1. Introduction
In the present era, many underdeveloped areas in China still use fossil fuels as the main part of the energy consumption structure, which increases the burden on environmental protection, and people urgently need to find green and environmentally friendly alternative fuels [1]. Ammonia is often seen as an alternative because of its high energy density and good hydrogen content. At the same time, novel nitrogen reduction reactions (NRR) are gaining popularity as a novel and environmentally friendly ammonia production process [2].
Among the types of nitrogen reduction reaction, electrocatalytic nitrogen reduction reaction is different from the traditional H-B method, using a new catalyst, under mild conditions can achieve high efficiency catalysis [3]. It has the advantages of lower cost, larger chemical space and sustainable proton source. Only by finding a suitable high-efficiency electrocatalyst can the large-scale production of NH3 in the electrochemical industry become possible [4].
In recent years, with regard to the development of electrocatalysts, researchers have proposed a series of strategies to improve their performance, so that the theoretical framework of the electronic structure of catalysts has been systematically optimized, and the study of electron spin for regulating the activity of catalysts has been further developed [5]. As a new field of modern chemistry, spin chemistry is a new field. It is related to the electron and nuclear spins and their behavior in chemical reactions. It is based on the basic principle that chemical reactions are only allowed for products whose total electron spin is the same as the spin state of the reagent, and in the reactions, often exhibit very high selectivity [6].
In this paper, the research progress of electrocatalytic nitrogen reduction reaction (NRR) is summarized firstly, and then the regulation mode of electron spin effect in electrocatalytic reaction is systematically introduced. The potential and path of spin effect in improving the activity and stability of electrocatalyst are discussed. Finally, the possible development direction of the electron spin effect in the electrocatalytic nitrogen reduction reaction is proposed, which provides theoretical support for the research and development of new electrocatalysts.
2. The research progress of electrocatalytic nitrogen reduction reaction (NRR)
To date, the Haber–Bosch process remains the dominant industrial method for ammonia synthesis, but its high temperature (500 °C) and pressure (15–30 MPa) requirements result in significant energy consumption and CO₂ emissions. To address these issues, alternative ammonia production methods have emerged, with electrochemical nitrogen fixation considered one of the most promising. Although this approach faces challenges such as competing hydrogen evolution reaction (HER) and the low solubility of N₂, it remains a viable green strategy for sustainable ammonia synthesis [7]. Electrocatalytic nitrogen reduction enables ammonia production under mild conditions, representing a major advancement in green chemistry. However, the development of efficient catalysts that function effectively in such environments remains difficult [8]. In recent years, proton ceramic electrolysis cells (PCEC) have been evaluated for ammonia synthesis, and Vieri et al. have studied transition metal-based electrocatalysts, identifying key influencing factors [9]. Electrocatalysis is particularly attractive due to its combination of catalytic and electrochemical advantages, and unlike the Haber–Bosch process, it can proceed in aqueous electrolytes, ionic liquids, or solid electrolytes [10]. In aqueous systems, temperature and pressure significantly affect performance. Tranchida et al. showed that operating a pressurized H-cell at 5 bar and 75 °C in a water-based electrolyte yielded 6.73 μg h⁻¹ cm⁻² of NH₃—over five times higher than under ambient conditions—demonstrating the benefits of moderate operating conditions [11]. Despite ongoing research, Faradaic efficiency and ammonia yield remain limited due to N₂–catalyst–H₂O competition and insufficient three-phase interfaces [2]. While Pt and other precious metals exhibit strong HER activity, their high cost motivates the search for more economical green alternatives [12]. Recent progress includes an anion exchange strategy using Ru/RuO₂ embedded in N/S Co porous carbon proposed by Samad et al. [13], and the identification of C₂N-based transition metal clusters (e.g., Mn₃-C₂N, Co₃-C₂N) by Xiao et al., which can suppress the potential favorable to HER [14].
3. Electron spin principle and regulation method
3.1. The physical nature and production mechanism of spin
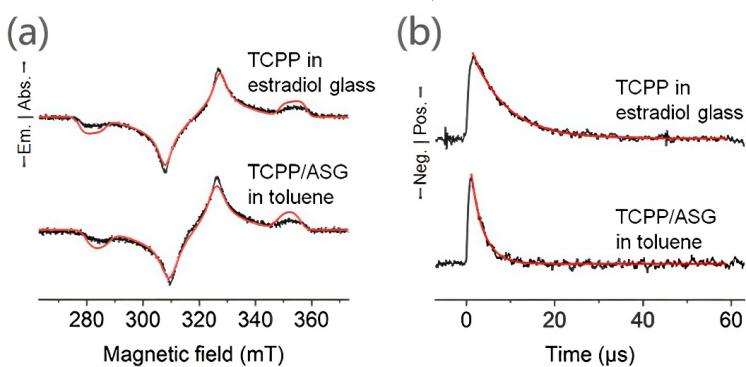
