cytotoxic which means that they harm human cells by disrupting membrane integrity, inducing apoptosis, or generating reactive oxygen species (ROS). Additionally, they can have genotoxic effects leading to DNA damage and affecting cell replication. Some researchers highlight their potential for inflammation, induce oxidative stress, and long-term genetic mutations in human cells [28].
5.2. Environmental impact
Silver nanoparticles release toxicity into the environment and can accumulate in water bodies, soil, and living organisms. For example, they can inhibit soil bacteria crucial for nutrient cycling or harm aquatic species by disrupting biological processes at sub-lethal concentrations. Their persistence can disrupt microbial ecosystems to reduce biodiversity and affect the health of both aquatic and terrestrial life [29].
Regulatory and Manufacturing Issues:
The synthesis, characterization, and application of silver nanoparticles is lack of standardized protocols. It is challenging to ensure consistency, safety, and efficacy in both clinical and industrial applications due to the variation in particle size, shape, coating, and concentration across different manufacturing processes. This inconsistency complicates regulatory approval processes [30].
Regulatory agencies like the European Medicines Agency (EMA) and the U. S. Food and Drug Administration (FDA) face challenges due to the limited availability of long-term safety data on AgNPs. This is particularly concerning for products involving human exposure, such as wound dressings and coatings for medical devices [30].
The cost of production is high due to the complex and multi-step processes involved in the synthesis of high-quality AgNPs particularly used for biomedical applications. Without cost-effective manufacturing methods, this makes widespread adoption in healthcare challenging.
6. Conclusion
Different kinds of AgNPs have different shapes and sizes that contribute to their physical physical, chemical, and antimicrobial properties to apply in medicine area. Especially, AgNPs have excellent antimicrobial ability. AgNPs interact with the microbial cell membrane and lead to increased permeability and structural damage. AgNPs can release Ag⁺ ions to enhance their antimicrobial activity.
AgNPs have proven to have an important role in modern healthcare like drug delivery, wound healing, cancer therapy, and diagnostics due to their physical, chemical and antimicrobial properties. Although they have many significant benefits, challenges that includes potential toxicity, environmental impact, and regulatory and manufacturing issues still remain. To address toxicity concerns, researchers are focusing on combining AgNPs with targeting ligands, such as peptides or antibodies. This can enhance therapeutic efficacy while minimizing cytotoxic effects on non-target cells. Some researchers try to use biodegradable polymers like chitosan or polylactic acid to reduce long-term ecological impact of AgNPs. Moreover, AgNPs combine with other nanomaterials such as gold nanoparticles or carbon nanotubes to reduce drug resistance and improve treatment outcomes. Overall, the future of AgNPs depends on the balance between their medical advantages and environmental and health safety concerns.
References
[1]. Salata, O. V. (2004). Applications of nanoparticles in biology and medicine. Journal of Nanobiotechnology, 2(1), 3.
[2]. Alexander, J. W. (2009). History of the medical use of silver. Surgical Infections, 10(3), 289–292.
[3]. Dakal, T. C., et al. (2016). Mechanistic basis of antimicrobial actions of silver nanoparticles. Frontiers in Microbiology, 7, 1831.
[4]. Pal, S., Tak, Y. K., & Song, J. M. (2007). Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and Environmental Microbiology, 73(6), 1712–1720.
[5]. Dakal, T. C., Kumar, A., Majumdar, R. S., & Yadav, V. (2016). Mechanistic basis of antimicrobial actions of silver nanoparticles. Frontiers in Microbiology, 7, 1831.
[6]. Burdușel, A.-C., Gherasim, O., Grumezescu, A. M., Mogoantă, L., Ficai, A., & Andronescu, E. (2018). Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials, 8(9), 679.
[7]. Lee, K. S., & El-Sayed, M. A. (2006). Journal of Physical Chemistry B, 110(39), 19220–19225.
[8]. Sardar, R., et al. (2009). Optical properties and applications of silver nanoparticles. Langmuir, 25(24), 13840–13851.
[9]. Zheng, Y., et al. (2018). Silver nanoparticles: Synthesis, properties, and applications in electronics and biomedicine. Materials Science and Engineering: R: Reports, 129, 14–43.
[10]. Ashassi-Sorkhabi, H., et al. (2014). Electrical properties and charge transport in silver nanoparticle films. Journal of Nanoparticle Research, 16(9), 2679.
[11]. Yang, X., et al. (2013). Application of silver nanoparticles in biosensors. Analytical Chemistry, 85(5), 2371–2380.
[12]. Kvítek, L., et al. (2008). Catalytic activity of silver nanoparticles for the reduction of dyes in the presence of NaBH₄. Journal of Physical Chemistry C, 112(15), 5852–5858.
