1. Introduction
The Drug Delivery System (DDS) is a technical system that regulates the overall distribution of drugs in organisms in space, time and dose. In general, when designing a drug, there are four main factors as discussed below.
First, drug release should be controlled. That is, to prolong the half-life of the drug, so that the concentration of the drug in the human body can be maintained in the appropriate concentration for a long period of time, so as to reduce the number of times the patient ingests the drug and prevent the accumulation of harmful substances in the drug in the human body. With the development of technology, drugs can be released in space, time and at a precise rate.
Second, targeted drug delivery. Targeted drug delivery allows the drug to recognize and concentrate only on cells or tissues with specific lesions, thus preventing the drug from damaging healthy cells.
Third, to enhance the stability and water solubility of the drug. When drugs enter the body, they will be digested and broken down by lysosomes and various enzymes in the cells, so the drug carrier usually has to protect the drug from being broken down. On the other hand, some drugs have poor solubility in the human fluid environment, which can be modified by the surface of drug carrier, such as connecting water-soluble polyethylene glycol chains on the surface. The combined use of these two points can greatly improve the efficiency of drugs.
Fourth, improve the efficiency of drug entry into the cell membrane. The active ingredients of drugs are usually macromolecules or substances that cannot be absorbed by cells. Modification of drugs can help drugs pass through the phosphate bilayer of cells and improve the therapeutic effect of drugs.
There are many materials that can be used to transport drugs; however, polymer compound in the application of drug delivery has disadvantages. Polymer compound synthesis requires a complex process, polymer molecular weight is difficult to control, and polymer-drug carrier is difficult to have uniform porosity. Synthetic products in the biological liquid environment are difficult to keep stable. In contrast, metal-organic frameworks (MOFs) have certain porosity and large specific surface areas, which ensures its load, particle size in nano MOFs, and tissue have a larger contact area with cells and interaction. Moreover, MOFs have strong flexibility, which can change the metal and ligand or through the surface, making the MOFs have different properties. Thus, under the above background, this paper studies and discusses the mechanism of MOFs drug transport, typical MOFs materials in transporting small and macromolecular substances, and several aspects of MOFs surface modification. This paper hopes to provide suggestions and guidance for designing future MOF materials for DDS, to promote the application efficiency of nanomaterials in relevant aspects, and to achieve efficient, highly selective and highly stable drug delivery.
2. The mechanism of nano MOFs for drug transportation
More and more studies have proved that MOFs have good development prospects in drug delivery, but when nano MOFs transport small molecules, macromolecular nucleic acids, proteins, etc., it is necessary to ensure that the effective substances can be transported to the diseased site as much as possible. And avoid drug degradation by lysosomes in cells. This requires us to have a clear understanding of the transport mechanism of drugs in cells.
Whether MOFs can enter cells is closely related to the structure of cells. When MOFs are outside the cell, the receptors on the cell membrane, membrane proteins, and the phospholipid structure of the bilayer are the first checkpoints. Cellular endocytosis plays a significant part in cellular signaling, ingesting and eliminating of nutrients and metabolic wastes. MOFs are often transported this way, and endocytosis produces vesicles that vary in size but often range from 50 to 150 nm in size.. The intracellular transport and absorption modes of MOFs are related to the specific biological environment of the cell and the type of drugs transported. This is mainly controlled by various organelles in the cell, and these organelles mainly include lysosomes, autophagosomes, Golgi apparatus, and early or late recycling endosomes. The process of endocytosis is a very complex biological activity that involves transmitting information and energy in cells and releasing and transporting substances. Studies have shown that major endocytosis pathways include phagocytosis, macrocytosis, and clathrin and vesicle-independent routes, as well as vesicle and clathrin-mediated processes[1].
3. MOF application in drug delivery systems
MOFs can be widely used to transport large and small molecules.
3.1 MOFs for small molecule delivery.
The traditional drugs used for drug delivery are organic, inorganic, and complex. The organic polymer system and liposome system can have good biocompatibility, but the porosity of the polymer cannot be determined, and the controlled release of the drug cannot be achieved [2]. In contrast, inorganic materials such as microporous zeolite and mesoporous silicon can achieve controlled drug release due to their definite relative porosity, but their drug-loading capacity and poor biocompatibility cannot be widely used for drug delivery [3]. Ferey and colleagues first introduced a third-step approach by using inorganic-organic "hybrid" coordination polymers. Among them, MOFs have been widely studied because they combine both advantages. High surface area and porosity, minimal toxicity to some transition metals, such as Fe and Zn, good physicochemical stability, and a crystal-clear chemical composition are all present [4].