Figure 1: (a)Tr-ESR spectra of 1 mM TCPP in estradiol glass (top)and 1 mM TCPP/ASG in toluene(bottom)at room temperature(296 K). The simulation results by using EasySpin in MATLAB areshown (red lines). (b) Decays of the ESR signal of 1 mM TCPP inestradiol glass at 327 mT(top) and TCPP/ASG in toluene at 327 mT(bottom)at room temperature. Single-exponential fitting results are also shown (red lines)[15]
Table 1: Zero-Field Splitting Parameters and Relative Zero Field Populations Derived from the Simulation of the Tr ESR Spectra of 1 mM TCPP in Estradiol Glass and 1 mM TCPP/ASG in Toluene Shown in Figure 1a [15]
|D|(MHz) | |E|(MHz) | Px | Py | Pz | |
TCPP in estradiol glass | 1130 | 195 | 0.21 | 0.75 | 0.04 |
TCPP/ASG in toluene | 1062 | 198 | 0.20 | 0.76 | 0.04 |
Electron spin generation lies at the core of quantum materials and is influenced by various external conditions [15]. Transient non-equilibrium polarized electron spins can be generated via photoexcitation even at room temperature. Yabuki et al. investigated triplet electron spin polarization at the solid–liquid interface and found that although spin generation and transfer are feasible, the low cross-relaxation rate presents a major limitation. They utilized time-resolved electron spin resonance (Tr ESR) to analyze TCPP triplet spin polarization on ASG surfaces in toluene at 296 K. The ESR spectra showed typical porphyrin triplet features, with zero-field splitting parameters and polarization ratios comparable to those in estradiol glass (Table 1, Figure 1a). Notably, the spin-lattice relaxation time of the TCPP triplet on the ASG surface was 2.8 μs, longer than in solution but shorter than in estradiol glass (7.7 μs), indicating partial molecular mobility at the solid–liquid interface (Figure 1b). These results suggest that such interfaces can support the formation of highly polarized triplet states that interact with liquid-phase radicals through spin exchange.In addition to interfacial effects, external macroscopic conditions can significantly influence electron spin dynamics and lifetime [16]. For instance, boron-doped graphene nanoribbons provide a tunable platform for localized magnetic states. Boto et al. studied the correlation between spin relaxation time and dopant location in graphene nanofragments. By combining magnetic Redfield theory and computational modeling, they demonstrated that certain configurations exhibit relaxation times on the millisecond scale. Further analysis revealed that spin decoherence is mainly driven by fluctuations in spin–orbit coupling, while thermal motion enhances hyperfine interactions. These findings highlight a strong interconnection among relaxation time, spin–orbit effects, and magnetic coherence, offering promising directions for designing organic spintronic materials with long-lived spin states.
3.2. Spin regulation methods
With the gradual development and application of electrocatalyst, people have gradually realized its internal working principle. Electron spin is beginning to receive more and more attention from researchers, and the structure of electron spin systems has practical implications for many applications, including dynamic nuclear polarization (DNP), enhanced nuclear magnetic resonance (NMR), generation of electron spin qubits for quantum information science (QIS), and production of electron spin qubits. And the quantitative study of paramagnetic systems by electron paramagnetic resonance (EPR)[17]. Therefore, in order to understand the unique role of electron spin in electrocatalysts, it is necessary to have a preliminary understanding of the spin regulation methods.
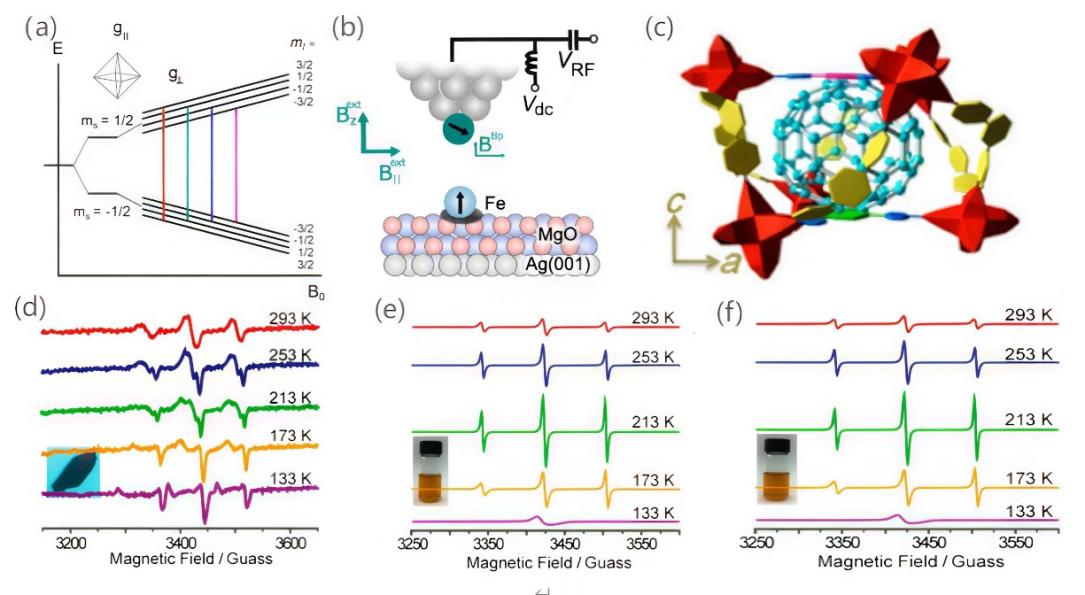
Figure 2: (a)Simulated EPR spectra of hyperfine coupled with spin S = 1/2 open-shell matter (local axisymmetric) and nuclear spin I = 3/2 in an octahedral environment[18] (b) Electron spin resonance in a scanning tunneling microscope with a vectormagnet[19]. (c) Schematic drawing of the inclusion of Y2@C79N inside the cavity of MOF-177. Temperature-dependent ESR spectra of Y2@C79N⊂MOF 177 single crystal,(d) Y2@C79NinCS2 solution (e) and Y2@C79N powders (f) at temperatures of 293, 253, 213, 173, and 133 K [20]
First of all, understand the specific environment of the electron spin, and it can be determined according to specific circumstances. For example, in EPR, the "fingerprint" of a particular unpaired electron spin environment takes the form of a g tensor, which depends on the oxidation state, coordination geometry, and spin-orbit coupling properties of the paramagnetic substance under consideration. Figure.2a shows a simulated EPR spectrum corresponding to an octahedral environment with spin S = 1/2 open-shell matter (local axisymmetrical) and a hyperfine coupled to a nuclear spin I = 3/2 [18]. This shows that the width of the EPR signal line is affected by the interaction with the spin of the adjacent electron.
