1. Introduction
Under the global energy transition paradigm, conventional energy sources are confronting dual challenges of resource depletion and environmental constraints, while new energy development has became a crucial driving factor in reshaping the global energy architecture and advancing sustainable development. However, the transition from conventional to renewable energy systems necessitates breakthroughs in large-scale energy storage technologies to address critical challenges such as energy intermittency and grid stabilization. This urgent demand has catalyzed comprehensive investigations into sustainable and high-efficiency energy storage solutions [1].
Due to their high energy density and long cycle life, lithium-ion batteries (LIBs) dominate the markets of portable electronic products and electric vehicles. Nevertheless, Lithium ions are easy to form needle-like “lithium dendrites” on the surface of the anode, thus piercing the diaphragm and causing short circuits, coupled with the use of flammable organic electrolyte greatly reduces the safety of lithium batteries [2]. Aqueous zinc ion batteries (ZIBs) have received much attention as a new generation of promising green and safe batteries. Zinc is an abundant and low-cost element, and aqueous ZIBs offer a number of advantages, including high theoretical specific capacity and low redox potential, and excellent safety features due to the use of an aqueous electrolyte. However, ZIBs also face a number of challenges that hinder their commercialization, such as dendrite growth on the zinc anode, because dendrites may penetrate the diaphragm, leading to short circuits and shortening battery life. In addition, side reactions such as hydrogen precipitation and zinc corrosion, as well as dissolution of the cathode material, can lead to capacity degradation and poor cycle stability [3].
Hydrogel materials show great potential in addressing these challenges. Hydrogels are water-retentive three-dimensional porous mesh materials composed of natural or synthetic polymers that combine ionic conductivity and physical flexibility. In aqueous zinc ion batteries, hydrogel electrolytes play several key roles [4]. First, they improve the mass transfer kinetics of zinc ions. The polymer chains in the hydrogel are able to interact with the zinc ions, providing specific ion channels for their diffusion. This interaction helps to homogenize the flux of zinc ions, ensuring more uniform deposition on the anode and thus effectively inhibiting dendrite growth. For example, some hydrogels containing limited functional groups can combine with the zinc ions and direct their more orderly deposition [5,6]. Second, hydrogels can inhibit side reactions. The water-encapsulating properties of hydrogels reduce the activity of free water, which typically induce side reactions such as hydrogen precipitation and zinc corrosion. By immobilizing water, hydrogels create a more stable electrochemical environment, minimizing the possibility of these side reactions and improving the cycle life of ZIBs [7]. Third, hydrogels can strengthen the stability of cathode materials. They can form a protective layer on the surface of the cathode material, preventing it from dissolving during charge/discharge cycling. This protective mechanism helps maintain the structural integrity of the cathode, resulting in improved cycling performance and capacity retention. Despite these advantages, there are still some obstacles to the application of hydrogels in aqueous zinc ion batteries [8]. The optimization of hydrogel properties, such as further improvement of ionic conductivity and mechanical strength, functions such as self-healing and anti-freezing, and their multifunctional applications, remains a challenge.
This paper intends to present a complete and in - depth review of the research progress on flexible hydrogel polymer electrolyte materials for zinc ion batteries. We will first discuss the basic properties of hydrogels and their mechanism of action in aqueous zinc ion batteries. Then, we will review recent advances in the design and synthesis of hydrogel electrolytes and their impact on the performance of ZIBs. In addition, we will analyze the existing challenges and limitations in this field and propose potential strategies for future development. Through this review, we hope to provide valuable insights and guidance for the further development of high-performance ZIBs using hydrogel materials.
2. Zinc anode challenges
ZIBs have attracted much attention due to their advantages of high safety, low cost and green environment. However, the zinc anode suffers from zinc dendrites, hydrogen precipitation reaction, and corrosion passivation, which severely limit the performance and service life of the battery. These side reactions will not only reduce the utilization efficiency, but also lead to capacity decline and even pose safety hazards. The challenges facing anodes from these aspects are described in detail below.
