1. Introductory
With the rise of solar power, artificial intelligence, and new energy automobile fields, coating technology has been more and more widely used, especially in the past few years due to the rise of thin film substrate, optical film, reflective film, automotive decorative film, and a number of new functions of the film to polyethylene terephthalate (PET) film is the most widely used. [1]
Polyethylene terephthalate (PET) is a linear saturated polyester, which is synthesized by esterification or transesterification of purified terephthalic acid (PTA) or its derivative dimethyl terephthalate (DMT) with ethylene glycol (EG) to form ethylene terephthalate ethylene terephthalate (BHET) monomer, which is then subjected to a polycondensation reaction to obtain PET. [2]PET is commonly used in various fields of industry and lifedue toits excellent mechanical properties, good dimensional stability, resistance to abrasion, aging and fatigue[3]. However, due to the high electrical resistivity of PET, in 65% relative humidity, 25 ℃ under the condition of moisture absorption rate of only 0.4% Resistivity up to1014Ω-cm[4], anti-static performance is poor, very easy in the production and processing and transportation, use of the process of generating and accumulating static electricity, resulting in electrostatic dust absorption, static discharge and other undesirable phenomena, the situation is serious and even cause fires, explosions and other safety accidents[5],therefore Therefore, the static hazard is a major problem that should not be ignored in the application process of PET materials.
Based on this, this article focuses on the causes of static electricity in PET polyester molecules, and reviews and analyzes the advantages and disadvantages of various types of surface-active agent-based PET antistatic agents, as well as the methods of use and shortcomings.
2. Reasons for PET static electricity
2.1. Molecular structure causes electrons to be unable to move freely
PET polyester molecules are linked by covalent bonds and are unable to ionize and transfer electrons. Due to the lack of polar groups in the PET material, it does not conduct electricity efficiently, which prevents the electrons generated during friction from moving freely and being released. Normally, polyester exhibits electroneutrality, i.e., it has an equal amount of positive and negative charges, but due to mutual contact or friction during use, electrons leave one polyester surface to attach to the other polyester surface, resulting in the formation of excess positive and negative charges on the two polyester surfaces, respectively.
2.2. The molecular structure is more rigid
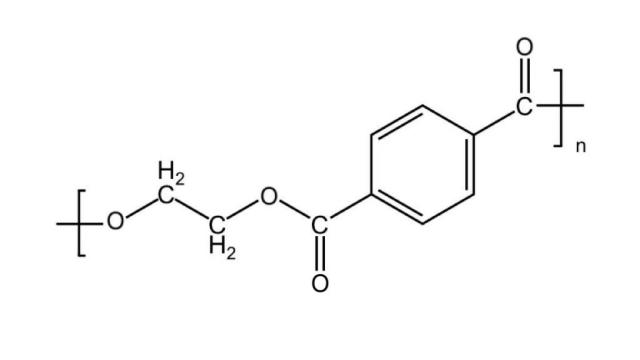
Figure 1: Molecular structure formula of PET molecule
The molecular structure formula of PET molecule is shown in Fig. (1), from which it can be seen that the repeating unit of PET molecular chain consists of three parts: the rigid benzene ring, the flexible methylene group and the polar ester group. [6] The benzenering structure is extremely rigid and exhibits a coplanar arrangement in the molecule, limiting the rotational freedom of the molecule, which, together with the combination of the ester group with the benzene ring, makes the movement of the entire chain segment even more restricted. Due to the rigidity of the PET molecular chain, the molecules are arranged more tightly, making its molecular structure dense, it is difficult for water molecules to penetrate or close to the molecular chain. Thisstructure results in a poor hydrophilicity of the PET polyester, which does not allow for the enhancement of surface conductivity through hygroscopicity。
2.3. High resistivity leads to static buildup
In standard environments, PET polyester has a surface resistance of up to1014Ω. When they come into contact with each other or rub together, electrons are transferred, creating an excess of positive and negative charges on the two polyesters, respectively. Due to the high resistivity of PET material, electrons cannot move freely and the charge accumulates on the surface of the material, forming an electrostatic charge.