[13]. Ashkarran, A. A., et al. (2012). Influence of surface plasmon resonance on the catalytic activity of silver nanoparticles decorated on graphene oxide. Nanotechnology, 23(4), 045705.
[14]. Franci, G., et al. (2015). Silver nanoparticles as potential antibacterial agents. Molecules, 20(5), 8856–8874.
[15]. Shrivastava, S., et al. (2007). Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology, 18(22), 225103.
[16]. Xu, L., Wang, Y. Y., Huang, J., Chen, C. Y., Wang, Z. X., & Xie, H. (2020). Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics, 10(20), 8996-9031.
[17]. Marambio-Jones, C., & Hoek, E. M. V. (2010). A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. Journal of Nanoparticle Research, 12(5), 1531–1551.
[18]. Jones, L. H., & Wang, X. P. (2019). Reduction methods in silver nanoparticle synthesis: The role of stabilizers and reducing agents. International Journal of Nanoscience, 10(2), 101-110.
[19]. Tsuji, M., et al. (2008). Preparation of silver nanoparticles by laser ablation in solution: Influence of laser wavelength on particle size. Applied Surface Science, 254(16), 5224–5230.
[20]. Duman, H., Eker, F., Akdaşçi, E., Witkowska, A. M., Bechelany, M., & Karav, S. (2024). Silver nanoparticles: A comprehensive review of synthesis methods and chemical and physical properties. Nanomaterials, 14(18), 1527.
[21]. Singh, P., Pandit, S., & Chudasama, B. (2020). Green synthesis of silver nanoparticles: An eco-friendly and sustainable approach. Environmental Chemistry Letters, 18(6), 1967-1979.
[22]. Duman, H., Eker, F., Akdaşçi, E., Witkowska, A. M., Bechelany, M., & Karav, S. (2024). Silver nanoparticles: A comprehensive review of synthesis methods and chemical and physical properties. Nanomaterials, 14(18), 1527.
[23]. Rai, M., et al. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances, 27(1), 76–83.
[24]. Kalantari, K., Mostafavi, E., Afifi, A. M., Izadiyan, Z., Jahangirian, H., Rafiee-Moghaddam, R., & Webster, T. J. (2020). Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale, 12(4), 2268–2291.
[25]. Shrivastava, S., et al. (2007). Enhanced antibacterial effects of silver nanoparticles. Nanotechnology, 18(22), 225103.
[26]. Ong, C., Lim, J. Z. Z., Ng, C. T., Li, J. J., Yung, L. Y., & Bay, B. H. (2013). Silver nanoparticles in cancer: Therapeutic efficacy and toxicity. Current Medicinal Chemistry, 20(6), 772–781.
[27]. Jain, K. K. (2008). Nanotechnology in clinical laboratory diagnostics. Clinica Chimica Acta, 395(1-2), 6–12.
[28]. Beer, C., Foldbjerg, R., Hayashi, Y., Sutherland, D. S., & Autrup, H. (2012). Toxicity of silver nanoparticles: Nanoparticle or silver ion? Toxicology Letters, 208(3), 286–292.
[29]. Ivask, A., Elbadawy, A., Kaweeteerawat, C., & Boren, D. (2014). Mechanisms of silver nanoparticle toxicity in environmental and clinical contexts. Environmental Science & Technology, 48(1), 129–136.
[30]. Ranjan, S., Dasgupta, N., & Ramalingam, C. (2016). Regulatory issues and safety concerns. Nanomaterials, 6(7), 136.
Cite this article
Gao,L. (2025). Revolutionizing Healthcare: The Role of Silver Nanoparticles in Modern Medicine. Applied and Computational Engineering,126,131-138.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Salata, O. V. (2004). Applications of nanoparticles in biology and medicine. Journal of Nanobiotechnology, 2(1), 3.
[2]. Alexander, J. W. (2009). History of the medical use of silver. Surgical Infections, 10(3), 289–292.
[3]. Dakal, T. C., et al. (2016). Mechanistic basis of antimicrobial actions of silver nanoparticles. Frontiers in Microbiology, 7, 1831.
[4]. Pal, S., Tak, Y. K., & Song, J. M. (2007). Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and Environmental Microbiology, 73(6), 1712–1720.
[5]. Dakal, T. C., Kumar, A., Majumdar, R. S., & Yadav, V. (2016). Mechanistic basis of antimicrobial actions of silver nanoparticles. Frontiers in Microbiology, 7, 1831.