The generation of spin polarization is closely related to the magnetic properties of substances. In recent years, the combination of electron spin resonance (ESR) and scanning tunneling microscopy (STM) has proven to be a technique for detecting the magnetic properties of individual atoms on a surface and achieving energy resolution for observing submicroelectron volts [19]. Willke et al. performed a single-atom ESR on a single Fe atom (Figure.2b) adsorbed to magnesium oxide (MgO) by using a two-dimensional vector magnetic field (Bz ext =6T, B∥ext =5T) and the local field of the magnetic STM tip in a commercially available STM. Show how the ESR amplitude can be greatly increased by optimizing the magnetic field, in particular revealing the enhanced signal under larger in-plane magnetic fields. This strongly demonstrates that ESR properties can be adjusted by combining vector magnets and pinhead fields, and provides a possible scheme for eventually achieving single-atom ESR with only pinhead fields.
It has also been found that the spin direction or state can be affected from the simplest external conditions, such as temperature, the physical state of matter, and so on[20]. Feng et al reported a solid spin system based on paramagnetic metal fullerenes by embedding paramagnetic metal fullerenes Y2@C79N(Figure 2c) into a cage hole in a metal-organic framework (MOF-177). However, when temperature dependent ESR spectroscopy was performed to detect the host-guest complex in different existing forms such as single crystal, solution, powder, etc., it was found that no matter what kind of physical state existed, when the temperature was from 299K to 253 K, Y2@C79N⊂MOF-177 begins to show an obvious anisotropic ESR peak from the original state, that is, the intensity of the ESR line increases particularly at a high field (Figure.2d, e, f). It can also be seen from many of the figures that the object behaves differently in the temperature-dependent ESR spectral image due to the different physical state. This shows that different physical states of the same substance will lead to changes in spin. The anisotropy shown in Y2@C79N can be attributed to changes in the interaction between spin angular momentum and orbital angular momentum at low temperatures.
3.3. Application of spin regulation
It is well established that chemical reactions are primarily governed by two fundamental parameters: the energy of reactants (including free energy and activation energy) and angular momentum, particularly electron spin. The conservation of total angular momentum is a widely accepted principle, leading to spin selectivity in chemical reactions—only reactants with spin states matching those of the products are chemically active. Thus, electron spin regulation plays a vital role in chemical reactions, either directly participating or functioning via catalytic pathways [21]. In spin-catalyzed reactions, magnetic field effects and changes in angular momentum can significantly influence reaction rates [22]. Hughes et al. demonstrated that applying a magnetic field affects the catalytic behavior of high- and low-spin Co (II) complexes differently. In the oxidation of 2,6-dimethylphenol to 2,6-dimethyl-1,4-benzoquinone, the relative reaction rates varied depending on the spin state of the Co (II) complex (Figure.1). The spin multiplicity of transition metal ions alters net angular momentum changes, determining whether the magnetic field accelerates or inhibits the reaction. These results provide a new approach to studying spin effects at catalytic sites and suggest that the differences between high- and low-spin Co (II) catalysts can be interpreted via magnetodynamic theory. Beyond fundamental research, electron spin also finds application in detection technologies, such as electron spin resonance (ESR) and electron spin echo modulation (ESEM) spectroscopy. For instance, in zeolite systems involving Ag⁺ exchange, ESR helps characterize the local environment of silver atoms, which is critical for understanding their catalytic role. ESEM further complements ESR by resolving weak hyperfine and quadrupole interactions that conventional ESR cannot detect[23]. These techniques enhance spin-resolved detection efficiency while reducing the labor and cost of future measurements.
4. Regulation mechanism of electron spin in NRR
With the further study of electron spin, we have a clearer understanding of its role in the electrocatalysis process. Therefore, applying the mechanism of electron spin regulation in catalytic NRR can help us make more progress in producing more efficient and environmentally friendly green electrocatalytic nitrogen reduction catalysts. Therefore, it is necessary to study the regulation mechanism of spin in NRR.
4.1. Limiting factors of NRR development
Electrochemical nitrogen fixation, conducted under mild conditions and powered by clean, renewable energy sources such as wind and hydropower, is considered a promising alternative to the traditional Haber–Bosch process. Unlike its conventional counterpart, it eliminates greenhouse gas emissions. However, limited catalytic efficiency remains a key bottleneck. Although precious metal-based electrocatalysts have shown potential in ammonia synthesis, their high cost and low Faradaic efficiency hinder large-scale application. Density functional theory has identified iron-based surfaces as viable candidates, and emerging materials—including single-atom catalysts, metal oxides, and porous composites—have begun to fill the gap in high-performance NRR systems. Despite these advancements, two fundamental challenges persist. First, the nonpolar nature and strong N≡N triple bond of nitrogen demand catalysts with high activity and stability to enable efficient activation under mild conditions. Second, the competing hydrogen evolution reaction (HER) on catalyst surfaces reduces ammonia yield and Faradaic efficiency by consuming shared protons and electrons [24]. Lowering the intrinsic activation barrier of N₂ remains difficult, and HER competition is primarily attributed to water serving as a proton donor in the NRR system. To address these issues, several strategies have been proposed. These include increasing alkali metal ion concentrations to restrict proton/electron mobility, constructing hydrophobic interfacial layers, and optimizing temperature and pressure to thermodynamically suppress HER. Additionally, rational catalyst design—particularly atomically dispersed systems with intrinsic HER-inhibiting characteristics—has emerged as a promising direction [25]. These approaches currently guide the development of electrocatalysts toward more efficient and selective nitrogen reduction.
