1. Introduction
BCIs have become revolutionary tools in the treatment and management of neurological diseases. Traditional BCI technologies, such as scalp electroencephalography (EEG) and invasive cortical EEG methods [1,2], have shown application potential, but they also possess some limitations. Scalp EEG can record electrical activity noninvasively through electrodes placed on the scalp. However, its low spatial resolution blocks its effect on precise neural mapping and control. In contrast, invasive techniques like Neurogrid and Neuralink can implant electrodes on or inside the cortical tissue [3-5]. Neurogrid is a flexible and grid-like electrode array, and Neuralink is a neural implant that can offer high-resolution recordings and stimulation. These techniques have shown effectiveness in capturing detailed neural signals and enabling neural control. However, their invasive nature poses some risks, including potential damage to neural tissue during implantation, inflammation, and biological compatibility issues [1].
Endovascular electrodes can address limitations of scalp EEG and invasive cortical electrodes [1, 6, 7]. EE are minimally invasive devices that can be delivered to the brain via blood vessels. This technique reduces the risk of surgery-related complications and damage to neural tissue. One of the representative EE devices, StentrodeTM [8], is a stent electrode array that can be implanted in the superior sagittal sinus (SSS) by percutaneous catheter venography [1]. Using the vascular network of the brain, EE can be put closely to the target neural regions without direct cortical penetration.
In recent years, the applications of EE have developed a lot. Research in animal models with StentrodeTM has demonstrated the safety, efficiency, and stability in recording neural activity and delivering electrical stimulation of EE. A recent study accomplished the installation of an endovascular neural interface using a micrometer-scale vasculature probe in mice [9]. This demonstrates that the range of BCI research tools can be broadened, and endovascular electrodes can be less invasive than traditional BCIs. Recent human clinical trials also verified these findings, showing that EE can treat paralysis by interfacing with neural circuits.
This review aims to summarize the current applications of EE technology in animal models and human clinical trials. And we explored the development of relevant hardware devices that can provide safer and more effective implantation. In the end, we discussed the current limitations of EE and their potential prospects. This review will have a guiding significance for the future development direction of EE technology, and provide possible research fields for subsequent researchers. For those who want to learn about EE, this review can also help them keep abreast of the latest clinical application of EE.
2. Early exploration for clinical application in animal models
2.1. Feasibility verification
Implanting endovascular electrodes in brain can be a promising treatment for neural diseases and paralysis. However, it is essential to verify whether it is suitable for humans, considering patients’ individual health and future lives. The technology can be decided based on two main aspects: implant feasibility and operational stability.
The implant feasibility is the criteria for the possibility of implanting electrode arrays in the motor cortex of the human brain. Researchers have conducted numerous related experiments on animals. In 2016, Thomas et al. proposed an ovine model for cerebral catheter venography [10]. In the experiments, they performed contrast-enhanced magnetic resonance imaging (MRI) on 13 animals and conducted cerebral catheter venography on 39 sheep. These experiments located the motor area, which directed the catheter to the superior sagittal sinus (SSS). The experiments also determined the largest wide-bore delivery catheter suitable for insertion into the SSS. It was reported that a 4-Fr catheter with a diameter of 1.1 mm was able to reach the SSS in every instance without complications. Because the position of the motor cortex is so similar in sheep and humans, this model demonstrated that implanting endovascular electrodes in the human brain is feasible.
It is also crucial to determine whether electrical stimulation can activate neuronal populations within blood vessels to see whether EE are necessary for treating brain disorders [11]. Based on this, Nicholas et al. developed a chronically implanted electrode array in 2018. In their study, they implanted the array in sheep, and then applied electrical stimulation by the array. It was reported that facial and limb reactions observed were similar to those induced by invasive electrodes. The finding indicates that EE could be a promising method for treating neural diseases by means of electrical stimulation. Because of its minimally invasiveness and low risk of complications, EE technology can be helpful to treat neural disorders.
