1. Introduction
China has a number of natural gas fields with high carbon dioxide content, such as Jiangsu Huangqiao gas field and Jilin Wanjinta gas field, of which the carbon dioxide content is 90~99%, and Guangdong Sanshui gas field, of which the carbon dioxide content is 84~99% [1-3]. After the carbon dioxide rich natural gas is extracted, it needs to be transported to the treatment plant through the gas collection pipeline to achieve the effective separation of carbon dioxide and natural gas. The separated carbon dioxide needs to be transported to the consumer market through various means of transportation such as pipelines, vehicles and ships, and is widely used in chemical manufacturing, oil and gas exploration, fire fighting and food processing [4, 5]. However, different from conventional natural gas, the transition between gas phase, liquid phase or supercritical state occurs easily in the process of pipeline transportation of carbon dioxide or high carbon dioxide content, which undoubtedly poses new challenges for the design of high carbon dioxide content pipeline and restricts the expansion of engineering application scenarios to a certain extent [6].
The physical property parameter with high carbon dioxide content is a function of temperature and pressure. Near the critical point, with the slight fluctuation of temperature and pressure, the physical property parameter will have a large nonlinear change. Therefore, mastering the physical property change law with high carbon dioxide content is the premise to ensure the accurate calculation results of temperature and pressure.
At present, the physical property parameters of carbon dioxide are generally calculated based on the equation of state, and the calculation method has been mature, but whether the change law of conventional physical property parameters of carbon dioxide is applicable to high carbon dioxide content needs further evaluation and analysis. In this paper, pure carbon dioxide and high carbon dioxide content (90%CO2, 10%CH4) are used as working medium. By invoking the data of density, specific heat capacity, viscosity, compression factor and other physical parameters in the built-in database of REFPROP software of the National Institute of Standards and Technology (NIST), the physical property parameters of the two are compared and analyzed.
2. High carbon dioxide content physical property parameter
Due to the mixture of methane, the critical point of high carbon dioxide content (90%CO2, 10%CH4) changes compared with carbon dioxide. The phase diagram of carbon dioxide and high carbon dioxide content is shown in Fig. 1, and the critical point of methane, carbon dioxide and high carbon dioxide content is shown in Table 1.
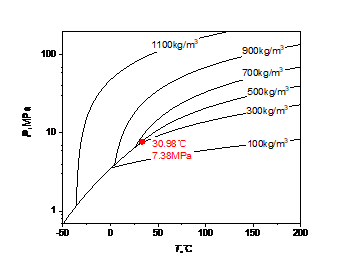
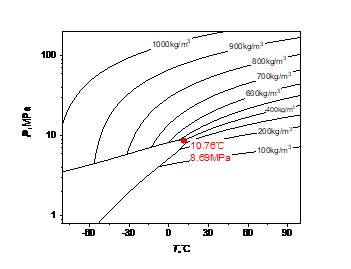
Figure 1: Carbon dioxide and high-carbon dioxide phases: (a) carbon dioxide; (b) high carbon dioxide content
Table 1: Critical points for methane, carbon dioxide, and high carbon dioxide content
Working medium | Critical pressure | Critical temperature |
Methane | 4.59MPa | -82.59℃ |
Carbon dioxide | 7.38MPa | 30.98℃ |
High carbon dioxide content 90%CO2+10%CH4 | 8.69MPa | 10.76℃ |
As can be seen from Fig. 1 and Table 1, with the increase of methane content, the critical temperature of the mixed fluid decreases, while the critical pressure increases, and a gas-liquid coexistence zone will appear, from a saturation line of pure carbon dioxide to a gas saturation line and a liquid saturation line with high carbon dioxide content.
2.1. Density
Many of the properties of high carbon dioxide content stem from the high sensitivity of density to temperature and pressure. The density data of pure carbon dioxide and high carbon dioxide content in the database were respectively called to draw the curve of their density changes with temperature and pressure, and analyze the law of their changes.
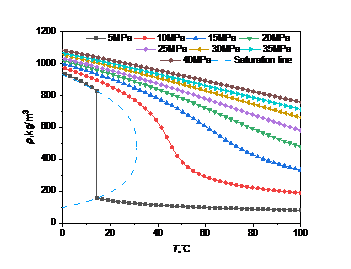
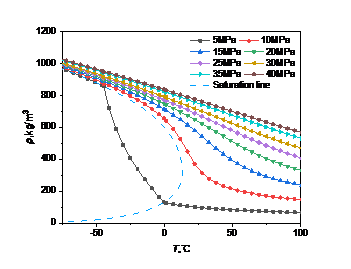
Figure 2: Density curve with temperature and pressure: (a) carbon dioxide; (b) high carbon dioxide content
The curve of carbon dioxide density with temperature and pressure presents a typical nonlinear change. When the pressure remains unchanged, the density will decrease with the increase of temperature. Under the condition of constant temperature, the density will increase with the increase of pressure. And when the pressure is high, the range of density change will be relatively small.