[6]. Burdușel, A.-C., Gherasim, O., Grumezescu, A. M., Mogoantă, L., Ficai, A., & Andronescu, E. (2018). Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials, 8(9), 679.
[7]. Lee, K. S., & El-Sayed, M. A. (2006). Journal of Physical Chemistry B, 110(39), 19220–19225.
[8]. Sardar, R., et al. (2009). Optical properties and applications of silver nanoparticles. Langmuir, 25(24), 13840–13851.
[9]. Zheng, Y., et al. (2018). Silver nanoparticles: Synthesis, properties, and applications in electronics and biomedicine. Materials Science and Engineering: R: Reports, 129, 14–43.
[10]. Ashassi-Sorkhabi, H., et al. (2014). Electrical properties and charge transport in silver nanoparticle films. Journal of Nanoparticle Research, 16(9), 2679.
[11]. Yang, X., et al. (2013). Application of silver nanoparticles in biosensors. Analytical Chemistry, 85(5), 2371–2380.
[12]. Kvítek, L., et al. (2008). Catalytic activity of silver nanoparticles for the reduction of dyes in the presence of NaBH₄. Journal of Physical Chemistry C, 112(15), 5852–5858.
[13]. Ashkarran, A. A., et al. (2012). Influence of surface plasmon resonance on the catalytic activity of silver nanoparticles decorated on graphene oxide. Nanotechnology, 23(4), 045705.
[14]. Franci, G., et al. (2015). Silver nanoparticles as potential antibacterial agents. Molecules, 20(5), 8856–8874.
[15]. Shrivastava, S., et al. (2007). Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology, 18(22), 225103.
[16]. Xu, L., Wang, Y. Y., Huang, J., Chen, C. Y., Wang, Z. X., & Xie, H. (2020). Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics, 10(20), 8996-9031.
[17]. Marambio-Jones, C., & Hoek, E. M. V. (2010). A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. Journal of Nanoparticle Research, 12(5), 1531–1551.
[18]. Jones, L. H., & Wang, X. P. (2019). Reduction methods in silver nanoparticle synthesis: The role of stabilizers and reducing agents. International Journal of Nanoscience, 10(2), 101-110.
[19]. Tsuji, M., et al. (2008). Preparation of silver nanoparticles by laser ablation in solution: Influence of laser wavelength on particle size. Applied Surface Science, 254(16), 5224–5230.
[20]. Duman, H., Eker, F., Akdaşçi, E., Witkowska, A. M., Bechelany, M., & Karav, S. (2024). Silver nanoparticles: A comprehensive review of synthesis methods and chemical and physical properties. Nanomaterials, 14(18), 1527.
[21]. Singh, P., Pandit, S., & Chudasama, B. (2020). Green synthesis of silver nanoparticles: An eco-friendly and sustainable approach. Environmental Chemistry Letters, 18(6), 1967-1979.
[22]. Duman, H., Eker, F., Akdaşçi, E., Witkowska, A. M., Bechelany, M., & Karav, S. (2024). Silver nanoparticles: A comprehensive review of synthesis methods and chemical and physical properties. Nanomaterials, 14(18), 1527.
[23]. Rai, M., et al. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances, 27(1), 76–83.
[24]. Kalantari, K., Mostafavi, E., Afifi, A. M., Izadiyan, Z., Jahangirian, H., Rafiee-Moghaddam, R., & Webster, T. J. (2020). Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale, 12(4), 2268–2291.
[25]. Shrivastava, S., et al. (2007). Enhanced antibacterial effects of silver nanoparticles. Nanotechnology, 18(22), 225103.
[26]. Ong, C., Lim, J. Z. Z., Ng, C. T., Li, J. J., Yung, L. Y., & Bay, B. H. (2013). Silver nanoparticles in cancer: Therapeutic efficacy and toxicity. Current Medicinal Chemistry, 20(6), 772–781.
[27]. Jain, K. K. (2008). Nanotechnology in clinical laboratory diagnostics. Clinica Chimica Acta, 395(1-2), 6–12.
[28]. Beer, C., Foldbjerg, R., Hayashi, Y., Sutherland, D. S., & Autrup, H. (2012). Toxicity of silver nanoparticles: Nanoparticle or silver ion? Toxicology Letters, 208(3), 286–292.
[29]. Ivask, A., Elbadawy, A., Kaweeteerawat, C., & Boren, D. (2014). Mechanisms of silver nanoparticle toxicity in environmental and clinical contexts. Environmental Science & Technology, 48(1), 129–136.
[30]. Ranjan, S., Dasgupta, N., & Ramalingam, C. (2016). Regulatory issues and safety concerns. Nanomaterials, 6(7), 136.