2.2. Accuracy verification
The accuracy of the signals can be evaluated in four aspects: bandwidth, signal-to-noise ratio (SNR), spatial resolution and decoding accuracy. The bandwidth of signals is used to evaluate the volume of accessible information while SNR reflects the efficiency of detecting and decoding neural events. Spatial resolution indicates the capacity of arrays to localize the sensed neural signals, and decoding accuracy determines if the BCI device can accurately decode information relevant to movement intent.
In one study, the spatial resolution is influenced by the location of arrays and the recording frequency. It was anticipated that subdural arrays would exhibit the highest spatial resolution, as the subdural electrodes are the closest to the brain and are in direct contact with the cortical surface. The difference in array locations is shown in Fig.1. However, minimal effects of array location on spatial resolution were observed at frequencies exceeding 24 Hz during the resting state. Furthermore, there is a direct correlation between SNR and classification accuracy. The bandwidth, SNR, spatial resolution, and decoding accuracy of subdural, epidural, and endovascular arrays are comparable [11]. Another study indicates that the signal quality of EE is comparable to that of sub-scalp EEG by recording the amplitudes, SNRs, and bandwidths of visual evoked potentials in sheep [12].
In conclusion, EE demonstrate equivalent signal quality to sub-scalp, subdural, and epidural arrays, which are widely used in invasive BCI applications. Future research should focus on comparing and evaluating the performance of EE against other invasive BCIs in detecting common paradigms such as motor imagery, steady-state visual evoked potentials, and P300, etc. Therefore, in anatomically constrained scenarios, EE as a reliable and minimally invasive device deserves priority.
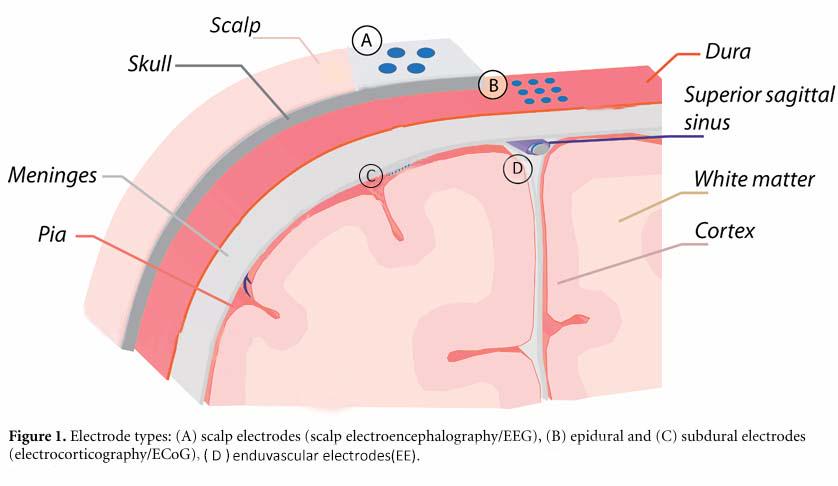
Figure 1. Different array locations. Electrode types: (A) sub-scalp electrodes, (B) epidural electrodes, (C) subdural electrodes, (D) endovascular electrodes (EE).
2.3. Long-term stability exploration
Electrodes implanted in the brain have been shown to induce chronic inflammation, leading to the failure of some electrode arrays within about six months [13]. Therefore, the service life of EE in the brain requires further study.
Animal experiments with StentrodeTM in the 2010s have preliminarily verified the long service life of EE. In 2016, Nicholas et al. implanted StentrodeTM in the superior sagittal sinus of the sheep motor cortex [14]. Through electrochemical impedance spectroscopy (EIS), they found that neural recordings maintained a stable maximum bandwidth during the six-month implantation period and exhibited high fidelity comparable to invasive electrode arrays.
In the same year, another study was conducted with StentrodeTM in 15 sheep, using EIS and an equivalent circuit model (ECM) to describe changes in EE during implantation. It also included animal histological assessments [15]. In histological assessment up to 190 days, the stent was covered by endothelial tissue within 9-14 days and over 85% of the stent struts were covered after two weeks. Endothelial binding was observed in all stent-implanted animals, and the neointima never fully blocked the 2-3 mm diameter SSS. This experiment illustrates that StentrodeTM can be tolerated long-term and stabilizes 9-14 days after implantation in the motor cortical superior sagittal sinus vessels.