3. Surface active agent type PET antistatic agent
3.1. Mechanism of surface-active agent type PET antistatic agent
Generally through the addition of antistatic agent to reduce the resistivity of PET, so that it can effectively dissipate static charges, thus avoiding the hazards caused by a large accumulation of static electricity. [7]One end of thesurfactantis a non-polar carbon-hydrogen bond, which has a very small affinity for water and is called a hydrophobic group; the other end is a polar group (e.g., -OH, -COOH,-NH2, etc.), which has a strong affinity for water, and is therefore called a hydrophilic group, and is always referred to as an amphiphilic molecule. As a surface-active agent type antistatic agent the mechanism of action is mainly hydrophilic-conductive mechanism[8],by increasing the hydrophilicity of PET polyester, so that it is able to absorb moisture and ionized substances in the air, which in turn increases its ionic conductivity .
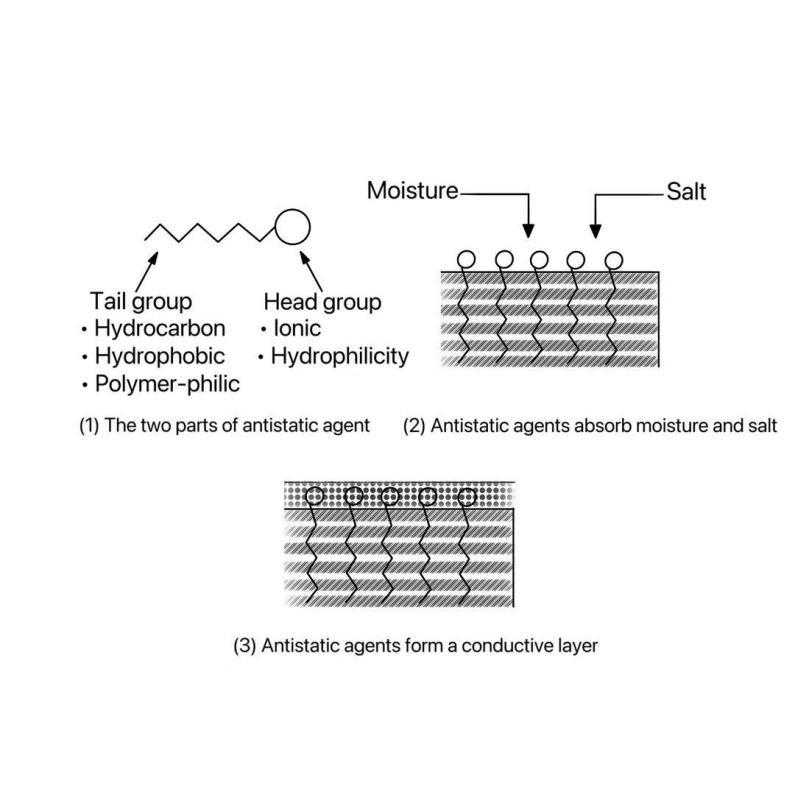
Figure 2: Hydrophilic-conducting mechanism diagram[9]
As shown in Figure (2) surface-active agent-type antistatic agent molecules in the hydrophobic groups are located in the polyester interior, and polyester to maintain a certain degree of compatibility; hydrophilic groups in the polyester and air contact interface directional arrangement, which can significantly improve the water absorption of polyester, its adsorption on the surface of the material can be ionized salts in the air or in the water molecules induced ionization, the surface of the polyester will be able to form a layer of conductive layer, able to conduct electricity through the ionic The polyester surface can form a conductive layer, which can release electrostatic charge in the form of ionic conductivity. The formation of this conductive layer significantly enhances the antistatic properties of polyester.
3.2. Classification of surface-active agent type PET antistatic agents
3.2.1. Cationic surfactants
The hydrophilic groups in the molecule are positively charged after ionization, and this type of antistatic agent usually contains compounds such as quaternary amine salts and quaternary salts, which have strong adhesion but poor stability, but Zhou Xiangdong [10] and othersfound that compounding the homemade polyurethane with the cationic antistatic agent KJD-1 to make an antistatic agent improves the antistatic effect of polyester fabrics and the washing resistance. Then after that, Yu Meiya [11] et al. found that in the application of blending and processing of cationic antistatic agent with PET matrix, appropriate amount of antioxidant can be added to inhibit the thermal and oxidative degradation of quaternary ammonium salts, so as to improve the thermal stability of antistatic agent.
3.2.2. Anionic surfactants
The hydrophilic groups in the molecule are negatively charged after ionization called anionic antistatic agents, the main components are sulfonate, phosphate compounds. Its heat resistance is excellent, and both lubrication and soft performance, but its compatibility with polyester is poor, the transparency of PET products have an impact. Yu Meiya [12] and othersfound that the anionic antistatic agent synthesized with the addition of sodium styrenesulfonate (SSS) monomer, in addition to enriching the hydrophilic groups of the antistatic agent to promote hygroscopicity and release of static charge, but also in the antistatic agent side chain endowed with a sulfonic acid group-metal-ionic bonding, which can be deconjugated to generate positive and negative ions to conduct electricity by ions, and thus improve the antistatic ability of the antistatic agent[13].