4.2. Influence of spin states on active sites
In order to improve the performance of electrocatalytic nitrogen reduction reaction (NRR), the local environment for N2 adsorption of efficient nanocatellites was improved by promoting N2 adsorption and selecting suitable catalysts to provide favorable active sites, so as to achieve atomic-level accurate regulation of electronic properties[26].
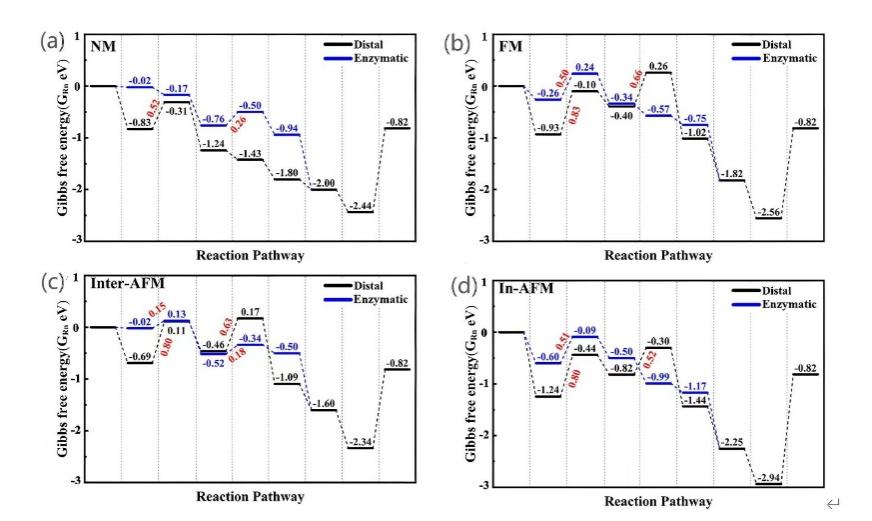
Figure 3: Gibbs free energy for electrocatalytic nitrogen reduction on Cr2CO2 surfaces with (a) NM, (b) FM, (c) Inter-AFM, and (d) In-AFM magnetic states[27]
As for the optimization of the performance of electrocatalytic NRR, it is also through magnetic regulation to change the coordination environment to achieve the regulation of polarization intensity and spin state, and improve the activity of the reaction. Solving the low adsorbability of N2 at the active site on the catalyst and the high bond energy of N≡N triple bond are the key problems to improve the performance of the catalyst[27]. In the study of Li et al., they explored the activation degree of Cr2CO2 in different magnetic states to reveal the different eNRR activity between different magnetic states, and carried out a detailed analysis of the charge, orbit and spin of N2. Figure.3 shows the Gibbs free energy barrier used to reduce N2 molecules on the surface of Cr2CO2 with different magnetic orders. While the increase of the potential barrier is an important factor in protonation hydrogenation and the generation of N2. For Cr2CO2 in the NM state, the decisive step of the distal mechanism is the first protonation process of N2 with a limiting potential of 0.52 V(Figure.3a), and in the subsequent reaction process, the limiting potential is only 0.26 V. In the FM and in-AFM magnetic states, N2 reduction on the surface of Cr2CO2 requires a higher limiting potential (Figure.3b, c). However, in the Inter-AFM magnetic sequence, the eNRR activity of Cr2CO2 was very high, and the limiting potential of the enzyme mechanism was only 0.18V. Comparing the limiting potential of N2 reduction on Cr2CO2 surface with different magnetic sequences, it can be found that the magnetic sequence has a significant effect on N2 molecular activation.
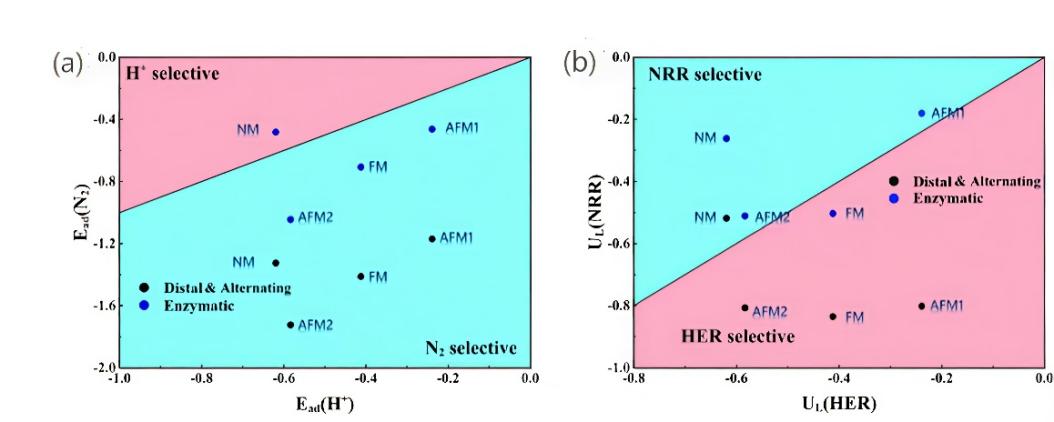
Figure 4: (a) Adsorption energy of N2 and H+ for selective screening. (b) Calculated potential vs. SHE for HER (U*H) and NRR (U*NNH) for selectivity screening [27]
However, in the first progenation process from *N2 to *NNH under acidic conditions, due to the adsorption competition between H at the reaction site and N2, the exposed Cr at the active site may participate more in HER reaction, which is unfavorable to the NRR process. By comparing the potential of the first protonation (U*NNH) on N2, the protonation of hydrogen at the Cr atomic site (U*H) and the adsorption energies of N2 and H. It was found that in terms of adsorption energy, N2 was more likely to be adsorbed on Cr2CO2 than H (except N2 would be adsorbed on NM-level Cr2CO2), indicating that the catalytic site on the surface of Cr2CO2 was not poisoned by H (Figure.4a). In addition, by comparing UL (NRR) and UL (HER), it was found that the NRR selection of Cr2CO2 catalysts with NM, Inter-AFM and in-AFM magnetic states was superior to HER (Figure.4b). The proposal of this study indicates that the problem of less effective active sites caused by the competition between N2 and HER with low adsorbability can be fundamentally solved by changing the magnetic order of matter electrons, and provides a new idea for the development of catalysts.