In 2019, the in vivo electrochemical impedance characteristics of nerve recording electrodes are evaluated at three different locations (epidural, subdural, and endovascular) [16]. The study compared impedance amplitudes and phase angles across various frequencies using EIS techniques after implanting the electrodes in sheep. It also investigated factors affecting electrode impedance and the electrode-tissue interface using a modified Randles circuit model. The results show that recorded brain signals decay as the distance of the recording electrode from the brain increases. Although the vasculature may affect the quality of the recorded signal, this effect is negligible compared to the impact of distance on signal quality. Thus, while impedance ranges are similar for the three electrodes' locations, intravascular electrodes demonstrate potential for long-term neuroprosthesis. Overall, we reviewed animal clinical trials in TABLE 1. These trials have demonstrated that EE are long-term, compatible with animal tissues, and less susceptible to the brain environment than subdural invasive neuroelectrodes, making them a promising candidate for human clinical trials.
Table 1. Animal Clinical Trials on Sheep.
Animal clinical trials (sheep) | Research Purpose | Results & Conclusions |
Nicholas et al., [11] | proof of feasibility and security | It may be possible to achieve reliable stimulation of specific brain areas, ensuring safety and reliability. |
Thomas et al., [10] | proof of feasibility and security | All surgical experiments were successful in accessing the SSS without associated complications, suggesting that the model under study will contribute to the development of EE implantation in the motor cortex. |
Nicholas et al., [14] | proof of feasibility and long-term use | During the 6-month implantation period, the maximum bandwidth of the fundamental frequency is stable, and no inflammatory response is caused. It proves EE can safely and effectively record long-term neural information, demonstrating the feasibility. |
Sam et al., [17] | proof of accuracy | The signal-to-noise ratio and bandwidth of EE showed no significant difference compared to traditional BCI, supporting the clinical application of EE in novel closed-loop neuromodulation techniques. |
Timothy et al., [12] | proof of accuracy | EEG and EE have almost the same effect. |
Sam et al., [16] | proof of long-term use | The position of the electrodes has little effect on the impedance of EE and can be permanently implanted in the sheep's brain. |
Nicholas et al., [15] | proof of long-term use | The use of endovascular electrodes in long-term neuroprosthesis demonstrates the potential. |
3. Clinical application in human
3.1. The StentrodeTM
StentrodeTM is a minimally invasive BCI that records brain signals from blood vessels [8]. It uses a stent as a scaffold to support the electrodes and is delivered through the jungle vein to the SSS without brain surgery. The device can sense or stimulate the activity of one or more neuronal cells. It also contains a microchip electrically connected to the electrode. The microchip includes a microprocessor, channel amplifier, digital signal converter and RF transmitter. Additionally, the device can be mounted on an expandable retainer, comprising a stent and includes an electronic system and external devices.
3.2. Human clinical research
Since the viability and safety of endovascular electrodes have been demonstrated in clinical trials, several studies have begun exploring their use in treating human diseases. In the first in-human experience, researchers used EE to help severely paralyzed patients perform daily tasks [18]. They designed a novel endovascular StentrodeTM BCI and implanted it in the SSS adjacent to the primary motor cortex of two participants with amyotrophic lateral sclerosis (ALS). The process of the experiment is shown in Fig.2. The external telemetry unit (ETU) powers the internal telemetry unit (ITU), which was placed within a subclavicular pocket. Participants received machine-learning-assisted training to manage different mouse-click motions like zoom and left-click, using an eye-tracker to control the cursor. Unsupervised home use began on day 86 for participant 1 and day 71 for participant 2. Participant 1 achieved an average click selection accuracy of 92.63% at a rate of 13.81 correct characters per minute (CCPM) without predictive text. Participant 2 achieved an average click selection accuracy of 93.18%. Both patients demonstrated the ability to manage daily tasks on the Windows 10 operating system.