2.1. Dendrites growth
While ZIBs are undergoing the cycling process, the problem of zinc anode dendrite growth severely limits their electrochemical performance and safety. In acidic or neutral electrolyte, Zn2+ is driven by the electric field to migrate toward the surface of the negative electrode during the progress of charging, and the surface defects (e.g., grain boundaries, dislocations) as the high-activity sites preferentially induce the nucleation of Zn2+, and the subsequent difference in ion diffusion rate triggers the uneven distribution of the local concentration gradient and the electric field, which in triggering a “tip effect” that leads to inhomogeneous Zn deposition and dendrite proliferation, particularly exacerbated at high current densities [9]. In the alkaline system, zinc exists in the form of complexed Zn(OH)42-, and its reduction needs to go through the desolvation and multi-step electron transfer process, the reaction kinetics hysteresis and the non-uniformity of zincate ion concentration distribution together lead to the destabilization of the deposition interface, accelerating the nucleation of dendritic crystals [10]. The continuous growth of dendrites may not only penetrate the diaphragm and cause internal short circuits, but also form “dead zinc” due to the weak bonding between the deposition layer and the substrate, which significantly reduces the Coulombic efficiency and cycling capacity. In addition, the porous structure of the dead zinc significantly increases the contact area between the active substance and the electrolyte, exacerbating side reactions (e.g., hydrogen precipitation, corrosion), leading to higher impedance at the electrode interface and a continuous decline in battery energy efficiency [11].
2.2. Hydrogen evolution reaction
In ZIBs, the hydrogen precipitation reaction (HER) at the zinc anode is another key side reaction. Thermodynamic analysis showed that the equilibrium potential of Zn2+/Zn (-0.76 V vs. SHE) was significantly lower than the equilibrium potential of H2O/H2 (0 V vs. SHE) in a mild electrolyte, and theoretically the HER should take precedence over the zinc deposition. However, limited by the low surface proton activity of the zinc electrode and the higher kinetic energy barrier of HER, the Zn2+ reduction reaction usually dominates the initial process. However, the HER continues to occur during long-term cycling: the anode comes into contact with free water in the bulk solution to trigger a reaction (2H2O + 2e- → H2↑ + 2OH-) [12]. In a closed cell system, the accumulation of H2 triggers an increase in internal pressure, leading to cell expansion or even rupture. In addition, OH- produced by HER will elevate the local pH at the interface, accelerating zinc anode corrosion and generating insulating by-products such as Zn4(OH)6SO4 and ZnO, which cover the electrode surfaces, increasing the interfacial polarization impedance and hindering ion transport, severely deteriorate the battery performance [13].
2.3. Corrosion and passivation
Corrosion of zinc metal in ZIBs is dominated by electrochemical corrosion accompanied by synergistic chemical corrosion. During charge-discharge cycling, electrochemical dissolution of the zinc anode occurs (Zn → Zn2+ + 2e-), whereas OH- from hydrogen precipitation reaction (HER) combines with Zn2+ to form products such as Zn(OH)2 and ZnO, and may further form complexes. The non-uniform deposition of such insoluble by-products on the electrode surface, on the one hand, covers the active sites and forms rough micro-convex structures, which induces local electric field distortion and exacerbates Zn dendrite nucleation [14]; on the other hand, its porous and loose characteristics fail to form a dense passivation layer similar to that of the solid electrolyte interphase (SEI) film formed in lithium-ion batteries, which results in the perpetuation of the corrosion reaction, along with the ongoing consumption of the electrodes and the electrolyte, and at the same time, it significantly elevating the interfacial impedance and destroying the long cycle stability of ZIBs. It is worth noting that in alkaline electrolyte, ZnO passivation layer is easily generated on the anode surface, but its high electronic impedance will hinder the bidirectional transmission of Zn2+ and electron, resulting in the “kinetic deactivation” on the surface of the electrodes, which leads to the attenuation of the discharge capacity and deterioration of the multiplicity performance. deterioration. In addition, localized rupture of the passivation layer exposes the fresh Zn surface, which further triggers self-corrosion and dendrite regeneration in a vicious circle [15].
3. Hydrogel for zinc anodes
In the pursuit of high-performance aqueous zinc ion batteries (ZIBs), hydrogel polymers have emerged as a promising class of materials for stabilizing zinc anodes. Their unique properties enable them to address key challenges facing zinc anodes, for instance, dendrite growth, side reactions and capacity degradation.
3.1. Reducing side reactions
Hydrogel polymers play a vital role in reducing the side reactions that plague the zinc anode in zinc ion batteries. One of the main side reactions is HER. Hydrogels can limit the contact of the zinc anode with free water molecules in the electrolyte. For example, some hydrogels with water inclusion properties can immobilize water and reduce the activity of free water molecules. As a result, the hydrogen precipitation reaction is effectively suppressed. Wang et al. [16] studied on water-poor hydrogel electrolytes for ZIBs which showed that water-poor hydrogel electrolytes can significantly reduce the rate of hydrogen precipitation during battery cycling.
In addition to the hydrogen precipitation reaction, zinc corrosion and passivation are also major problems. Hydrogels form a protective film on the surface of the zinc anode. This film prevents the zinc anode from reacting directly with the electrolyte, thus reducing electrochemical and chemical corrosion. For example, the study of Dong et al. [17] biomass-based hydrogel electrolytes used as natural stabilized cathodes for zinc ion batteries found that biomass-based hydrogel electrolytes can form a self-healing protective the surface of the zinc anode. This film not only reduced corrosion, but also minimized the formation of insulating by-products, which are common during zinc anode corrosion and passivation.