Ibuprofen 30 and other proof-of-concept medications, as well as a-CHC, doxorubicin, and Fluorouraciland , have all been delivered using small molecule pharmaceuticals using organometallic frameworks (5-FU). Additionally, by optimizing synthesis and processing, it can carry more drugs.
3.2 MOF delivery of nitric oxide (NO) molecules
Nitric oxide (NO) is a crucial component of the immunological, neurological, and cardiovascular systems. Solid NO carriers' antimicrobial, antithrombotic, and wound-healing qualities make them potentially useful in biomedicine. The properties of solid NO are very different from gaseous NO commonly seen [5]. Coordination polymers have definite porosity and chemical composition and can be used as good carriers for solid NO. The first example used to deliver NO was HKUST-1, which was proposed by Morris and colleagues. Morris and colleagues studied the released storage and adsorption of NO on HKUST-1. Experimental results demonstrate that this kind of MOF has a higher adsorption capacity than most zeolites and organic polymers, with an adsorption capacity of over 3 mmol/g per bar at the temperature of 298 K. However, HKUST-1 has strong irreversibility, namely: HKUST-1 cannot release the drug completely. Additionally, HKUST-1 is reactive in a physiological fluid environment and could be hazardous to humans [6].
To remedy this shortcoming, Morris and his colleagues have produced two other coordination polymers, CPO-27-Ni and CPO-27-Co. And as depicted in Figure1, under the same experimental conditions, coordinating polymers are able to adsorb 7.0 mmol NO per gram of this MOF, and the polymers are able to release the same amount of NO per gram, which indicates that the above two coordination polymers are reversible during adsorption, extraction, and desorption. Under humid circumstances, CPO-27-Ni/Co may deliver 7 mmol NO/g solid, a quantity that is three orders of magnitude greater than HKUST-1 and seven times greater than the greatest zeolite. In addition, since the interaction between NO and Ni is weaker than that between NO and Co, the rate of NO release from CPO-27-Ni is larger than that of CPO-27-Co [7].
But Co and Ni have certain toxicity to the human body, so they cannot be directly used in biomedical applications, but the principle used in the above study can be used in studies in other polymers made of less harmful metals like Zn or Fe.
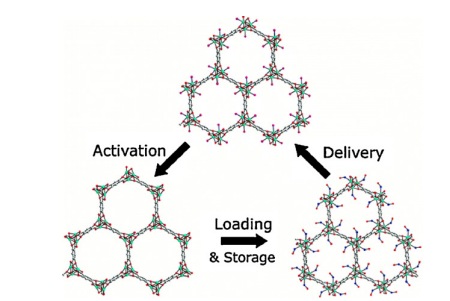
Figure1.The cycle of activation, delivery, loading, storage
3.3 MOFs for macromolecule delivery
MOFs can also be used for the transport of macromolecules, including nucleic acid and proteins. The challenge of protein delivery is that the protein size is relatively large, and the complex charge state of the protein surface makes it difficult to pass through the cell membrane. In addition, the protein is easily decomposed and inactivated by enzymes in the cell after entering the cell. Therefore, the protein needs to be encapsulated, and different nano MOF formulations can be used to deliver the protein when considering the MOFs' stability and loading capacity. These include employing mesoporous crystal nano MOFs NU-1000, and using ZIF-8 to encapsulate cytochrome C for cancer therapy[8].
Additionally, antisense oligonucleotides, siRNA, synthetic amiRs, and mRNA are other techniques used for nucleic acids. They can be targeted and function by gene specificity. This strategy mainly affects cellular functions like transcription, mRNA splicing, gene translation, and epigenetic regulation. The limitations of nucleic acid-related therapeutics are that the double-layered structure of the cell membrane hinders the entry of charged macromolecules such as RNA, and after entering the cell, the nucleic acid will be decomposed by the enzymes in the cell, making it unable to function. And using MOFs can solve this problem.