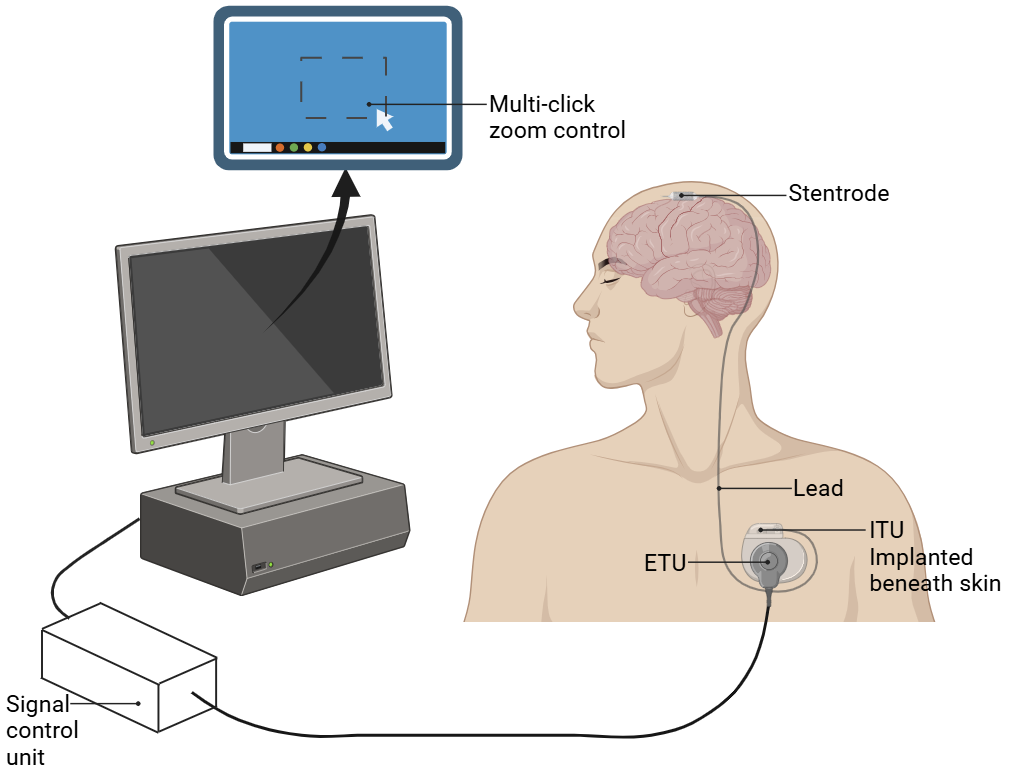
Figure 2. Endovascular motor neuroprosthesis system. Participants are trained to manage various mouse-click motions, using an eye-tracker to control the cursor.
Based on the above research, another team evaluated the safety of a fully implanted endovascular BCI in four patients with severe paralysis [19]. They assessed five patients with serious upper-limb paralysis over a 12-month trial period. The primary safety endpoint was serious adverse events related to the device, such as death or permanently worsened disability. Secondary endpoints included device migration and blood vessel blockage. Exploratory endpoints examined the number of unique commands produced by neural activity, signal quality and stability over 12 months, and system utilization for digital device control. The 4 patients analyzed were all male and their mean age was 61 years old, and all of them finished the 12-month follow-up without experiencing any major side effects, device migration or vessel blockage. For all four patients under investigation, the mean signal bandwidth was 233Hz and it remained constant throughout the study. Moreover, each patient effectively operated a computer with BCI and at least 5 different movement types were decoded offline. Overall, the existing clinical trials not only achieved the application of EE in humans but also demonstrated its long-term stability.