3.2.3. Amphoteric surfactants
The hydrophilic groups in the molecule are both positively and negatively charged after ionization and are called amphoteric electrostatic agents, which are mainly composed of monoalkyl fatty acids, polyol esters, and alkanol amide compounds. This kind of antistatic agent can play the role of both cationic and anionic role, its polymer material adhesion, heat resistance between cationic and anionic type, but its price is more expensive.
3.2.4. Nonionic surfactants
Hydrophilic groups in the molecule after ionization of the surfactant is called nonionic antistatic agent, its main component is quaternary ammonium compounds, antistatic effect is not as good as ionic antistatic agent, but has a strong adhesion, usually used for coating antistatic agent, and nonionic surfactants are generally non-toxic or low-toxicity, and can be used for food packaging materials, but its own charge, experiments are often combined with cationic or anionic surfactants to use better antistatic effect. However, it does not have its own charge, so in the experiment, it is often used in combination with cationic or anionic surfactants, and the antistatic effect is better. [14]
3.3. Use of surface-active agent type PET antistatic agents
Surface-active agent type antistatic agent is mainly used in two ways: one is the surface coating method that is coated by the method of antistatic agent coated on the surface of PET products, forming a layer of monomolecular layer to reduce the accumulation of static electricity; the second is the internal additive method that is added to the antistatic agent through the blending of the way directly to the PET polyester, antistatic agent in the use of the process of the material will be migrated to the surface, the formation of a layer of antistatic protective layer. The second is the internal addition method, in which the antistatic agent is added directly to the PET polyester by co-blending.
The advantage of coating type antistatic agent lies in the simple operation, wide range of use, antistatic effect is good, just add the surfactant to water, alcohols and other solvents according to a certain ratio, direct spraying, impregnation or brushing on the surface of the material, to be solvent evaporation, it will be able to form a layer of uniformity on the surface of the material protective layer, and at the same time, through the hydrophilic groups to enhance the material's moisture absorption, and to promote the leakage of electric charge. However, most of these antistatic agents are hydrophilic, resulting in the material in the wash or friction is easy to fall off, poor durability, antistatic effect will be with the use of time and friction times increase and weaken, so the coated antistatic agent is also known as temporary antistatic agent. [15]
The internal mixed antistatic agent, more in the polyester process, because the surfactant and PET polyester is not soluble, so it has a tendency to migrate to the surface of the polyester material, through the adsorption of water molecules in the air, the formation of a layer of water-slip layer on the surface of the antistatic layer, so as to reach the transfer of electric charge, reduce the surface resistivity of the purpose of the plastic. When the surface protection layer is damaged, the antistatic agent inside the material will penetrate and migrate to the surface, constantly replenishing the damaged antistatic layer on the surface, so that its antistatic effect can last for a long time. [16]