In addition, plasmid DNA in bacteria has a fixed replication initiation site and easy access to cleavage enzymes, making it easy to manipulate. Therefore, plasmid DNA is of great significance in the study of intracellular nucleic acid activity.
style='position:absolute;left:0pt;margin-left:104.5pt;margin-top:11.65pt;height:29pt;width:22.4pt;mso-wrap-style:none;z-index:251660288;mso-width-relative:page;mso-height-relative:page;' />
Figure 2 (a.) the structure of ZIF-8 (b.) the process of transfection
In recent research, ZIF-8 was reported to be used by Tang et al. to transport plasmid DNA (pDNA). The second approach of delivery differs from the first type because it contains an extra polymer. The structure of ZIF-8 and the transfection procedure are depicted in Figure 2 [9]. Capping ZIF-8 with polyethyleneimine (PEI) improved its capacity for loading and affinity for binding to pDNA. In cytotoxicity assays, lipofectamine-2000, pEGFP-C1@ZIF-8-PEI 25 kD and pEGFP-C1@ZIF-8 were administered to MCF-7 cells at different dosages (80, 100, and 120 g/mL, respectively).
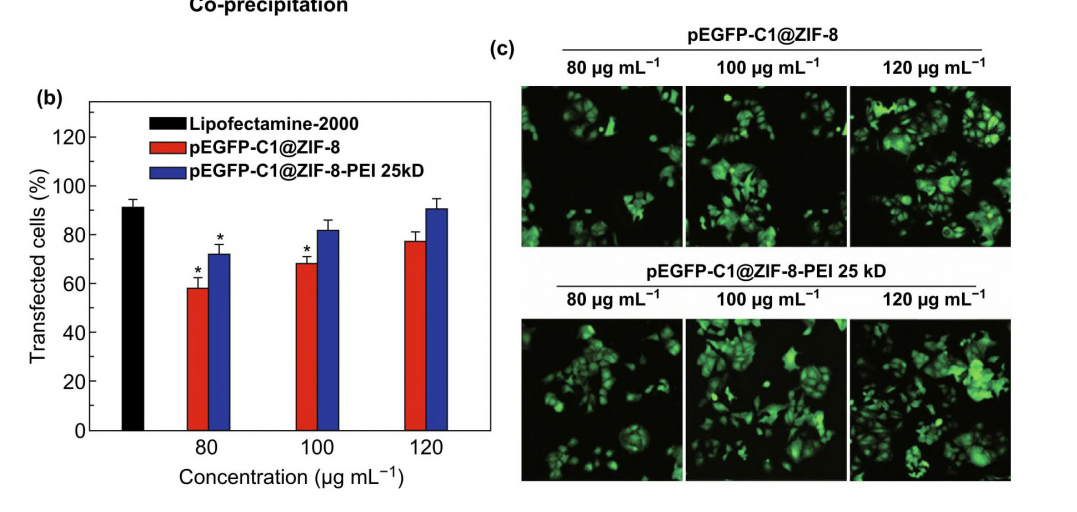
Figure3. Transfection in MCF-7 cells with different concentrations [10]
And in light of the results of the transfection experiment with MCF-7 cells. In MCF-7 cells, both drugs demonstrated effective gene transport and expression. According to the graph, pEGFP-C1@ZIF-8-PEI 25 kD had a 10% higher one-dose transfection effectiveness. And the confocal pictures in Figure 3 further supported the findings.
3.4 Typical MOF materials for drug delivery systems
MIL-100 and MIL101 have good drug-loading capacity. The content of MIL-100 ibuprofen/carrier is 35% (w/w), and the content of MIL-101 ibuprofen is 140% (w/w). Additionally, the two materials' X-ray powder diffraction (XRPD) results showed that their framework structures were still present, proving that both structures have good stability. The release rate of the drug is also one of the important indicators of MOFs.