4.3. The effect of spin regulation on catalytic stability
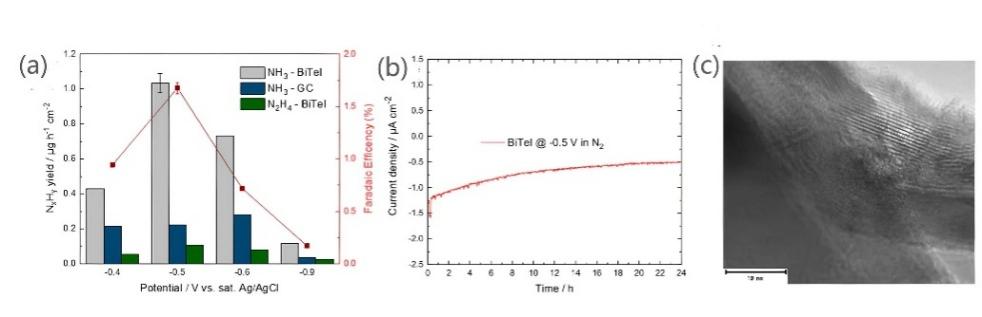
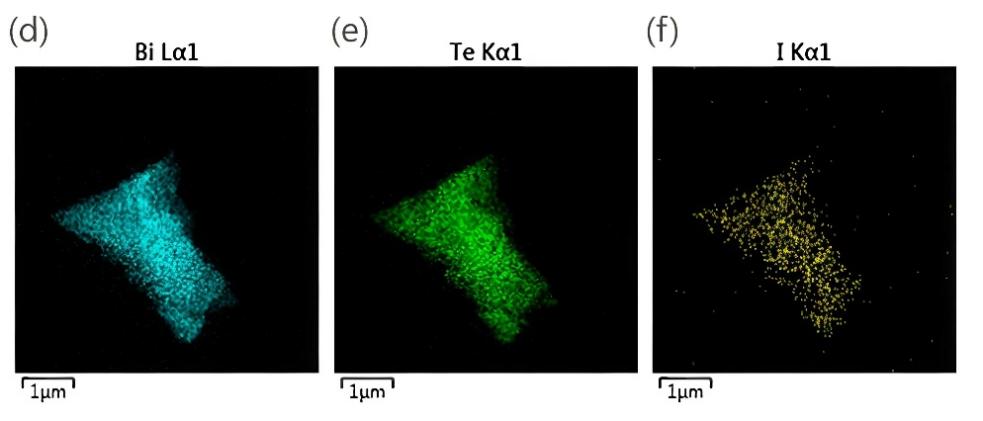
Figure 5: (a)NH3 and N2H4 generation rates of stripped BiTeI at different potentials and the corresponding NRR Faraday efficiency. The blue bar chart shows the NH3 production rate of the bare GC electrode for comparison.(b) Long term chronoamperometry graph of exfoliated BiTeI electrode over the period of 24 h indicating good stability.(c) HR-TEM image of exfoliated BiTeI nanosheets after the 24 h stability test.(d-f) the corresponding elemental maps of bismuth, tellurium, and iodine of exfoliated BiTeI nanosheets after the 24 h stability test.[28]
Among many new electrocatalysts, it is found that semiconductor metal-based 2D materials such as bismuth nanosheets can be used as catalysts with good performance. Antonatos et al. performed catalytic NRR tests on BiTeI sheets in the same electrolyte of H cells at potentials of -0.3 and -0.6 V (before the reduction peak) and -0.9 V (after the reduction peak), and compared the NRR performance with bare GC electrodes. It was found that the highest ammonia yield was achieved at −0.5 V, as shown in Figure 5a, with a production rate of 1.04 ± 0.05 μgh-1 cm-2, corresponding to a Faraday efficiency of 1.67 ± 0.10%, and a turnover frequency of 96.3h-1. At the same time, the stability of the material in the NRR was tested within 24 hours, where the potential remained stable at -0.5V and the electrolyte was changed in a short period of time without changing the electrode or membrane. No significant loss of current density was recorded within 1 day (Figure.5b). Moreover, the measured HR-TEM images of BiTeI nanosheets showed that the nanosheets still maintained the original hexagonal structure (Figure.5c) and the ideal distribution of elements throughout the material (Figure.5d), indicating that the BiTeI nanosheets had high stability in nitrogen electrocatalytic reduction.
The reason why BiTeI nanosheets show high stability and high catalytic efficiency in the electrocatalytic nitrogen reduction reaction is that bismuth atoms in BITEI nanosheets can cause strong spin-orbit interaction (SOI), and the structures that usually lack inversion symmetry tend to show strong SOI. BiTeI has a large Rashba splitting, and its inversion asymmetry induces SOI, which leads to spin splitting of electronic states. This phenomenon is called the Rashba effect, and it causes bands with opposite spin polarization to shift in the opposite direction along the momentum kR, and also depends on the size of the atom. The present study explains how to achieve high stability of electrocatalysts in the electrocatalytic nitrogen reduction reaction from the perspective of spin effect, and provides theoretical guidance for the design of high stability catalysts[28].