4. Limitations and Future prospects
4.1. Limitations
Preliminary animal experiments demonstrated the feasibility, accuracy and long-term use of StentrodeTM implantation in the animal brain [9-16], laying the groundwork for human clinical studies. Although the first in-human experiment and the assessment of long-term stability of StentrodeTM in human have been carried out in 2020 and 2022 [18,19], the number of human clinical experiments is not sufficient. Thus, to determine whether EE technology is safe and reliable when applied to humans, more human clinical trials are needed. They are also important for exploring more areas and ways of endovascular electrodes' application.
4.2. Future prospects
Endovascular electrodes technology, with its minimally invasive nature, and low surgical risk, may extend BCI technology to more application scenarios. The future directions can be summarized in two points: apply to more areas and be less invasive. Existing research in the two directions and analyses of their results were described in detail in the following text.
4.2.1. New direction: stimulate rather than collect signals
Traditional BCI technologies are used for collecting brain signals and trying to monitor patients' brain activities. Then the data would guide treatments or command neuroprosthesis. Deep Brain Stimulation (DBS) is a surgical intervention to modulate abnormal information processing within functional neural circuits. By giving out electrical stimulation in deep brain areas, DBS has been proven effective in treating movement disorders. However, traditional DBS is invasive and usually causes serious associated problems. EE technology with its minimally invasive nature, may be an alternative to DBS and opens new possibilities for BCI applications.
Clemens et al. conducted an experiment to examine whether EE stimulation can replace DBS in certain situations [20]. They overlaid probabilistic vascular atlases from 42 healthy subjects with neuroanatomical targets used during deep brain stimulation. After that, they decided on suitable vessels for implantation according to the distance and electrical field needed. The team identified 6 DBS targets sufficiently near a vessel to be an acceptable choice for endovascular DBS. That is a positive result revealing the potential of EE to effectively stimulate the target tissue. Due to its nature of reducing stimulation-induced side effects, EE may serve as a complementary technique in the DBS area.
4.2.2. Less-invasive exploration
One of the key advantages of EE technology is its minimally invasive nature, which is crucial for its further development. A novel micron-scale neuroelectronic interface platform technology has been developed in 2023 [9]. This approach uses the cerebral vascular system as a natural delivery system, enabling high-resolution neural signal recording and intervention. The researchers introduced a model of epilepsy in anesthetized mice by locally injecting penicillin. And they performed EEG recordings using probes, the process of the implantation surgery is shown in Fig.3. The researchers successfully observed bilateral spike and spike wave activity.
The study demonstrates that this new technology can be effectively used as a research tool and medical device for the detection and intervention of neurological diseases. It extends the application of EE to small, cost-effective animal models like mice, broadening the range of tools available for neuroengineering and neuroscience research. Additionally, this technique addresses the challenges of stiffness and bulkiness associated with conventional metal catheters and stents, reducing the risk of tissue damage and inflammation in micrometer-sized cerebral blood vessels.
Figure 3. Lateral (A) and overhead (B) figures of endovascular implantation surgery. The probes are inserted from internal carotid artery (ICA) to the bifurcation of middle cerebral artery (MCA) and anterior cerebral artery (ACA).
5. Conclusion
As a new technology in the field of BCIs, EE develop the treatment of neurological disorders and paralysis. This review has summarized the progress made in the application of EE in both animal models and human clinical trials. These studies highlight the safety and viability of EE, demonstrating its high-quality neural interface signals with low invasive nature to overcome the limitations of traditional BCI methods, such as scalp and cortical EEG. Furthermore, the development of relevant devices and techniques has enhanced the functionality and potential applications of EE, opening a way for minimally invasive treatments.
Reviewed studies proved that EE can interface with neural circuits, offering high-resolution neural signal recording and intervention capabilities, reducing the risks like surgical injury. As technology develops, the range of applications for EE is expected to expand, offering new treatment options for various neurological conditions. These improvements indicate a promising future for EE in the broader medical landscape, opening possibilities for more widespread and effective neurological interventions.
However, human clinical trials of this technology are not sufficient. To ensure the feasibility and safety and to explore more applications in humans of this technology, we need to conduct more human clinical trials.