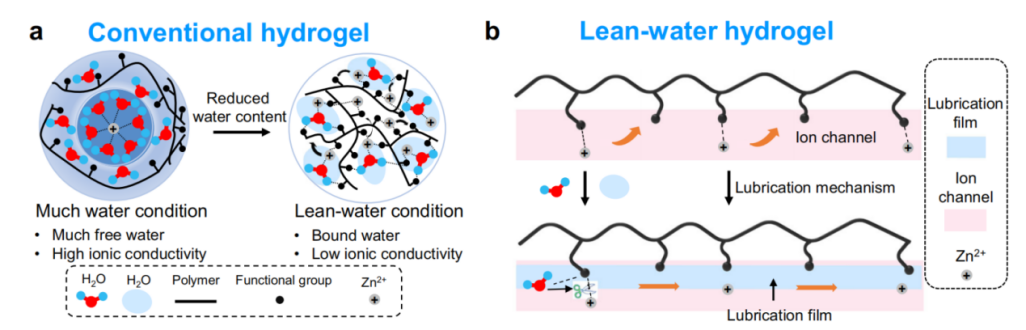
Figure 1: Ionic transport mechanism schematics of hydrogels. (a) ionic transport in regular hydrogel electrolytes under high and low water conditions (with restricted Zn²⁺). (b) anticipated ionic transport in low - water hydrogels: ion channel formation and lubrication effects
3.2. Stabilizing the cycling life of zinc anodes
Hydrogel polymers play a crucial role in stabilizing the cycle life of zinc anodes. They can regulate the deposition of zinc ions during charging and discharging. The polymer chains in the hydrogel can interact with Zn2+, providing specific ion channels for their diffusion. This interaction helps to homogenize the flux of zinc ions, ensuring a more uniform deposition on the anode. As a result, the dendrite growth is effectively suppressed. Chen et al. [18] introduced the ultra-high-modulus hydrogel electrolyte for dendrite-free ZIBs reported that the ultra-high-modulus hydrogel electrolyte can achieve dendrite-free zinc deposition even at high current densities. The unique structure of the hydrogel electrolyte guides the orderly deposition of Zn2+, preventing the formation of dendrites that could pierce the diaphragm and cause a short circuit.
In addition, the hydrogel strengthens the stability of the anode-electrolyte interface. By forming a stable interface, hydrogels reduce interfacial impedance and increase charge transfer efficiency. This leads to more stable cycling performance of zinc anodes. For example, Xu et al. [19] developed a superelastic hydrogel electrolyte containing functional protein molecules. These molecules acted as zinc ion channels, improving the stability of the anode-electrolyte interface and enhancing the cycling stability of ZIBs.
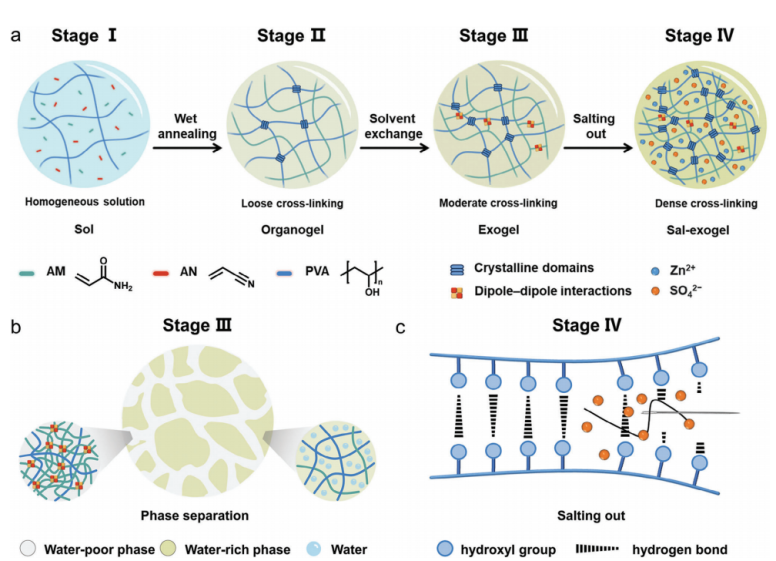
Figure 2: (a) Preparation of ultra-tough PVA hydrogel by combination of wet-annealing, solvent-exchange, and salting-out processes. (b) the interaction of the hydrophobic polymer network interpenetrating with the hydrophilic network leads to the formation of water-poor and water-rich phases. (c) formation of hydrogen bonds between the PVA chains because of the Hofmeister effect
3.3. Multifunctional hydrogel electrolytes
Multifunctional hydrogel electrolytes offer many advantages for stabilizing zinc anodes. Some hydrogels are self-healing, which is ideal for maintaining the integrity of the electrolyte and the anode-electrolyte interface.