As shown in Table 1, the factors that affect drug release are the binding energy of drug molecules and MOFs, the size of the gaps of MOFs, and the ionic state of drug molecules in different MOFs. As shown in the table below, the experimentally measured binding energy of MIL-101 to Ibuprofen is greater than that of MIL-53 and Ibuprofen, but the release rate of Ibuprofen from MIL-101 is greater than that of Ibuprofen from MIL-53, this is because MIL-101 voids are larger than MIL-100 voids. This does not, however, account for why MIL-100 (which has smaller voids) releases ibuprofen more quickly than MIL-101 and MIL-35. Ibuprofen has several ionic states, which explains why. Ibuprofen has two separate protonated ionic states in MIL-100 and MIL-101. Because of a weak contact between the two ions, the medication is released in two stages. MIL-53 has only the neutral Ibuprofen, which is linked to the MOFs relatively strongly, thus showing a 0-order release when releasing the drug [11].
Table1.a list of the ways coordination polymers transport drugs
Coordination polymer | Drug | Delivery type | Drug loading (drug/carrier) | Drug release | Conditions |
MIL-100 (Cr) | Ibuprofen | Adsorption/desorption | 35%, w/w | Zero-order (for the 1st 2 h) 100% discharged in 3 days | SBF/37 ◦C |
MIL-101 (Cr) | Ibuprofen | A/D | 140%, w/w | "Higuchi" (for the 1st 8 h) 100% discharged in 6 days | SBF/37 ◦C |
MIL-53 (Cr) | Ibuprofen | A/D | 20%, w/w | In three weeks, zero orders will be released in full. | SBF/37 ◦C |
MIL-53 (Fe) | Ibuprofen | A/D | 20%, w/w | In three weeks, zero orders will be released in full. | SBF/37 ◦C |
ZIF-8 has the properties of lower toxicity and high thermal and hydrothermal stability, and ZIF-8 is Non-toxic and biocompatible. So, they are also considered to be a very promising MOF materials in the future.
4. Surface functionalization of the MOFs
MOFs have excellent performance in drug delivery, but the use of MOFs in biological environments also requires good stability and dispersion of MOFs and specific tissue targeting. By modifying the surface of MOFs, the MOFs can discriminate against diseased cells, thereby avoiding drug damage to healthy cells.
Unsaturated metal sites on MOFs and groups that are prone to covalent bonding with other species, such as carboxylic acids, make MOFs easy for surface modification. Moreover, there are many ways to functionalize the surface of MOFs. Such as adding surface-active layers, the most typical of which is polyethylene glycol.
4.1 Polyethylene glycol
Studies have shown that polyethylene glycol can be added to the surface of nanoparticles. Polyethylene glycol is hydrophilic, which can enhance the steric hindrance effect, making it difficult for particles to aggregate, and reducing the adhesion of hemoglobin in biological fluids. Because of such properties, the modified MOFs nanoparticles are not easily phagocytosed by macrophage discovery, which ensures the stability of the drug[12,13].
In the first study using polyethylene glycol to modify MOFs, click-chemistry was used to graft polyethylene glycol chains of different lengths to the surface of UiO-66. With the growth of the polyethylene glycol chain, it can be observed that the ionic profile of the MOF becomes blurred and rounded. The grafted polyethylene glycol chains have molecular weights from 550 to 2000. The size of the particles and the associated chemical reactions can affect the interaction with the cell membrane. In vitro tests using calcein-loaded particles revealed MOFs without grafted polyethylene glycol chains and with grafted polyethylene glycol chains of molecular weight 500 were mainly transported through protein networks in cells. Only a small amount of particles were transported through macropinocytosis. In contrast, UiO-66-PEG2000 mainly flows through the clathrin, vesicular protein and macropinocytosis pathways. The original and PEG chain-branched UiO-66 were co-incubated with lysosomes for two hours, and the distribution of nanoparticles in lysosomes demonstrated that UiO-66 before and after modification could be transported through protein networks. The distribution of nanoparticles outside the lysosome was observed when the molecular weight of polyethylene glycol was 2000, which demonstrated the unique transport mechanism of the modified MOFs.
