Nitration and Flow Chemistry
Jingyi Lu1* Chennan He2
1Beijing International Bilingual Academy, Beijing 101300, China, jingy.lu@outlook.com
2Baylor university, waco 76706, US, chennan_he2@baylor.edu
*Correspondence author email: jingy.lu@outlook.com
Abstract: The paper introduced the principle of nitration reaction and flow chemistry, beginning with background information and the reaction mechanism of nitration. Then the correlation between nitration and flow chemistry is further explored by comparing the nitration reaction in conventional batch mode and under continuous flow. As the dangerous corrosive acids and strong exotherms make the reaction especially difficult to increase production scale, flow chemistry is utilized and proved to be a better alternative to the conventional production method to perform nitration. Using flow chemistry to scale up the exothermic and hazardous nitration reactions offer advantages including better yield, higher production rate, enhanced safety, etc.
1 Introduction
1.1 Background
Nitration, introducing a nitro (NO2) group to a molecule, is one of the most industrially essential processes in all the electrophilic aromatic substitution reactions in organic chemistry [1,2]. The first nitration to be reported was the nitration of benzene in 1834, of which nitrobenzene was prepared by treating benzene with fuming nitric acid. Soon after the method of using "mixed acid", a mixture of nitric and sulfuric acid, was introduced, and since then nitration has been a continuously investigated subject [3].
Nitration is an indispensable reaction in the production of certain explosives. Alfred Nobel, well known for being the founder of the renowned Nobel Prize, has had such an outstanding achievement because of the development of nitroglycerin, which is commercialized as a powerful explosive. Nitroglycerin, as its name, is a chemical made from nitrating glycerin. Ascanio Sobrero first discovered nitroglycerine in 1847 by experimenting with the nitration of glycerol using a mixture of nitric and sulfuric acids (Figure 1) [4].
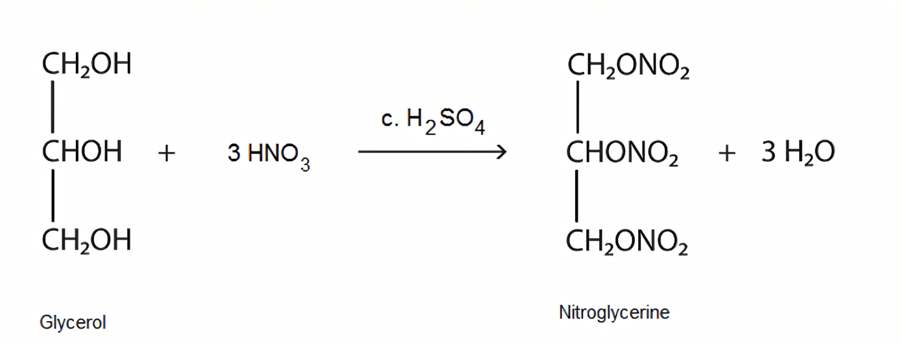
Figure 1. Nitration of glycerol [5]
The reaction results in detonation as it is highly exothermic. Afterward, Alfred Nobel recognized the potential of nitroglycerin, who then started experimentation to tackle the problem of uncontrollable detonation. Then, in 1863, the Nobel patent detonator was invented, and the new explosive had been a great success and contributed to the renown of the Nobel family [4].
Another strong explosive that gained a lot of scientific and industrial interest is trinitrotoluene, TNT. First synthesized in the 19th century, it was a primarily produced explosive during World War I and World War II and is still used as part of many explosive mixtures today in militaries and certain branches of industry. TNT can be synthesized through the nitration of 2,4-dinitrotoluene (DNT), and this can be achieved by the safer, faster, and more efficient approach to flow chemistry (Figure 2) [6].
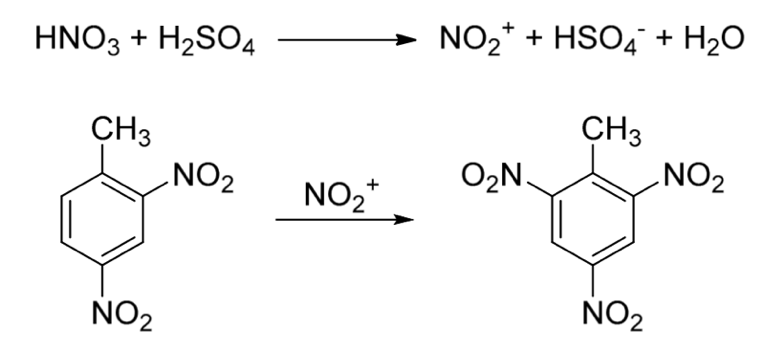
Figure 2. Synthetic path for the conversion of 2,4-dinitrotoluene (2,4-DNT) to 2,4,6-trinitrotoluene (TNT) [6]
Other than explosives, nitration is also significant in the medicinal field as nitrate-containing compounds for medicinal purposes are extensively used and had a long history that dates to the 19th century. For instance, nitroglycerin was used as a treatment for the relief of anginal pain. Nobel had himself been prescribed nitroglycerin when he suffered from the intense pain of angina pectoris, which he found ironic [4].