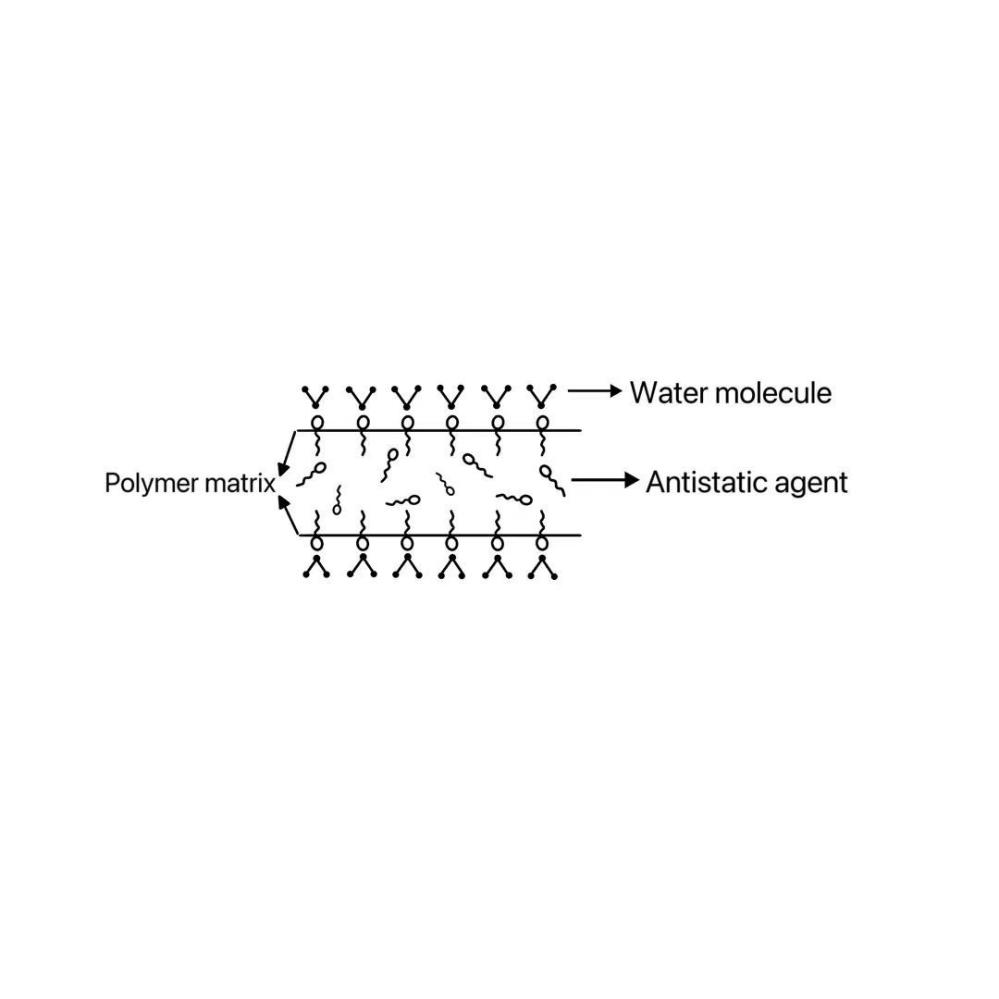
Figure 3: Mechanism of in-mixed surface-active agent type antistatic agents[17]
It is also known as permanent antistatic agent because of its better durability. When exploring the performance of cationic antistatic agents, Yu Meiya et al. used the internal addition method to prepare antistatic agent PET injection molding samples by blending cationic antistatic agents containing 14% quaternary ammonium salt with PET slices, and found that when the mass fraction of the blended cationic antistatic agent reaches 20%, the surface resistivity of injection molding samples is 5.58×1011Ω, which is a decrease of 4 orders of magnitude compared with that of PET and satisfy the antistatic requirement.
Xiaoru Wang et al[18] studiedthe effect of sodium polystyrene sulfonate on polyethylene terephthalate (PET) resin, when 2% sodium polystyrene sulfonate and 8% small molecule sulfonate were added for antistatic, the surface resistance of the specimen was reduced by three orders of magnitude to 109 Ω as compared to that of no sodium polystyrene sulfonate and it also improved the thermal stability and the crystallization temperature of the antistatic PET resin.
4. Advantages and shortcomings of surface-active agent-based PET antistatic agents
Surface-active agent type PET antistatic agents have the following advantages, which make them favored in the antistatic application of PET materials. First of all, this type of antistatic agent can significantly reduce the static buildup on the surface of PET materials, thus effectively reducing problems such as dust adsorption, static sparking and static damage, and enhancing the appearance and service life of the materials. Secondly, the surface-active agent type antistatic agent can be added in a flexible way, and can be added directly to PET resin for blending without complicated process. In addition, the surface-active agent type antistatic agent usually contains polar groups or ionic groups, these groups in the PET surface to form a thin and uniform conductive layer, so that the surface of the material has excellent antistatic and conductive, enhance the stability and durability of the antistatic effect. Because of its good thermal stability, it performs particularly well in applications such as textiles, packaging and electronics, which require long-term antistatic properties. Overall, surface-active agent-based PET antistatic agents are an efficient, economical and widely used antistatic material option.
However, combined with the method of use and the mechanism of action, the coated surfactant antistatic agent is greatly affected by the external environment, which is seriously dependent on the humidity of the environment, the humidity is too low will affect the arrangement of hydrophilic groups, is not conducive to the formation of conductive protective layer, and ultimately affects the antistatic properties[19],and its durability is poor, easy to come off in the process of use by water washing and friction.