In conclusion, endovascular electrodes represent a significant breakthrough in BCI technology. With more human trials to conduct in the future, EE are likely to show the potential to revolutionize the treatment of neural diseases and paralysis. The ongoing research and development in this field suggest that EE could become a cornerstone of future neurological therapies, offering safer, more effective, and less invasive options for patients. Thus, this review can indicate the future development direction of EE.
Acknowledgments
All the authors contributed equally to this work and should be considered as co-first authors.
References
[1]. B. Thielen et al., “Making a case for endovascular approaches for neural recording and stimulation,” J. Neural Eng., vol. 20, no. 1, p. 011001, Feb. 2023, doi: 10.1088/1741-2552/acb086.
[2]. K. Värbu, N. Muhammad, and Y. Muhammad, “Past, Present, and Future of EEG-Based BCI Applications,” Sensors, vol. 22, no. 9, 2022, doi: 10.3390/s22093331.
[3]. D. Khodagholy et al., “NeuroGrid: recording action potentials from the surface of the brain,” Nat. Neurosci., vol. 18, no. 2, pp. 310–315, Feb. 2015, doi: 10.1038/nn.3905.
[4]. E. Musk and Neuralink, “An Integrated Brain-Machine Interface Platform With Thousands of Channels,” J. Med. Internet Res., vol. 21, no. 10, p. e16194, Oct. 2019, doi: 10.2196/16194.
[5]. R. F. Kirsch, A. B. Ajiboye, and J. P. Miller, “The Reconnecting the Hand and Arm with Brain (ReHAB) Commentary on ‘An Integrated Brain-Machine Interface Platform With Thousands of Channels,’” J Med Internet Res, vol. 21, no. 10, p. e16339, 2019, doi: 10.2196/16339.
[6]. Q. He et al., “The brain nebula: minimally invasive brain–computer interface by endovascular neural recording and stimulation,” J. NeuroInterventional Surg., p. jnis-2023-021296, Feb. 2024, doi: 10.1136/jnis-2023-021296.
[7]. R. K. Sefcik, N. L. Opie, S. E. John, C. P. Kellner, J. Mocco, and T. J. Oxley, “The evolution of endovascular electroencephalography: historical perspective and future applications,” Neurosurg. Focus, vol. 40, no. 5, p. E7, May 2016, doi: 10.3171/2016.3.FOCUS15635.
[8]. Thomas James Oxley, “Sensing or Stimulating Ativity of Tissue,” US20140288667A1, Sep. 25, 2014
[9]. A. Zhang, E. T. Mandeville, L. Xu, C. M. Stary, E. H. Lo, and C. M. Lieber, “Ultraflexible endovascular probes for brain recording through micrometer-scale vasculature,” Science, vol. 381, no. 6655, pp. 306–312, Jul. 2023, doi: 10.1126/science.adh3916.
[10]. T. J. Oxley et al., “An ovine model of cerebral catheter venography for implantation of an endovascular neural interface,” J. Neurosurg., vol. 128, no. 4, pp. 1020–1027, Apr. 2018, doi: 10.3171/2016.11.JNS161754.
[11]. N. L. Opie et al., “Focal stimulation of the sheep motor cortex with a chronically implanted minimally invasive electrode array mounted on an endovascular stent,” Nat. Biomed. Eng., vol. 2, no. 12, pp. 907–914, Dec. 2018, doi: 10.1038/s41551-018-0321-z.
[12]. T. B. Mahoney, P.-C. Liu, D. B. Grayden, and S. E. John, “Comparison of Sub-Scalp EEG and Endovascular Stent-Electrode Array for Visual Evoked Potential Brain-Computer Interface,” in 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia: IEEE, Jul. 2023, pp. 1–4. doi: 10.1109/EMBC40787.2023.10340834.
[13]. V. S. Polikov, P. A. Tresco, and W. M. Reichert, “Response of brain tissue to chronically implanted neural electrodes,” J. Neurosci. Methods, vol. 148, no. 1, pp. 1–18, Oct. 2005, doi: 10.1016/j.jneumeth.2005.08.015.