In addition, there are also studies that graft polyethylene glycol onto iron-based MIL-101, which can reduce protein adsorption and phagocytic uptake, thereby improving the stability of iron-based MIL-101 [14]. However, MOFs tend to be unstable in phosphate-based and other buffers because phosphate ions interact with metal groups in MOFs, which leads to the structural disintegration of MOFs. In the study of Chen et al., Zr-based MOF coatings, including NU-901, MOF-808, UiO-66 and PCN-128, 222. were prepared. A PEG chain is attached to the head of the phosphate, which makes the MOFs exist as colloids in the buffer solution, which makes the MOFs more stable in the buffer solution. Chen et al. have also conducted in vitro experiments, and mPEG-PO3 will not release the drug explosively when loaded with high concentrations of anti-cancer drugs, which increases the effective time of this drug. In addition, Chen et al. also freeze-dried the drug. The experiment showed that the structure of MOFs does not agglomerate and can be dispersed effectively, which shows the stability of the structure of this MOFs material [15].
4.2 Folic acid
With folate receptors on the surface of malignant cells, including colorectal, ovarian, lung, cervical, epithelial cells, breast, and brain cancers, modification of MOFs with folic acid is also a frequently employed strategy [16,17]. At the same time, this receptor is extremely rare in healthy tissue. Therefore, many studies are devoted to modifying MOFs with folic acid as targeted drugs. Wang and colleagues used folic acid and polyethylene glycol chains to functionalize a newly synthesized bioMOF (CaZol nano MOFs). This bioMOF is prepared from calcium and an agent called zoledronate (Zol). This agent is widely used to treat metastatic bone cancer. MOFs targeted by folic acid can be more easily recognized by cancer cells and enter into cells through endocytosis. MOFs can be slowly released in cells, which prolongs the half-life of drugs and makes drugs have better anti-cancer efficiency. The experimental results showed that the bioMOF modified by folic acid and polyethylene glycol showed higher efficiency in inhibiting the hyperplasia of PC3 and H460 cancer cells and promoting their apoptosis compared with the small molecule Zol. There are also quantitative experiments to prove this conclusion. First, PC3 and H460 cancer cells were transplanted into mice, and then the above-mentioned folic acid and polyethylene glycol-modified MOFs were injected into mice, respectively. The results showed that the therapeutic efficiency of Zol increased by 80%, while the non-targeted CaZol drug in the control experiment did not have an anti-cancer effect [18].
4.3 NanoMOF camouflage
This method coats nano MOFs with a bio-derived coating material composed of exosomes and phospholipid cell membranes, where exosomes are composed of vesicles secreted by different organelles [19]. Studies have shown that this membrane, which resembles the phosphate structure of the cell membrane, contains its native cell's proteins and nucleic acids, which play a message-transmitting role in both healthy and diseased cells [20]. Compared with synthetic coating materials, this bio-derived coating material does not cause the rejection of cells, and the coating of MOFs with this coating material also forms camouflage and enhances the stability of MOFs [21]. In the experiment, Khashab and coworkers encapsulated ZIF-8, carried particular proteins on phosphate membranes derived from MCF-7 HeLa cervical and breast cancer cells, and then passed it through T-lymphocyte cells, HeLa, MCF-7 and human dermal fibroblast. And the experimental results showed that the protein absorbed by the group after the bio-derivatived membrane camouflage was significantly increased.
However, the exosomes used in the experiments were derived from cancer cells, which may affect the life activities of normal cells and may also lead to the proliferation of cancer cells. Therefore, the researchers considered the use of healthy biofilms to transport MOFs, which ensures the stability and concealment of MOFs under the premise of ensuring safety.
5. Conclusion
MOFs have been widely studied because of their large specific surface area, especially in drug delivery. The large specific surface area of MOFs makes them have excellent drug-carrying capacity. MOFs also have excellent biocompatibility. Nano-sized MOFs particles can interact better with cell binding and can be targeted for drug delivery by modifying the surface of MOFs. In this paper, the research progress of MOFs in drug transport is mainly introduced from the transport mechanism of MOFs in cells, the transport of macromolecules and small molecules by MOFs, and the surface modification of MOFs materials. The transport of MOFs in cells is mainly through endocytosis, which includes phagocytosis, macrocytosis, and clathrin and vesicle-independent routes, as well as vesicle and clathrin-mediated processes. In addition, the influencing factors of drug release rate from MOFs and different MOFs materials for transporting NO gas are mainly discussed. In the third chapter, the application of MOFs in transporting macromolecules is mainly discussed, and the potential of MOFs in transporting proteins, nucleic acids and other macromolecules is illustrated. In the fourth chapter, the stability of MOFs and the performance of targeted drug delivery are enhanced by different surface modification methods of MOFs, and three methods of polyethylene glycol, folic acid, and the use of bio-derivative membranes are mainly analyzed. Today, the main challenge of MOFs in drug delivery is how to make the drug can be accurately transported to the lesion site while ensuring the stability of the drug.