In Fig. 2(a), when the pressure is less than the critical point (5MPa), the density changes abruptly at 14.3℃, from 827.3kg/m3 to 156.7kg/m3, because carbon dioxide is transformed from liquid to gas at this time, and the density changes little in the gaseous state. When the pressure is greater than the critical point, the density changes less and less with the temperature as the pressure increases.
In Fig. 2(b), when the pressure is less than the critical point (5MPa), the density no longer changes abruptly at the same temperature, but decreases continuously with the increase of temperature in the range of -45.0 ° C to -4.4 ° C until it becomes a gaseous state. When the pressure is greater than the critical point, the trend is the same as that in Figure 2(a), that is, as the pressure increases, the density changes less and less with the temperature.
2.2. Viscosity
Viscosity is one of the important physical properties of fluid, which reflects the interaction between the particles inside the fluid and the fluidity of the fluid. The greater the viscosity, the weaker the fluidity of the fluid, and the smaller the viscosity, the better the fluidity.
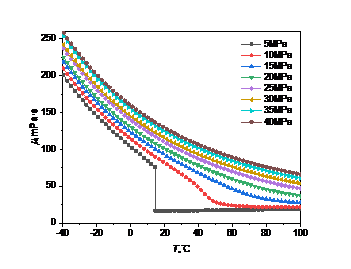
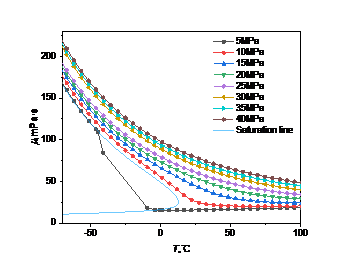
Figure 3: Viscosity curve with temperature and pressure: (a) carbon dioxide; (b) high carbon dioxide content
When the pressure is constant, the viscosity of carbon dioxide will gradually decrease with the increase of temperature. If the temperature remains the same, increasing the pressure will cause the viscosity of carbon dioxide to rise. In the high pressure environment, the viscosity gradually decreases with the increase of temperature, and there is no abrupt change.
In Fig. 3(a), when the pressure is less than the critical point (5MPa), the viscosity changes abruptly at 14.3℃, dropping from 75.6mPa·s to 16.8mPa·s. This is because at this time, carbon dioxide is transformed from liquid to gas. In the gaseous state, the viscosity changes little and increases very slowly with temperature. This is mainly due to the different interaction modes and motion states between gas and liquid molecules, resulting in the opposite trend of gas viscosity and liquid viscosity with temperature. When the pressure is greater than the critical point, the density changes less and less with the temperature as the pressure increases.
In Fig. 3(b), when the pressure is less than the critical point (5MPa), the viscosity no longer changes abruptly at the same temperature, but decreases continuously with the increase of temperature in the range of -45.0℃ to -4.4 ℃ until it becomes a gaseous state. When the pressure is greater than the critical point, the trend is the same as that in Fig. 3(a), that is, as the pressure increases, the density changes less and less with the temperature.
2.3. Specific heat capacity at constant pressure
Specific heat capacity at constant pressure refers to the amount of heat required to raise the temperature of a unit mass of a substance by 1 K under constant pressure. It is related to the substance's microscopic structure and thermal motion.
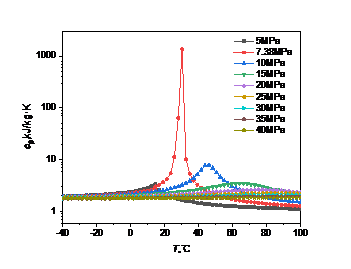
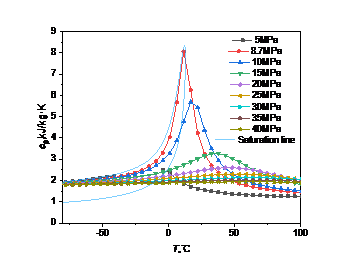
Figure 4: Curve of specific heat capacity at constant pressure with temperature and pressure: (a) carbon dioxide; (b) high carbon dioxide content
Fig. 4 shows specific heat capacity at constant pressure of carbon dioxide is less affected by temperature and pressure changes. When the pressure is constant, the specific heat capacity at constant pressure first rises to a peak with the increase of temperature, and then gradually decreases. The temperature corresponding to the peak is called the quasi-critical temperature, and the point is called the quasi-critical point. When the temperature is below the quasi-critical temperature, the fluid has a "liquid-like" property [7]. When the temperature is above the quasi-critical temperature, the fluid has a "gas-like" property [8]. When the temperature is constant, the specific heat capacity at constant pressure will decrease with the increase of pressure, and the amplitude is small.