For internal mixed surfactant antistatic agent, if the antistatic molecules and polyester compatibility is good, then when the antistatic molecular layer on the surface of PET polyester material is subjected to ring-breaking, the internal molecules migrate too slowly to the surface, and can not replenish the loss of antistatic agent in a timely manner; if the compatibility is poor, but also due to the antistatic molecules have a tendency to migrate to the surface, resulting in the migration of antistatic molecules is too fast, and the antistatic effect will be lost prematurely Loss. [20]
5. Reach a verdict
At present, in the field of surface-active agent type PET antistatic agent, although many types of antistatic agent and the use of methods have been developed, but a variety of surface-active agent type antistatic agent have their shortcomings exist, and many methods are still in the research stage. In the future, as PET materials become more widely used, our ultimate goal is to develop industrialized PET polyester products that combine good antistatic effects, low cost, and excellent mechanical and thermal stability. I think that in the surface-active agent type antistatic agent this single track, we can have some innovation and progress, in the original advantages of surfactants, try to gradually overcome its shortcomings, improve its durability and processing adaptability; at the same time should also focus on the green development, improve its environmental protection, and how to achieve this goal on the basis of the existing methods still need a lot of work.
References
[1]. Bai Mei, Qiu Shanghuang. Static safety prevention of PET film in coating production[J]. Modern Occupational Safety, 2020(07):89-90.
[2]. Zhang Da Province. Fundamentals of polyethylene terephthalate synthesis I. Synthesis of ethylene terephthalate[J]. Synthetic Fiber Industry, 2023,46(1) :43-48.
[3]. Li Xuelian, Xue Xueming, Fu Yongsheng. Preparation of novel antistatic PET fibers[J]. Synthetic Resins and Plastics, 2017,34(3):49-52.
[4]. ZHAO He-Ying. Synthesis and properties of antistatic PET resin[J]. Synthetic Resins and Plastics, 2003(02):1-5
[5]. LIU Y, LU S, LUO J, et al. Research progress of antistatic-reinforced polymer materials. A review[J]. Polymers for Advanced Technologies, 2023, 34(4): 1393-1404.
[6]. Levi. Research on the synthesis process and kinetics of PET/PEG co-polyester [D]. Shanghai: East China University of Science and Technology, 2020(01):1-73
[7]. SONG Xiangyi, WU Shuizhu, ZHAO Jianqing et al. Research progress of antistatic agents for PET[J]. Polyester Process, 2008 ,21(4):5-8
[8]. PIONTECK J,WYPYCH G.Antistatic agents and other components of formulation[A].Handbok of Antistatics. ChemTec Publishing,2016:141-147
[9]. ZHANG Tao, WANG Shuxia, SI Hu et al. Research progress of antistatic PET polyester[J]. Synthesis Technology and Application, 2024 ,39 (01):21-27
[10]. Zhou Xiangdong, Li Chunqing, Wu Jianguo et al. Development of end-capping aqueous polyurethane antistatic agent and antistatic agent finishing of polyester fabric[J]. Printing and dyeing auxiliaries,2003,20(3):15-18
[11]. Yu Meiya, Huang Wangsen, Mao Lijuan, et al. Synthesis of cationic antistatic agents and their properties[J]. Printing and dyeing,2023,49(11):63-67+73
[12]. Meiya Yu, Lijuan Mao, Jingjin Wu, et al. Synthesis of anionic antistatic agents and their properties[J]. Modern Textile Technology, 2023,31(02):177-184
[13]. SU Y F , YIN H, WANG X L, et al. Preparation and properties of ethylene-acrylate salt ionomer/polypropylene antistatic alloy[J]. Advanced Composites and Hybrid Materials, 2021, 4(1): 104-113
[14]. Zhang HW, Xia P, Ni ZB, et al. Preparation of antistatic polypropylene[J]. Guangzhou Chemical Industry, 2011,39(03):85-86
[15]. Han Zhuo-Shan; Guo Jia-Mei; Zhang Sheng-Wen; Dong Wei-Fu;, Progress of Antistatic Coating for Plastic Packaging, Plastic Packaging, 2022, 7
[16]. Markus C, Grob, Minder E. Permanent antistatic additives. new developments[J]. Plastics Additives & Compounding, 1999,1(3):20-26.