5. Summary and outlook
Spin chemistry is an important frontier in catalysis, offering a pathway to enhance electrocatalyst performance by leveraging spin-regulated reaction mechanisms. In electrocatalytic nitrogen reduction reaction (NRR), regulating the spin state of catalysts or modifying the adsorption behavior of active sites can optimize the reaction pathway and accelerate the development of efficient, green ammonia synthesis processes. This review outlines recent theoretical and experimental advances in spin-regulated NRR, including novel electrocatalysts and anion exchange strategies based on heteroatom-doped porous carbon. It further summarizes electron spin generation mechanisms, regulation strategies, and influencing factors. The g-tensor is recognized as a critical parameter reflecting spin-specific environments. Applications of spin effects are also discussed in reaction mechanism studies and detection technologies such as ESR and ESEM. The role of electron spin in electrochemical nitrogen fixation is reviewed from two key perspectives:
(1) Regulating active sites in NRR. Due to the inertness and high bond energy of nonpolar N₂ molecules, identifying effective active sites remains a major bottleneck in industrial NRR. Spin regulation, particularly through the application of external magnetic fields, can alter the surface electronic states of catalysts and enhance N₂ adsorption. This strategy facilitates the development of multi-site catalysts with improved selectivity and more precise binding between the reactants and the active sites.
(2) Expanding to other reactions. As an intrinsic electron property, spin also affects reactions such as HER, ORR, and CO₂RR. Understanding spin regulation mechanisms across systems could provide broader insight into reaction kinetics and catalyst design.
Although spin-regulation research is still in its early stages, it shows strong potential for advancing both fundamental understanding and practical applications in electrocatalysis.
References
[1]. Zhong S. and Shi M.(2025), "Evaluation and Obstacle Factors of Renewable Energy Substitution Potential in Underdeveloped Rural Areas of China," Sustainability, 17(3),1315.
[2]. Moon Y. H., Kim N. Y., Kim S. M., and Jang Y. J.,(2022) "Recent Advances in Electrochemical Nitrogen Reduction Reaction to Ammonia from the Catalyst to the System," Catalysts, 12(9),1015.
[3]. Sun J.,Kong W., Jin W.,Han Y.,Ma L.Ding X., Niu Y. and Xu Y..(2020), "Recent advances of MXene as promising catalysts for electrochemical nitrogen reduction reaction," Chinese Chemical Letters, 31(4),953-956
[4]. Xu C.,Su H.,Zhao S.,Nilghaz A.,Tang K.,Ma L. and Zou Z. (2025), "Electrocatalytic and Photocatalytic N2 Fixation Using Carbon Catalysts," Nanomaterials, 15(1),0065.
[5]. Sun S. Zhang Y.,Shi X.,Sun W.,Felser C.,Li W. and Li G. (2024), From Charge to Spin An In‐Depth Exploration of Electron Transfer in Energy, Advanced Materials 12(5),0024
[6]. Buchachenko A. L. and Berdinsky V. L.(1996), "Spin Catalysis of Chemical Reactions," The Journal of Physical Chemistry, 100(47), 18292-18299
[7]. Rasheed H. U.,Kim J. H.,Kiim T.,Lee K.,Shim J.,Kim S. H. and Yoon H. C. (2024), "Electrochemical Ammonia Synthesis from Dilute Gaseous Nitric Oxide Reduction at Ambient Conditions," Catalysts, 14(11),0838.
[8]. Iqbal A., Skúlason E., and Abghoui Y.(2024), "Understanding the Mechanistic Pathways of N2 Reduction to Ammonia on (110) Facets of Transition Metal Carbides," Crystals, 14(9),0770.
[9]. Vieri H. M.,. Kim M.-C, Badakhsh A., and Choi S. H.(2024), "Electrochemical Synthesis of Ammonia via Nitrogen Reduction and Oxygen Evolution Reactions—A Comprehensive Review on Electrolyte-Supported Cells," Energies, 17(2),0441.
[10]. Kuznetsova I., Lebedeva O., Kultin D., Mashkin M., Kalmykov K., and Kustov L.(2024), "Enhancing Efficiency of Nitrate Reduction to Ammonia by Fe and Co Nanoparticle-Based Bimetallic Electrocatalyst," International Journal of Molecular Sciences, 25(13),7089.
[11]. Tranchida G., Milazzo R. G., Lombardo S. A., and Privitera S. M. S.(2024), "Combined Effect of Pressure and Temperature on Nitrogen Reduction Reaction in Water," Energies, 17(12),2963.
[12]. Sharma R. K.,Patel H.,Mushtaq U.,Kyriakou V.,Zafeiropoulos G.,Peeters F.,Welzel S.,(2021), "Plasma Activated Electrochemical Ammonia Synthesis from Nitrogen and Water," ACS Energy Letters, 6(2),313-319
[13]. S. A. Samad.Ye X.,Han Z.,Huang S.,Lu C.,Hou J.,Yang M.,Zhang Z.,Qiu F. and Zhuang X. (2025), "Anion-Exchange Strategy for Ru/RuO2-Embedded N/S-Co-Doped Porous Carbon Composites for Electrochemical Nitrogen Fixation," Polymers, 17(4),0543.
[14]. Xiao S., Zhang D., Wang G., Zhou T., and Wang N.,(2024) "Density Functional Theory Study of Triple Transition Metal Cluster Anchored on the C2N Monolayer for Nitrogen Reduction Reactions," Molecules, 29(14),3314.