3.3.1. Ultra-tough hydrogel electrolytes
In practical applications, ZIBs often faces external forces, for example extrusion, stretching, and bending, etc. Therefore, a hydrogel electrolyte with ultra-strong mechanical properties is essential to guarantee the structural integrity and long-term stable operation of batteries. Xu et al. [20] prepared an ultra-tough hydrogel electrolyte by constructing a multi-bonding network. This hydrogel formed a single network (PAA network) containing sparse covalent cross-links as well as two dynamic cross-links (conventional hydrogen bonding between PAA chains and quadruple hydrogen bonding in UPy dimers. Thanks to the high bonding energy of the UPy dimer, the dissociation of the UPy dimer can dissipate energy when the hydrogel is stretched, and the test demonstrates that the tensile strength of the hydrogel is up to 2.47 MPa at 75 wt% water content. In simulated real-world applications, UPyGel-10 (containing 10 mol% UPy monomer) was subjected to multiple load-unload tensile tests and showed excellent fatigue resistance without network collapse. The hydrogel also showed good resistance to alkaline conditions, maintaining its shape after swelling in 1 M KOH. At the same time, the ionic conductivity of the swollen hydrogel reached ~17 mS cm-1, and the conductivity remained unchanged when stretched to 500% strain. These properties enable the zinc anode to maintain good contact with the electrolyte when subjected to mechanical deformation and in alkaline environments.
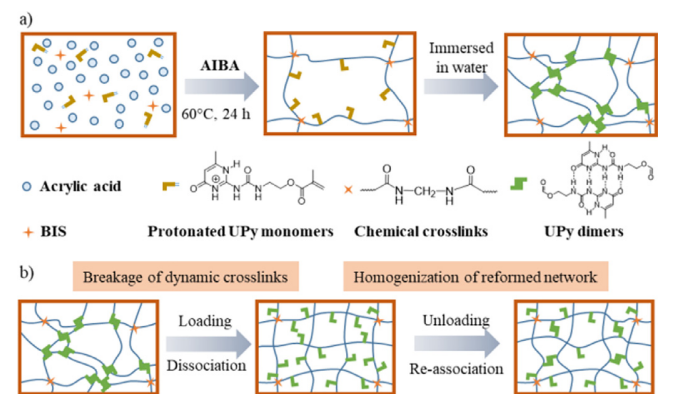
Figure 3: (a) Preparation routes of MBN hydrogels. (b) loading - unloading causes UPy dimers to split, then partially re - associate uniformly when unloaded, homogenizing the network
3.3.2. Self-healing hydrogel electrolytes
Self-healing hydrogel electrolytes (SHE) play a key role in maintaining the long-term stable operation of zinc-ion batteries, which are capable of repairing themselves upon damage. Ling et al. [21] synthesized a SHE by cross-linking polymerization and composite reinforcement. The dynamic hydrogen bonding generated by the hydroxyl groups on the main chain of this hydrogel polymer endowed the SHE with self-healing properties. Experimental results show that it can efficiently repair various types of damages. In zinc ion batteries, SHE quickly repairs damage even when the battery suffers pinprick-like damage during cycling. After 1500 cycles at the current density of 5.0 A g-1, the battery capacity retention rate reached 83.1%, which is much higher than that of conventional electrolytes. This indicates that the self-healing property can effectively maintain the stability of the zinc anode and significantly enhance the reliability of ZIBs.