[17]. Zhengwu Xu, Jiamei Guo, Jun Lu, et al. Research progress of antistatic masterbatch for plastic packaging[J]. Plastic Packaging, 2020, 30(05):1-5
[18]. Wang, Xiaoru, Wu, Shuizhu, Zhao, Jianqing, et al. Effect of polystyrene sulfonate nanoparticles on the properties of antistatic PET resins[J]. Plastics Processing, 2008(04):20-22
[19]. Wang Fanshu, Zhou Lei, Bie Zhiwei, et al. Recent research progress of antistatic agents[J]. Plastics Science and Technology, 2013,41(12):85-90
[20]. Hu Lianjuan. Research on new antistatic materials[D]. Hunan. Hunan Normal University, 2015(05): 1-73
Cite this article
Wang,W. (2025). Research on Surface-active Agent Type PET Antistatic Agent. Applied and Computational Engineering,136,219-225.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Bai Mei, Qiu Shanghuang. Static safety prevention of PET film in coating production[J]. Modern Occupational Safety, 2020(07):89-90.
[2]. Zhang Da Province. Fundamentals of polyethylene terephthalate synthesis I. Synthesis of ethylene terephthalate[J]. Synthetic Fiber Industry, 2023,46(1) :43-48.
[3]. Li Xuelian, Xue Xueming, Fu Yongsheng. Preparation of novel antistatic PET fibers[J]. Synthetic Resins and Plastics, 2017,34(3):49-52.
[4]. ZHAO He-Ying. Synthesis and properties of antistatic PET resin[J]. Synthetic Resins and Plastics, 2003(02):1-5
[5]. LIU Y, LU S, LUO J, et al. Research progress of antistatic-reinforced polymer materials. A review[J]. Polymers for Advanced Technologies, 2023, 34(4): 1393-1404.
[6]. Levi. Research on the synthesis process and kinetics of PET/PEG co-polyester [D]. Shanghai: East China University of Science and Technology, 2020(01):1-73
[7]. SONG Xiangyi, WU Shuizhu, ZHAO Jianqing et al. Research progress of antistatic agents for PET[J]. Polyester Process, 2008 ,21(4):5-8
[8]. PIONTECK J,WYPYCH G.Antistatic agents and other components of formulation[A].Handbok of Antistatics. ChemTec Publishing,2016:141-147
[9]. ZHANG Tao, WANG Shuxia, SI Hu et al. Research progress of antistatic PET polyester[J]. Synthesis Technology and Application, 2024 ,39 (01):21-27
[10]. Zhou Xiangdong, Li Chunqing, Wu Jianguo et al. Development of end-capping aqueous polyurethane antistatic agent and antistatic agent finishing of polyester fabric[J]. Printing and dyeing auxiliaries,2003,20(3):15-18
[11]. Yu Meiya, Huang Wangsen, Mao Lijuan, et al. Synthesis of cationic antistatic agents and their properties[J]. Printing and dyeing,2023,49(11):63-67+73
[12]. Meiya Yu, Lijuan Mao, Jingjin Wu, et al. Synthesis of anionic antistatic agents and their properties[J]. Modern Textile Technology, 2023,31(02):177-184
[13]. SU Y F , YIN H, WANG X L, et al. Preparation and properties of ethylene-acrylate salt ionomer/polypropylene antistatic alloy[J]. Advanced Composites and Hybrid Materials, 2021, 4(1): 104-113
[14]. Zhang HW, Xia P, Ni ZB, et al. Preparation of antistatic polypropylene[J]. Guangzhou Chemical Industry, 2011,39(03):85-86
[15]. Han Zhuo-Shan; Guo Jia-Mei; Zhang Sheng-Wen; Dong Wei-Fu;, Progress of Antistatic Coating for Plastic Packaging, Plastic Packaging, 2022, 7
[16]. Markus C, Grob, Minder E. Permanent antistatic additives. new developments[J]. Plastics Additives & Compounding, 1999,1(3):20-26.
[17]. Zhengwu Xu, Jiamei Guo, Jun Lu, et al. Research progress of antistatic masterbatch for plastic packaging[J]. Plastic Packaging, 2020, 30(05):1-5
[18]. Wang, Xiaoru, Wu, Shuizhu, Zhao, Jianqing, et al. Effect of polystyrene sulfonate nanoparticles on the properties of antistatic PET resins[J]. Plastics Processing, 2008(04):20-22
[19]. Wang Fanshu, Zhou Lei, Bie Zhiwei, et al. Recent research progress of antistatic agents[J]. Plastics Science and Technology, 2013,41(12):85-90
[20]. Hu Lianjuan. Research on new antistatic materials[D]. Hunan. Hunan Normal University, 2015(05): 1-73