[14]. N. L. Opie et al., “Feasibility of a chronic, minimally invasive endovascular neural interface,” in 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA: IEEE, Aug. 2016, pp. 4455–4458. doi: 10.1109/EMBC.2016.7591716.
[15]. N. L. Opie et al., “Chronic impedance spectroscopy of an endovascular stent-electrode array,” J. Neural Eng., vol. 13, no. 4, p. 046020, Aug. 2016, doi: 10.1088/1741-2560/13/4/046020.
[16]. S. E. John et al., “In Vivo Impedance Characterization of Cortical Recording Electrodes Shows Dependence on Electrode Location and Size,” IEEE Trans. Biomed. Eng., vol. 66, no. 3, pp. 675–681, Mar. 2019, doi: 10.1109/TBME.2018.2854623.
[17]. S. E. John et al., “Signal quality of simultaneously recorded endovascular, subdural and epidural signals are comparable,” Sci. Rep., vol. 8, no. 1, p. 8427, May 2018, doi: 10.1038/s41598-018-26457-7.
[18]. T. J. Oxley et al., “Motor neuroprosthesis implanted with neurointerventional surgery improves capacity for activities of daily living tasks in severe paralysis: first in-human experience,” J. NeuroInterventional Surg., vol. 13, no. 2, pp. 102–108, Feb. 2021, doi: 10.1136/neurintsurg-2020-016862.
[19]. P. Mitchell et al., “Assessment of Safety of a Fully Implanted Endovascular Brain-Computer Interface for Severe Paralysis in 4 Patients: The Stentrode With Thought-Controlled Digital Switch (SWITCH) Study,” JAMA Neurol., vol. 80, no. 3, p. 270, Mar. 2023, doi: 10.1001/jamaneurol.2022.4847.
[20]. C. Neudorfer et al., “Endovascular deep brain stimulation: Investigating the relationship between vascular structures and deep brain stimulation targets,” Brain Stimulat., vol. 13, no. 6, pp. 1668–1677, Nov. 2020, doi: 10.1016/j.brs.2020.09.016.
Cite this article
Zhang,Z.;Zhang,R.;Chen,Z.;Meng,J. (2025). A Review of the Clinical Application of Endovascular Electrodes and Its Future Prospects. Applied and Computational Engineering,132,27-35.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Machine Learning and Automation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. B. Thielen et al., “Making a case for endovascular approaches for neural recording and stimulation,” J. Neural Eng., vol. 20, no. 1, p. 011001, Feb. 2023, doi: 10.1088/1741-2552/acb086.
[2]. K. Värbu, N. Muhammad, and Y. Muhammad, “Past, Present, and Future of EEG-Based BCI Applications,” Sensors, vol. 22, no. 9, 2022, doi: 10.3390/s22093331.
[3]. D. Khodagholy et al., “NeuroGrid: recording action potentials from the surface of the brain,” Nat. Neurosci., vol. 18, no. 2, pp. 310–315, Feb. 2015, doi: 10.1038/nn.3905.
[4]. E. Musk and Neuralink, “An Integrated Brain-Machine Interface Platform With Thousands of Channels,” J. Med. Internet Res., vol. 21, no. 10, p. e16194, Oct. 2019, doi: 10.2196/16194.
[5]. R. F. Kirsch, A. B. Ajiboye, and J. P. Miller, “The Reconnecting the Hand and Arm with Brain (ReHAB) Commentary on ‘An Integrated Brain-Machine Interface Platform With Thousands of Channels,’” J Med Internet Res, vol. 21, no. 10, p. e16339, 2019, doi: 10.2196/16339.
[6]. Q. He et al., “The brain nebula: minimally invasive brain–computer interface by endovascular neural recording and stimulation,” J. NeuroInterventional Surg., p. jnis-2023-021296, Feb. 2024, doi: 10.1136/jnis-2023-021296.