References
[1]. Doherty G. J, McMahon H. T. Annual review of biochemistry, 2009, 78(1): 857-902.
[2]. Soppimath K.S, Aminabhavi T.M, Kulkarni A.R, Rudzinski W.E, 2001, J. Control. Release, 70(1).
[3]. Munoz B, Ramila A, Perez-Pariente J, Diaz I, Vallet-Regi M.2003, Chem. Mater, 15:500.
[4]. Horcajada P, Serre C, Vallet-Regi M, Sebban M, Taulelle F, Férey G. 2006, Angew. Chem. Int. Ed. Engl, 45:5974.
[5]. Moncada S, Palmer R.M.J, Higgs E.A. 1991, Pharmacol. Rev. 43: 109.
[6]. Xiao B, Wheatley P.S, Zhao X.B, Fletcher A.J, Fox S, Rossi A.G, Megson I.L, Bordiga S, L. Regli, Thomas K.M, Morris R.E. 2007, J. Am. Chem. Soc. 129: 1203.
[7]. McKinlay A.C, Xiao B, Wragg D.S, Wheatley P.S, Megson I.L, Morris R.E.2008, J. Am. Chem. Soc. 130: 10440.
[8]. Chen Y, Li P, Modica J.A, Drout R.J, Farha O.K. 2018, J. Am. Chem. Soc. 140: 5678-5681
[9]. Xiao B, Wheatley P.S, Zhao X.B, Fletcher A.J, Fox S, Rossi A.G, Megson I.L, Bordiga S, Regli L, Thomas K.M, Morris R.E. 2007, J. Am. Chem. Soc. 129: 1203.
[10]. Zhang Li, Liu P, Chen M, Zhong Y. 2019, Advanced Materials, 31(29)
[11]. Horcajada P, Serre C, Vallet-Regi M, Sebban M, Taulelle F, Férey G.2006, Angew. Chem. Int. Ed. Engl. 45: 5974.
[12]. Horcajada P, Serre C, Maurin G, Ramsahye N.A, Balas F, Vallet-Regi M, Sebban M, Taulelle F, G. Férey. 2008, J. Am. Chem. Soc. 130: 6774.
[13]. HarrisJ. M, Chess R. B. 2003, Nat. Rev. Drug Discovery, 2: 214–221.
[14]. Qie Y, Yuan H, von Roemeling C. A. Y, Chen X. Liu K. D, Shih, J. A, Knight H. W, Tun R. E, Wharen, Kim B. Y. 2016, Sci. Rep, 6: 26269.
[15]. Abanades Lazaro I, Haddad S, Sacca S, Orellana-Tavra C, Fairen-Jimenez D, Forgan R. S. 2017, Chem, 2: 561–578.
[16]. Chen X, Zhuang Y, Rampal N, Hewitt R, Divitini G, O’Keefe C. A, Liu X, Whitaker D. J, Wills J. W, Jugdaohsingh R, Powell J. J, Yu H, Grey C. P, Scherman O. A, Fairen-Jimenez D. 2021, J. Am. Chem. Soc, 143: 13557–13572.
[17]. Xia W, Low P. S, Med J. 2010, Chem, 53:6811–6824.
[18]. Sudimack J, Lee R. J. 2000, Adv. Drug Delivery Rev, 41: 147–162.
[19]. Au K. M, Satterlee A, Min Y, X, Tian Y. S, Kim J, Caster M, Zhang L, Zhang T, Huang L, Wang A.Z. 2016, Biomaterials, 82: 178–193.
[20]. Illes B, HirschleP, Barnert S, Cauda V, Wuttke S, Engelke H. 2017, Chem Mater, 29: 8042–8046.