[15]. Yabuki R., Nishimura K., Hamachi T., Matsumoto N., and Yanai N.(2023), "Generation and Transfer of Triplet Electron Spin Polarization at the Solid–Liquid Interface," The Journal of Physical Chemistry Letters,14(20),4754-4759
[16]. Boto R. A., Cebreiro-Gallardo A., Menchón R. E., and Casanova D.(2024), "Electron–Spin Relaxation in Boron-Doped Graphene Nanoribbons," Journal of Chemical Theory and Computation,20(22),9906-9916,
[17]. Equbal A., Ramanathan C., and Han S.(2024), "Dipolar Order Induced Electron Spin Hyperpolarization," The Journal of Physical Chemistry Letters, 15(20), 5397-5406,
[18]. Nguyen H. and Clément R. J.(2020), "Rechargeable Batteries from the Perspective of the Electron Spin," ACS Energy Letters, 05(12), 3848-3859
[19]. Willke P. ,Singha A.,Zhang X.,Esat T.,P.Lutz C.,Heinrich A. J. and Choi T.(2019), "Tuning Single-Atom Electron Spin Resonance in a Vector Magnetic Field," Nano Letters, 19(11), 8201-8206
[20]. Feng Y. ,Wang T.,Li Y.,Li J.,Wu J.,Wu B.,Jiang L. and Wang C. (2015), "Steering Metallofullerene Electron Spin in Porous Metal–Organic Framework," Journal of the American Chemical Society,137(47),15055-15060
[21]. Buchachenko A. L. and Berdinsky V. L.(2002), "Electron Spin Catalysis," Chemical Reviews, 102(3), 603-612
[22]. Hughes M. E. and Corden B. B.(1989), "Magnetic field effects in cobalt(II)-catalyzed oxidations: the role of electron spin angular momentum," Journal of the American Chemical Society, 111(11),4110-4111
[23]. Narayana M. and Kevan L.(1985), "Role of magnesium(2+) ion in modifying the environment of radiolytically produced silver atoms in A zeolites: electron spin resonance and electron spin echo modulation spectroscopy studies," Journal of the American Chemical Society, 107(16),4643-4647
[24]. Majumder M.,Saini H.,Dedek I.,Schneemann A.,Chodankar N. R.,Ramarao V.,Santosh M. S.,Nanjundan A. K.,Kment S.,Dubal D.,Otyepka M. ,Zbořil R. and Jayaramulu K.(2021), "Rational Design of Graphene Derivatives for Electrochemical Reduction of Nitrogen to Ammonia," ACS Nano,15(11), 17275-17298
[25]. Hu J.,Zou H.,Li F.,Wei S.,Cheng M.,Dai H.,Song T. and Duan L.(2023), "Review on Electrochemical Reduction of Nitrogen by Graphdiyne-Based Catalysts: Recent Advances and Outlook," Energy & Fuels,37(5), 3501-3522
[26]. Tan W. ,Zhao H.,Ding L.,Ren N.,Wang A.and Zhao M.(2025), "Advancements in Electrocatalytic Nitrogen Reduction Reaction: A Review on the Role of Catalyst Electronic Structure and Design Strategies," ACS Applied Nano Materials, 8(6),2632-2651
[27]. Li N.,Zhang Z.,Wang Z.,Liu B.,Zhou D.,Zhou X.,Zhang P. and Zhao X.(2024), "Novel magneto-electrocatalyst Cr2CO2-MXene for boosting nitrogen reduction to ammonia," Materials Horizons, 11(7),1769-1778
[28]. Antonatos N.,Kovalska E., Mazánek V., Veselý M., Sedmidubský D.,Wu B. and Sofer Z.(2021), "Electrochemical Exfoliation of Janus-like BiTeI Nanosheets for Electrocatalytic Nitrogen Reduction," ACS Applied Nano Materials,4(1),590-599
Cite this article
Wei,B. (2025). Electron Spin in Electrocatalytic Nitrogen Reduction Reactions from Mechanistic Understanding to Catalyst Design. Applied and Computational Engineering,155,181-190.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-FMCE 2025 Symposium: Semantic Communication for Media Compression and Transmission
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zhong S. and Shi M.(2025), "Evaluation and Obstacle Factors of Renewable Energy Substitution Potential in Underdeveloped Rural Areas of China," Sustainability, 17(3),1315.
[2]. Moon Y. H., Kim N. Y., Kim S. M., and Jang Y. J.,(2022) "Recent Advances in Electrochemical Nitrogen Reduction Reaction to Ammonia from the Catalyst to the System," Catalysts, 12(9),1015.
[3]. Sun J.,Kong W., Jin W.,Han Y.,Ma L.Ding X., Niu Y. and Xu Y..(2020), "Recent advances of MXene as promising catalysts for electrochemical nitrogen reduction reaction," Chinese Chemical Letters, 31(4),953-956
[4]. Xu C.,Su H.,Zhao S.,Nilghaz A.,Tang K.,Ma L. and Zou Z. (2025), "Electrocatalytic and Photocatalytic N2 Fixation Using Carbon Catalysts," Nanomaterials, 15(1),0065.
[5]. Sun S. Zhang Y.,Shi X.,Sun W.,Felser C.,Li W. and Li G. (2024), From Charge to Spin An In‐Depth Exploration of Electron Transfer in Energy, Advanced Materials 12(5),0024
[6]. Buchachenko A. L. and Berdinsky V. L.(1996), "Spin Catalysis of Chemical Reactions," The Journal of Physical Chemistry, 100(47), 18292-18299
[7]. Rasheed H. U.,Kim J. H.,Kiim T.,Lee K.,Shim J.,Kim S. H. and Yoon H. C. (2024), "Electrochemical Ammonia Synthesis from Dilute Gaseous Nitric Oxide Reduction at Ambient Conditions," Catalysts, 14(11),0838.
[8]. Iqbal A., Skúlason E., and Abghoui Y.(2024), "Understanding the Mechanistic Pathways of N2 Reduction to Ammonia on (110) Facets of Transition Metal Carbides," Crystals, 14(9),0770.