It can be found that the higher the pressure, the higher the corresponding quasi-critical temperature. Near the critical point, the specific heat capacity will rise sharply, and the closer the pressure is to the critical point, the more drastic the change of the specific heat capacity.
In Fig. 4(a), when the pressure is 7.38MPa and the temperature is 31.1℃, the peak specific heat capacity of carbon dioxide is 1731.9kJ/(kg·K), which is significantly higher than the peak specific heat capacity under other pressures. In Fig. 4(b), when the pressure is 8.69MPa and the temperature is 10.8℃, the peak specific heat capacity of carbon dioxide is 7.98kJ/(kg·K), which is about 200 times smaller than that of pure carbon dioxide.
2.4. Compression factor
The compression factor represents the deviation in the volume between the real gas and the ideal gas when subjected to the same pressure compression, that is, the ratio of the molar volume of a real gas to that of an ideal gas under the same conditions [9]. It is mainly designed to correct the application of the ideal gas state equation in real gas.
If the value of the compression factor is greater than 1, then the volume of the real gas is greater than that of the ideal gas at the same temperature and pressure, so the gas is difficult to be compressed; On the contrary, if the value of the compression factor is less than 1, the gas is relatively easy to be compressed.
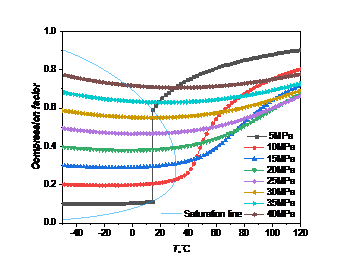
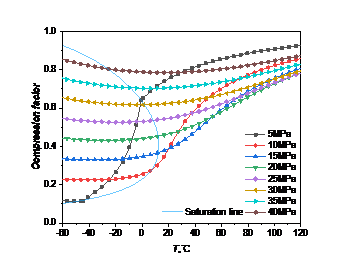
Figure 5: Change curve of compression factor with temperature pressure: (a) carbon dioxide; (b) high carbon dioxide content
As can be seen from the Fig. 5, the change trend of compression factor of carbon dioxide and high carbon dioxide content with temperature and pressure are basically consistent. The compression factor of carbon dioxide and high carbon dioxide content are both less than 1, both have compressibility.
When the pressure is much greater than the critical pressure, the compression factor decreases slowly and then increases with the temperature increase, indicating that the compression factor will not change significantly due to the temperature change. When the temperature is constant, the compression factor increases with the increasing pressure, indicating that the greater the pressure is, the easier to be compressed, and the closer the gas is to the ideal gas. When the pressure is close to the critical pressure (10MPa-25MPa), the compression factor mutates as the temperature rises. When the pressure is less than the critical pressure (5MPa), the compression factor changes dramatically due to the phase change, and as the temperature increases, the compression factor will be even greater than the compression factor corresponding of the high pressure under isothermal conditions.
3. Conclusion
To sum up, the physical property of high carbon dioxide content will change significantly with the change of temperature and pressure, especially in the area near the critical point, even small temperature and pressure fluctuation, also can lead to dramatic changes in properties such as density, viscosity, specific heat capacity at constant pressure, compression factor and so on, the change may reach several times or even hundreds of times. The critical property parameters of carbon dioxide and high carbon dioxide content are shown in Table 21 below.
Table 2: Critical property parameters of carbon dioxide and high carbon dioxide content
Working medium | Carbon dioxide | High carbon dioxide content (90%CO2+10%CH4) |
Pressure | 7.38MPa | 8.69MPa |
Temperature | 30.98℃ | 10.76℃ |
Density | 427.55kg/m3 | 431.67 kg/m3 |
Dynamic | 30.21mPa·s | 31.67mPa·s |
Specific heat at constant | 1371.90 kJ/(kg·K) | 7.98 kJ/(kg·K) |
Compression factor | 0.30 | 0.32 |
The carbon dioxide extracted from the carbon dioxide gas field generally contains methane, ethane, nitrogen and other components, and the internal gathering and transportation pipeline of the carbon dioxide gas field is generally transported in the gas phase, and after purification, it is transported to a distant market or injection point. According to the studies of many scholars, it is more economical to adopt liquid phase or supercritical transport. Therefore, in the process of the whole pipeline design operation, the high carbon dioxide content near the physical property with the change of temperature, pressure, the balance of supercritical phase, heat transfer properties still need further research, for the effective development and utilization of high carbon dioxide content gas field and safe, economic and efficient pipeline transport to lay the theoretical foundation.