[7]. R. K. Sefcik, N. L. Opie, S. E. John, C. P. Kellner, J. Mocco, and T. J. Oxley, “The evolution of endovascular electroencephalography: historical perspective and future applications,” Neurosurg. Focus, vol. 40, no. 5, p. E7, May 2016, doi: 10.3171/2016.3.FOCUS15635.
[8]. Thomas James Oxley, “Sensing or Stimulating Ativity of Tissue,” US20140288667A1, Sep. 25, 2014
[9]. A. Zhang, E. T. Mandeville, L. Xu, C. M. Stary, E. H. Lo, and C. M. Lieber, “Ultraflexible endovascular probes for brain recording through micrometer-scale vasculature,” Science, vol. 381, no. 6655, pp. 306–312, Jul. 2023, doi: 10.1126/science.adh3916.
[10]. T. J. Oxley et al., “An ovine model of cerebral catheter venography for implantation of an endovascular neural interface,” J. Neurosurg., vol. 128, no. 4, pp. 1020–1027, Apr. 2018, doi: 10.3171/2016.11.JNS161754.
[11]. N. L. Opie et al., “Focal stimulation of the sheep motor cortex with a chronically implanted minimally invasive electrode array mounted on an endovascular stent,” Nat. Biomed. Eng., vol. 2, no. 12, pp. 907–914, Dec. 2018, doi: 10.1038/s41551-018-0321-z.
[12]. T. B. Mahoney, P.-C. Liu, D. B. Grayden, and S. E. John, “Comparison of Sub-Scalp EEG and Endovascular Stent-Electrode Array for Visual Evoked Potential Brain-Computer Interface,” in 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia: IEEE, Jul. 2023, pp. 1–4. doi: 10.1109/EMBC40787.2023.10340834.
[13]. V. S. Polikov, P. A. Tresco, and W. M. Reichert, “Response of brain tissue to chronically implanted neural electrodes,” J. Neurosci. Methods, vol. 148, no. 1, pp. 1–18, Oct. 2005, doi: 10.1016/j.jneumeth.2005.08.015.
[14]. N. L. Opie et al., “Feasibility of a chronic, minimally invasive endovascular neural interface,” in 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA: IEEE, Aug. 2016, pp. 4455–4458. doi: 10.1109/EMBC.2016.7591716.
[15]. N. L. Opie et al., “Chronic impedance spectroscopy of an endovascular stent-electrode array,” J. Neural Eng., vol. 13, no. 4, p. 046020, Aug. 2016, doi: 10.1088/1741-2560/13/4/046020.
[16]. S. E. John et al., “In Vivo Impedance Characterization of Cortical Recording Electrodes Shows Dependence on Electrode Location and Size,” IEEE Trans. Biomed. Eng., vol. 66, no. 3, pp. 675–681, Mar. 2019, doi: 10.1109/TBME.2018.2854623.
[17]. S. E. John et al., “Signal quality of simultaneously recorded endovascular, subdural and epidural signals are comparable,” Sci. Rep., vol. 8, no. 1, p. 8427, May 2018, doi: 10.1038/s41598-018-26457-7.
[18]. T. J. Oxley et al., “Motor neuroprosthesis implanted with neurointerventional surgery improves capacity for activities of daily living tasks in severe paralysis: first in-human experience,” J. NeuroInterventional Surg., vol. 13, no. 2, pp. 102–108, Feb. 2021, doi: 10.1136/neurintsurg-2020-016862.
[19]. P. Mitchell et al., “Assessment of Safety of a Fully Implanted Endovascular Brain-Computer Interface for Severe Paralysis in 4 Patients: The Stentrode With Thought-Controlled Digital Switch (SWITCH) Study,” JAMA Neurol., vol. 80, no. 3, p. 270, Mar. 2023, doi: 10.1001/jamaneurol.2022.4847.
[20]. C. Neudorfer et al., “Endovascular deep brain stimulation: Investigating the relationship between vascular structures and deep brain stimulation targets,” Brain Stimulat., vol. 13, no. 6, pp. 1668–1677, Nov. 2020, doi: 10.1016/j.brs.2020.09.016.