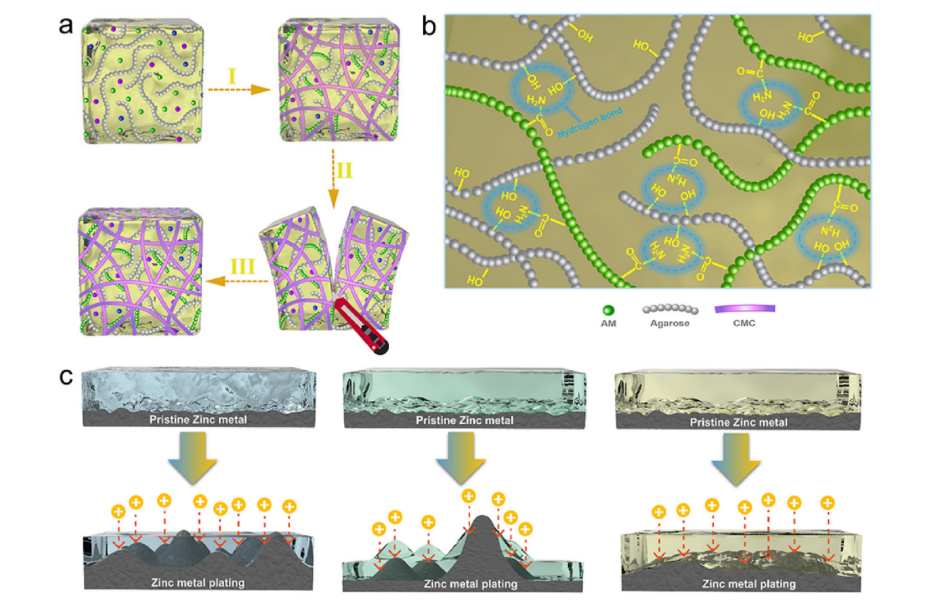
Figure 4: (a) SHEs' composition and self-healing illustration. (b) origin of self-healability (hydrogen bond). (c) schematic of Zn foil morphology evolution with LEs, pure PAMs, and SHEs in stripping/plating cycles
3.3.3. Anti -- freezing hydrogel electrolytes
In cold environments, conventional aqueous electrolytes are prone to freezing, which hinders ion transport and severely degrades battery performance. Hence, the advancement of antifreeze hydrogel electrolytes is crucial for expanding the application scope of ZIBs. Shi et al. [22] developed an antifreeze hydrogel electrolyte based on zinc tetrafluoroborate (Zn(BF4)2) and polyacrylamide (PAM). The strong electronegativity of the F atom in the BF4- anion enables it to coordinate with water molecules, break hydrogen bonds and inhibit dendrite growth. The strong electronegativity of the F atoms in the BF 4-anion allows it to interact with water molecules, replacing the hydrogen bonds between them and inhibiting the formation of ice crystals. At -70°C, this hydrogel electrolyte remains unfrozen and flexible with an ionic conductivity of 2.38 mS cm-1. Zinc ion batteries equipped with this electrolyte can operate stably at -70°C, showing enhanced electrochemical performance and high capacity retention, greatly expanding the application of ZIBs in cold regions.
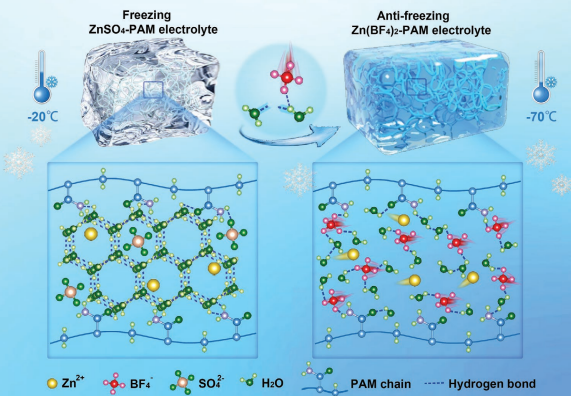
Figure 5: Schematic diagram and mechanism of electrolytes
3.3.4. Thermo-responsive hydrogel electrolytes
Thermo-responsive hydrogel electrolytes (TRHE) can automatically adjust their performance according to the change of ambient temperature, which provides the possibility of efficient operation of ZIBs under different temperature conditions. Meng et al. [23] constructed a TRHE by incorporating phase change chains with heat - absorbing properties into the agarose backbone via hydrogen - bonding interactions. When the temperature rises, the PEG in TRHE transforms from the crystalline state to the amorphous form and absorbs heat. This not only buffers the temperature rise of the cell, but also promotes ion transport due to the change in the hydrogel network structure. In practice, TRHE-equipped batteries maintain a certain capacity retention rate at high temperatures (e.g. 100°C) and ensure stable operation at low temperatures. Cycling tests show that the battery can maintain good performance within a wide temperature range, providing an effective solution for the stable operation of ZIBs in complex temperature environments.