[21]. Alyami M. Z, Alsaiari S. K, Li Y, Qutub S. S, Aleisa F. A, Sougrat R, Merzaban J. S, Khashab N. M. 2020, J. Am. Chem. Soc, 142: 1715–1720.
Cite this article
Song,W. (2023). The mechanism and design strategy of metal-organic frameworks in drug delivery. Applied and Computational Engineering,7,188-195.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Doherty G. J, McMahon H. T. Annual review of biochemistry, 2009, 78(1): 857-902.
[2]. Soppimath K.S, Aminabhavi T.M, Kulkarni A.R, Rudzinski W.E, 2001, J. Control. Release, 70(1).
[3]. Munoz B, Ramila A, Perez-Pariente J, Diaz I, Vallet-Regi M.2003, Chem. Mater, 15:500.
[4]. Horcajada P, Serre C, Vallet-Regi M, Sebban M, Taulelle F, Férey G. 2006, Angew. Chem. Int. Ed. Engl, 45:5974.
[5]. Moncada S, Palmer R.M.J, Higgs E.A. 1991, Pharmacol. Rev. 43: 109.
[6]. Xiao B, Wheatley P.S, Zhao X.B, Fletcher A.J, Fox S, Rossi A.G, Megson I.L, Bordiga S, L. Regli, Thomas K.M, Morris R.E. 2007, J. Am. Chem. Soc. 129: 1203.
[7]. McKinlay A.C, Xiao B, Wragg D.S, Wheatley P.S, Megson I.L, Morris R.E.2008, J. Am. Chem. Soc. 130: 10440.
[8]. Chen Y, Li P, Modica J.A, Drout R.J, Farha O.K. 2018, J. Am. Chem. Soc. 140: 5678-5681
[9]. Xiao B, Wheatley P.S, Zhao X.B, Fletcher A.J, Fox S, Rossi A.G, Megson I.L, Bordiga S, Regli L, Thomas K.M, Morris R.E. 2007, J. Am. Chem. Soc. 129: 1203.
[10]. Zhang Li, Liu P, Chen M, Zhong Y. 2019, Advanced Materials, 31(29)
[11]. Horcajada P, Serre C, Vallet-Regi M, Sebban M, Taulelle F, Férey G.2006, Angew. Chem. Int. Ed. Engl. 45: 5974.
[12]. Horcajada P, Serre C, Maurin G, Ramsahye N.A, Balas F, Vallet-Regi M, Sebban M, Taulelle F, G. Férey. 2008, J. Am. Chem. Soc. 130: 6774.
[13]. HarrisJ. M, Chess R. B. 2003, Nat. Rev. Drug Discovery, 2: 214–221.
[14]. Qie Y, Yuan H, von Roemeling C. A. Y, Chen X. Liu K. D, Shih, J. A, Knight H. W, Tun R. E, Wharen, Kim B. Y. 2016, Sci. Rep, 6: 26269.
[15]. Abanades Lazaro I, Haddad S, Sacca S, Orellana-Tavra C, Fairen-Jimenez D, Forgan R. S. 2017, Chem, 2: 561–578.
[16]. Chen X, Zhuang Y, Rampal N, Hewitt R, Divitini G, O’Keefe C. A, Liu X, Whitaker D. J, Wills J. W, Jugdaohsingh R, Powell J. J, Yu H, Grey C. P, Scherman O. A, Fairen-Jimenez D. 2021, J. Am. Chem. Soc, 143: 13557–13572.
[17]. Xia W, Low P. S, Med J. 2010, Chem, 53:6811–6824.
[18]. Sudimack J, Lee R. J. 2000, Adv. Drug Delivery Rev, 41: 147–162.
[19]. Au K. M, Satterlee A, Min Y, X, Tian Y. S, Kim J, Caster M, Zhang L, Zhang T, Huang L, Wang A.Z. 2016, Biomaterials, 82: 178–193.
[20]. Illes B, HirschleP, Barnert S, Cauda V, Wuttke S, Engelke H. 2017, Chem Mater, 29: 8042–8046.
[21]. Alyami M. Z, Alsaiari S. K, Li Y, Qutub S. S, Aleisa F. A, Sougrat R, Merzaban J. S, Khashab N. M. 2020, J. Am. Chem. Soc, 142: 1715–1720.