[9]. Vieri H. M.,. Kim M.-C, Badakhsh A., and Choi S. H.(2024), "Electrochemical Synthesis of Ammonia via Nitrogen Reduction and Oxygen Evolution Reactions—A Comprehensive Review on Electrolyte-Supported Cells," Energies, 17(2),0441.
[10]. Kuznetsova I., Lebedeva O., Kultin D., Mashkin M., Kalmykov K., and Kustov L.(2024), "Enhancing Efficiency of Nitrate Reduction to Ammonia by Fe and Co Nanoparticle-Based Bimetallic Electrocatalyst," International Journal of Molecular Sciences, 25(13),7089.
[11]. Tranchida G., Milazzo R. G., Lombardo S. A., and Privitera S. M. S.(2024), "Combined Effect of Pressure and Temperature on Nitrogen Reduction Reaction in Water," Energies, 17(12),2963.
[12]. Sharma R. K.,Patel H.,Mushtaq U.,Kyriakou V.,Zafeiropoulos G.,Peeters F.,Welzel S.,(2021), "Plasma Activated Electrochemical Ammonia Synthesis from Nitrogen and Water," ACS Energy Letters, 6(2),313-319
[13]. S. A. Samad.Ye X.,Han Z.,Huang S.,Lu C.,Hou J.,Yang M.,Zhang Z.,Qiu F. and Zhuang X. (2025), "Anion-Exchange Strategy for Ru/RuO2-Embedded N/S-Co-Doped Porous Carbon Composites for Electrochemical Nitrogen Fixation," Polymers, 17(4),0543.
[14]. Xiao S., Zhang D., Wang G., Zhou T., and Wang N.,(2024) "Density Functional Theory Study of Triple Transition Metal Cluster Anchored on the C2N Monolayer for Nitrogen Reduction Reactions," Molecules, 29(14),3314.
[15]. Yabuki R., Nishimura K., Hamachi T., Matsumoto N., and Yanai N.(2023), "Generation and Transfer of Triplet Electron Spin Polarization at the Solid–Liquid Interface," The Journal of Physical Chemistry Letters,14(20),4754-4759
[16]. Boto R. A., Cebreiro-Gallardo A., Menchón R. E., and Casanova D.(2024), "Electron–Spin Relaxation in Boron-Doped Graphene Nanoribbons," Journal of Chemical Theory and Computation,20(22),9906-9916,
[17]. Equbal A., Ramanathan C., and Han S.(2024), "Dipolar Order Induced Electron Spin Hyperpolarization," The Journal of Physical Chemistry Letters, 15(20), 5397-5406,
[18]. Nguyen H. and Clément R. J.(2020), "Rechargeable Batteries from the Perspective of the Electron Spin," ACS Energy Letters, 05(12), 3848-3859
[19]. Willke P. ,Singha A.,Zhang X.,Esat T.,P.Lutz C.,Heinrich A. J. and Choi T.(2019), "Tuning Single-Atom Electron Spin Resonance in a Vector Magnetic Field," Nano Letters, 19(11), 8201-8206
[20]. Feng Y. ,Wang T.,Li Y.,Li J.,Wu J.,Wu B.,Jiang L. and Wang C. (2015), "Steering Metallofullerene Electron Spin in Porous Metal–Organic Framework," Journal of the American Chemical Society,137(47),15055-15060
[21]. Buchachenko A. L. and Berdinsky V. L.(2002), "Electron Spin Catalysis," Chemical Reviews, 102(3), 603-612
[22]. Hughes M. E. and Corden B. B.(1989), "Magnetic field effects in cobalt(II)-catalyzed oxidations: the role of electron spin angular momentum," Journal of the American Chemical Society, 111(11),4110-4111
[23]. Narayana M. and Kevan L.(1985), "Role of magnesium(2+) ion in modifying the environment of radiolytically produced silver atoms in A zeolites: electron spin resonance and electron spin echo modulation spectroscopy studies," Journal of the American Chemical Society, 107(16),4643-4647
[24]. Majumder M.,Saini H.,Dedek I.,Schneemann A.,Chodankar N. R.,Ramarao V.,Santosh M. S.,Nanjundan A. K.,Kment S.,Dubal D.,Otyepka M. ,Zbořil R. and Jayaramulu K.(2021), "Rational Design of Graphene Derivatives for Electrochemical Reduction of Nitrogen to Ammonia," ACS Nano,15(11), 17275-17298
[25]. Hu J.,Zou H.,Li F.,Wei S.,Cheng M.,Dai H.,Song T. and Duan L.(2023), "Review on Electrochemical Reduction of Nitrogen by Graphdiyne-Based Catalysts: Recent Advances and Outlook," Energy & Fuels,37(5), 3501-3522
[26]. Tan W. ,Zhao H.,Ding L.,Ren N.,Wang A.and Zhao M.(2025), "Advancements in Electrocatalytic Nitrogen Reduction Reaction: A Review on the Role of Catalyst Electronic Structure and Design Strategies," ACS Applied Nano Materials, 8(6),2632-2651
[27]. Li N.,Zhang Z.,Wang Z.,Liu B.,Zhou D.,Zhou X.,Zhang P. and Zhao X.(2024), "Novel magneto-electrocatalyst Cr2CO2-MXene for boosting nitrogen reduction to ammonia," Materials Horizons, 11(7),1769-1778
[28]. Antonatos N.,Kovalska E., Mazánek V., Veselý M., Sedmidubský D.,Wu B. and Sofer Z.(2021), "Electrochemical Exfoliation of Janus-like BiTeI Nanosheets for Electrocatalytic Nitrogen Reduction," ACS Applied Nano Materials,4(1),590-599