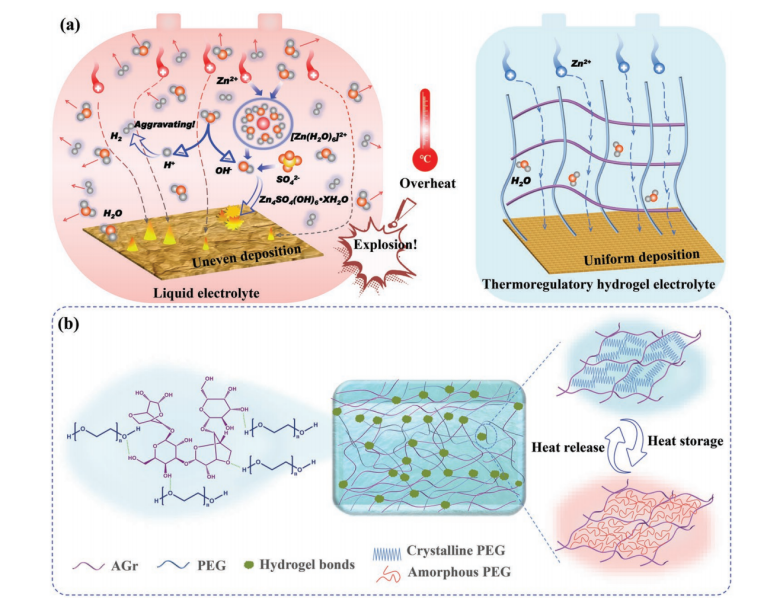
Figure 6: (a) Sketches showing thermal issues in liquid versus thermoregulatory hydrogel electrolytes. (b) structure and heat - regulating principle of the thermoregulatory hydrogel electrolyte
Overall, the unique properties of multifunctional hydrogel electrolytes, such as high ionic conductivity, mechanical durability, degradability, temperature adaptability, etc., bring a rich variety of solutions for stabilizing zinc anodes in ZIBs. These properties effectively overcome many of the problems faced by zinc anodes, such as improved charge transfer efficiency, enhanced structural stability of the battery, reduced environmental impact, and expanded temperature range of the battery. This series of advantages has greatly contributed to the advancement of ZIBs technology, allowing zinc ion batteries to show broad application prospects in many fields, such as wearable devices, large-scale energy storage, and extreme environmental applications, and is expected to evolve into one of the core technologies for energy storage in the future.
4. Summary and perspective
In conclusion, hydrogel polymer electrolytes show great potential in solving the zinc anode problem in ZIBs. It can regulate the zinc ion transport and make the zinc ion migration more orderly to improve the battery charging and discharging efficiency; it can also inhibit the side reactions for example hydrogen precipitation reaction and zinc corrosion to reduce the irreversible loss; and at the same time, it can form a protective barrier on the surface of the anode material to stabilize and prolong the ZIBs service life.
However, its application is currently facing many challenges, such as optimizing the hydrogel properties (improving ionic conductivity, enhancing mechanical strength, and conferring functions such as self-healing and anti-freezing), and improving the interfacial compatibility between the hydrogel and the electrodes so as to solve the problems of low charge transfer efficiency and poor interfacial stability. Looking ahead, the focus of scientific research should be on the development of new high-performance hydrogel materials. For one side, innovative synthesis methods and formulations are needed to enhance the stability of hydrogels to meet the needs of different scenarios and expand their multifunctional properties. On the other hand, the interfacial behavior of hydrogels and electrodes should be studied in depth to optimize the interfacial structure, enhance compatibility, and improve charge transfer efficiency and interfacial stability. After solving these problems, hydrogel polymer electrolytes will significantly improve the performance of ZIBs, enhance their competitiveness in the energy storage market, and facilitate ZIBs to move towards high efficiency and greenness, safety and sustainability.
References
[1]. Ding Y, Ling B, Zhao X, et al. Porous zinc metal anodes for aqueous zinc-ion batteries: Advances and prospectives[J]. Energy Materials and Devices, 2024, 2(3): 9370040.
[2]. Lu H, Hu J, Wang L, et al. Multi‐Component Crosslinked Hydrogel Electrolyte toward Dendrite‐Free Aqueous Zn Ion Batteries with High Temperature Adaptability[J]. Advanced Functional Materials, 2022, 32(19): 2112540.
[3]. Li L, Jia S, Yue S, et al. Hydrogel-stabilized zinc ion batteries: progress and outlook[J]. Green Chemistry, 2024, 26(11): 6404-6422.
[4]. Wang H, Riaz M S, Ali T, et al. Organo‐Hydrogel Electrolytes with Versatile Environmental Adaptation for Advanced Flexible Aqueous Energy Storage Devices[J]. Small Science, 2023, 3(5): 2200104.
[5]. Zhang H, Gan X, Gao Y, et al. Carboxylic Acid‐Functionalized Cellulose Hydrogel Electrolyte for Dual‐Interface Stabilization in Aqueous Zinc‐Organic Batteries[J]. Advanced Materials, 2025, 37(1): 2411997.
[6]. Leng K, Li G, Guo J, et al. A Safe Polyzwitterionic Hydrogel Electrolyte for Long‐Life Quasi‐Solid State Zinc Metal Batteries[J]. Advanced Functional Materials, 2020, 30(23): 2001317.