Acknowledgements
The project is supported by Director Fund of National Key Laboratory for Efficient Development of Marine Oil and Gas in 2024.
References
[1]. Luo W. Research on The Natural Gas with High Content of CO₂ Pipeline Transportation Technology[D]. Southwest Petroleum University, 2013.
[2]. Xu J, Zhang J Y, Pan X, Zhang C G. Research status of carbon dioxide storage technology[J]. Coal Conversion, 2005.28 (3): 79-85.
[3]. Zhang X Y, Cheng J M, Liu J, Wang Y Z. CO, Research progress of geological disposal[J]. Hydrogeology Engineering Geology,2006,19(4):85-88.
[4]. DONG Y. Research on viscosity reducing transport technology of supercritical CO2 heavy oil[D]. University of Petroleum,2011.
[5]. ROY B C, GOTO M, HIROSE T. Extraction of ginger oil with supercritical carbon dioxide: Experiments and modeling[J]. Industrial and Engineering Chemistry Research, 1996, 35(2): 607⁃612.
[6]. Zhuang Z J, Li M, Zhang L Q, et al. Summary of Physical Parameters of Supercritical Carbon Dioxide[J]. Low Temperature and Specialty Gases,2023,41(05):8-15+20.
[7]. Kim J K, Jeon H K, Lee J S. Wall temperature measurement and heat transfer correlation of turbulent supercritical carbon dioxide flow in vertical circular/non-circular tubes[J]. Nuclear Engineering & Design, 2007, 237(15-17): 1795-1802. DOI: 10.1016/j.nucengdes.2007.02.017.
[8]. Pettersen J. Flow vaporization of CO2 in Microchannel Tubes[J]. Experimental Thermal & Fluid Science 2002, 28(2):111-121. DOI: 10.1016/S0894-1777(03)00029-3.
[9]. Wang M D. Compression factors and compressibility of gases [J]. Chinese Chemical Bulletin, 2002, 65(5): 358⁃360.
Cite this article
Yu,J.;Du,X.;Hao,T.;Zhu,W.;Yuan,X.;Wu,J.;Xu,Q. (2025). Review of Physical Properties of High Carbon Dioxide Content. Applied and Computational Engineering,127,187-192.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Luo W. Research on The Natural Gas with High Content of CO₂ Pipeline Transportation Technology[D]. Southwest Petroleum University, 2013.
[2]. Xu J, Zhang J Y, Pan X, Zhang C G. Research status of carbon dioxide storage technology[J]. Coal Conversion, 2005.28 (3): 79-85.
[3]. Zhang X Y, Cheng J M, Liu J, Wang Y Z. CO, Research progress of geological disposal[J]. Hydrogeology Engineering Geology,2006,19(4):85-88.
[4]. DONG Y. Research on viscosity reducing transport technology of supercritical CO2 heavy oil[D]. University of Petroleum,2011.
[5]. ROY B C, GOTO M, HIROSE T. Extraction of ginger oil with supercritical carbon dioxide: Experiments and modeling[J]. Industrial and Engineering Chemistry Research, 1996, 35(2): 607⁃612.
[6]. Zhuang Z J, Li M, Zhang L Q, et al. Summary of Physical Parameters of Supercritical Carbon Dioxide[J]. Low Temperature and Specialty Gases,2023,41(05):8-15+20.
[7]. Kim J K, Jeon H K, Lee J S. Wall temperature measurement and heat transfer correlation of turbulent supercritical carbon dioxide flow in vertical circular/non-circular tubes[J]. Nuclear Engineering & Design, 2007, 237(15-17): 1795-1802. DOI: 10.1016/j.nucengdes.2007.02.017.
[8]. Pettersen J. Flow vaporization of CO2 in Microchannel Tubes[J]. Experimental Thermal & Fluid Science 2002, 28(2):111-121. DOI: 10.1016/S0894-1777(03)00029-3.
[9]. Wang M D. Compression factors and compressibility of gases [J]. Chinese Chemical Bulletin, 2002, 65(5): 358⁃360.