[7]. Ren H, Li S, Wang B, et al. Mapping the design of electrolyte additive for stabilizing zinc anode in aqueous zinc ion batteries[J]. Energy Storage Materials, 2024, 68: 103364.
[8]. Tian H, Yao M, Guo Y, et al. Hydrogel Electrolyte with Regulated Water Activity and Hydrogen Bond Network for Ultra‐Stable Zinc Electrode[J]. Advanced Energy Materials, 2025, 15(9): 2403683.
[9]. Verma V, Kumar S, Manalastas W, et al. Undesired Reactions in Aqueous Rechargeable Zinc Ion Batteries[J]. ACS Energy Letters, 2021, 6(5): 1773-1785.
[10]. Yang J, Yin B, Sun Y, et al. Zinc Anode for Mild Aqueous Zinc-Ion Batteries: Challenges, Strategies, and Perspectives[J]. Nano-Micro Letters, 2022, 14(1): 42.
[11]. Zhao X, Liang X, Li Y, et al. Challenges and design strategies for high performance aqueous zinc ion batteries[J]. Energy Storage Materials, 2021, 42: 533-569.
[12]. Wang J, Yang Y, Zhang Y, et al. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries[J]. Energy Storage Materials, 2021, 35: 19-46.
[13]. Wen Q, Fu H, Cui R de, et al. Recent advances in interfacial modification of zinc anode for aqueous rechargeable zinc ion batteries[J]. Journal of Energy Chemistry, 2023, 83: 287-303.
[14]. Du W, Ang E H, Yang Y, et al. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries[J]. Energy & Environmental Science, 2020, 13(10): 3330-3360.
[15]. Zhu C, Li P, Xu G, et al. Recent progress and challenges of Zn anode modification materials in aqueous Zn-ion batteries[J]. Coordination Chemistry Reviews, 2023, 485: 215142.
[16]. Wang Y, Li Q, Hong H, et al. Lean-water hydrogel electrolyte for zinc ion batteries[J]. Nature Communications, 2023, 14(1): 3890.
[17]. Dong H, Li J, Zhao S, et al. Investigation of a Biomass Hydrogel Electrolyte Naturally Stabilizing Cathodes for Zinc-Ion Batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(1): 745-754.
[18]. Chen Z, Shen T, Xiao X, et al. An Ultrahigh‐Modulus Hydrogel Electrolyte for Dendrite‐Free Zinc Ion Batteries[J]. Advanced Materials, 2024, 36(52): 2413268.
[19]. Xu X, Li S, Yang S, et al. Superelastic hydrogel electrolyte incorporating helical protein molecules as zinc ion transport pathways to enhance the cycling stability of zinc metal batteries[J]. Energy & Environmental Science, 2024, 17(20): 7919-7931.
[20]. Xu H, Liu Y, Xie X M. Stretchable alkaline quasi-solid-state electrolytes created by super-tough, fatigue-resistant and alkali-resistant multi-bond network hydrogels[J]. Chinese Chemical Letters, 2023, 34(4): 107470.
[21]. Ling W, Mo F, Wang J, et al. Self-healable hydrogel electrolyte for dendrite-free and self-healable zinc-based aqueous batteries[J]. Materials Today Physics, 2021, 20: 100458.
[22]. Shi Y, Wang R, Bi S, et al. An Anti‐Freezing Hydrogel Electrolyte for Flexible Zinc‐Ion Batteries Operating at −70 °C[J]. Advanced Functional Materials, 2023, 33(24): 2214546.
[23]. Meng Y, Zhang L, Peng M, et al. Developing Thermoregulatory Hydrogel Electrolyte to Overcome Thermal Runaway in Zinc‐Ion Batteries[J]. Advanced Functional Materials, 2022, 32(46): 2206653.
Cite this article
Li,S. (2025). The Progress in Advanced Hydrogel Polymer Electrolytes for ZIBs. Applied and Computational Engineering,144,204-212.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ding Y, Ling B, Zhao X, et al. Porous zinc metal anodes for aqueous zinc-ion batteries: Advances and prospectives[J]. Energy Materials and Devices, 2024, 2(3): 9370040.
[2]. Lu H, Hu J, Wang L, et al. Multi‐Component Crosslinked Hydrogel Electrolyte toward Dendrite‐Free Aqueous Zn Ion Batteries with High Temperature Adaptability[J]. Advanced Functional Materials, 2022, 32(19): 2112540.
[3]. Li L, Jia S, Yue S, et al. Hydrogel-stabilized zinc ion batteries: progress and outlook[J]. Green Chemistry, 2024, 26(11): 6404-6422.
[4]. Wang H, Riaz M S, Ali T, et al. Organo‐Hydrogel Electrolytes with Versatile Environmental Adaptation for Advanced Flexible Aqueous Energy Storage Devices[J]. Small Science, 2023, 3(5): 2200104.
[5]. Zhang H, Gan X, Gao Y, et al. Carboxylic Acid‐Functionalized Cellulose Hydrogel Electrolyte for Dual‐Interface Stabilization in Aqueous Zinc‐Organic Batteries[J]. Advanced Materials, 2025, 37(1): 2411997.
[6]. Leng K, Li G, Guo J, et al. A Safe Polyzwitterionic Hydrogel Electrolyte for Long‐Life Quasi‐Solid State Zinc Metal Batteries[J]. Advanced Functional Materials, 2020, 30(23): 2001317.
[7]. Ren H, Li S, Wang B, et al. Mapping the design of electrolyte additive for stabilizing zinc anode in aqueous zinc ion batteries[J]. Energy Storage Materials, 2024, 68: 103364.
[8]. Tian H, Yao M, Guo Y, et al. Hydrogel Electrolyte with Regulated Water Activity and Hydrogen Bond Network for Ultra‐Stable Zinc Electrode[J]. Advanced Energy Materials, 2025, 15(9): 2403683.
[9]. Verma V, Kumar S, Manalastas W, et al. Undesired Reactions in Aqueous Rechargeable Zinc Ion Batteries[J]. ACS Energy Letters, 2021, 6(5): 1773-1785.
[10]. Yang J, Yin B, Sun Y, et al. Zinc Anode for Mild Aqueous Zinc-Ion Batteries: Challenges, Strategies, and Perspectives[J]. Nano-Micro Letters, 2022, 14(1): 42.
[11]. Zhao X, Liang X, Li Y, et al. Challenges and design strategies for high performance aqueous zinc ion batteries[J]. Energy Storage Materials, 2021, 42: 533-569.
[12]. Wang J, Yang Y, Zhang Y, et al. Strategies towards the challenges of zinc metal anode in rechargeable aqueous zinc ion batteries[J]. Energy Storage Materials, 2021, 35: 19-46.
[13]. Wen Q, Fu H, Cui R de, et al. Recent advances in interfacial modification of zinc anode for aqueous rechargeable zinc ion batteries[J]. Journal of Energy Chemistry, 2023, 83: 287-303.
[14]. Du W, Ang E H, Yang Y, et al. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries[J]. Energy & Environmental Science, 2020, 13(10): 3330-3360.
[15]. Zhu C, Li P, Xu G, et al. Recent progress and challenges of Zn anode modification materials in aqueous Zn-ion batteries[J]. Coordination Chemistry Reviews, 2023, 485: 215142.
[16]. Wang Y, Li Q, Hong H, et al. Lean-water hydrogel electrolyte for zinc ion batteries[J]. Nature Communications, 2023, 14(1): 3890.
[17]. Dong H, Li J, Zhao S, et al. Investigation of a Biomass Hydrogel Electrolyte Naturally Stabilizing Cathodes for Zinc-Ion Batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(1): 745-754.
[18]. Chen Z, Shen T, Xiao X, et al. An Ultrahigh‐Modulus Hydrogel Electrolyte for Dendrite‐Free Zinc Ion Batteries[J]. Advanced Materials, 2024, 36(52): 2413268.
[19]. Xu X, Li S, Yang S, et al. Superelastic hydrogel electrolyte incorporating helical protein molecules as zinc ion transport pathways to enhance the cycling stability of zinc metal batteries[J]. Energy & Environmental Science, 2024, 17(20): 7919-7931.
[20]. Xu H, Liu Y, Xie X M. Stretchable alkaline quasi-solid-state electrolytes created by super-tough, fatigue-resistant and alkali-resistant multi-bond network hydrogels[J]. Chinese Chemical Letters, 2023, 34(4): 107470.
[21]. Ling W, Mo F, Wang J, et al. Self-healable hydrogel electrolyte for dendrite-free and self-healable zinc-based aqueous batteries[J]. Materials Today Physics, 2021, 20: 100458.
[22]. Shi Y, Wang R, Bi S, et al. An Anti‐Freezing Hydrogel Electrolyte for Flexible Zinc‐Ion Batteries Operating at −70 °C[J]. Advanced Functional Materials, 2023, 33(24): 2214546.
[23]. Meng Y, Zhang L, Peng M, et al. Developing Thermoregulatory Hydrogel Electrolyte to Overcome Thermal Runaway in Zinc‐Ion Batteries[J]. Advanced Functional Materials, 2022, 32(46): 2206653.