Flow Chemistry refers to the chemistry performed in a continuous flow using pipe, tube, or more complex microstructure devices, contrasting the conventional batch chemistry [6,7]. In the pharmaceutical sector, Flow Chemistry has emerged to be a valuable technology for research and development chemists [8]. The convergence of the microfluidic system of reactions from fabricating materials like glass to the adoption of continuous flow by organic chemistry gives rise to “Flow Chemistry”. The advantages of continuous flow have stimulated the evolution of micro-reaction technology [7]. Flow chemistry is significant to photochemistry as well as nitration. Flow chemistry enables scientists and industries to perform reactions in small reaction volumes and scale up by increasing flow rate, numbering up by using multiple reactors, and prolonging running time [8]. The small reaction volume contributes to the increased safety and continuously removed and quenched product enables automation of the production [6,8]. The synthesis of nitroglycerin has also attracted significant scientific interest in utilizing a flow process as a better alternative to conventional batch synthesis [6].
1.2 Significance
Nitration has always been a popular topic to investigate and is an active and rewarding subject of a large quantity of literature, especially of organic compounds. People utilize the nitration of aromatic organic compounds extensively to produce a wide range of useful materials such as dyes, pharmaceuticals, perfumes, plastics, and many chemical intermediates [9]. In addition, as introduced in previous sections, explosives synthesized via nitration are of great interest to military and other industrial applications.
As nitration is usually a difficult process due to its exothermic nature, nitration in large volumes imposes safety hazards and intense safety management is required. Flow chemistry is a safer alternative for such a reaction as it has a small reaction volume and better heat dissipation. Flow chemistry development also enables continuous processing of ideas and concepts to be adopted that were previously limited to large plants. Besides pharmaceutical and explosives applications, flow chemistry is utilized in specialty chemicals, polymers, and nanomaterials synthesis, and it also led to a renaissance in photochemical applications. As batch reactions are not favorable for reactions that require exposure to light, flow chemistry brought various advantages to photochemical applications. Photochemistry in flow employs fluorinated ethylene propylene (FEP) tube that could around the light source, then scaled up by arranging in axial around a high-power UV source, which the system will have the advantages of short exposures, short optical path, and wavelength filtering [7].
1.3 Problem
Even though chemists have been performing nitration for centuries, the industry still relies on certain early technologies that have many disadvantages, including being notoriously unselective (which leads to undesirable by-product formation), which can also cause environmental pollution [2,3]. Furthermore, the corrosive by-products and wastes of nitration are a big problem and added expense for safety management. Many nitration processes are done using mixed acid, of which an example is the industrial nitration of toluene that is performed with a mixture of nitric acid, sulfuric acid, and water, and this will result in excessively acidic waste streams. Moreover, the nitrating mixture of concentrated nitric acid and sulfuric acid is particularly corrosive and dangerous to handle, mix, transport, and dispose of [6,9]. Moreover, nitration reaction is an exothermic reaction and the amount of heat released for batch reaction usually exceeds 300/g, which will require further safety assessments [8]. For instance, during the investigation of nitroglycerin in the 19th century, multiple victims were killed during the nitroglycerin explosion, and Sobrero himself was severely injured [4]. All the factors mentioned have contributed to manufacturing and safety management costs, which are the imputes for people to endeavor to improve and find the optimal conditions of reaction or an alternative method to overcome the limitations and hazards [9].
1.4 Purpose
The significance of nitration and the problems scientists face are thoroughly discussed. It is essential to investigate how to improve nitration in manufacturing to cause less pollution, lower the cost of production to have economic advantages, decrease safety hazards to make it easier to handle, and enhance the efficiency of production which indicates a higher yield [9]. Several measures are utilized to minimize the impact of nitration on the environment, including replacing bulk acidic media with an acidic sonic and utilizing catalyst to allow it to proceed under milder, safer conditions [2].
Flow chemistry is significant in addressing the problem of the dangerousness of specific reactions such as nitration. Flow reactors, compared to the reactions in batch mode, have a small reaction volume that enables the highly exothermic or hazardous reactions to proceed safely as there will be well-controlled pressure and temperature and fast dissipation of heat, which is the primary advantage of performing nitration in flow [6,8]. The currently investigated aspects of flow chemistry are focused on automation and optimization, which includes extraction of kinetics, optimization of the process concerning continuous variables (temperature, pressure, composition, flow rate), and discrete parameters (solvents, catalysts) [7].
2 Reaction Mechanism of Nitration
Nitration, as mentioned, is an electrophilic substitution reaction. In short, nitration is the introduction of a nitro (NO2) group [1]. The mechanism of nitration can be explained using the nitration of benzene. Electrophilic substitution is one of the characteristic reactions of benzene. Benzene (C6H6) has a ring structure and a high unsaturation. However, it does not behave like alkenes as it has a highly stable aromatic ring structure, and thus substitution reaction is favored over addition reactions. On the other hand, like alkenes, benzene is attractive to electrophiles as the aromatic ring is a region of electron density, where the delocalized cloud of π electrons will seek electron-deficient species to form new bonds as hydrogen atoms are lost. Therefore the reaction is called electrophilic substitution (Figure 3) [10].
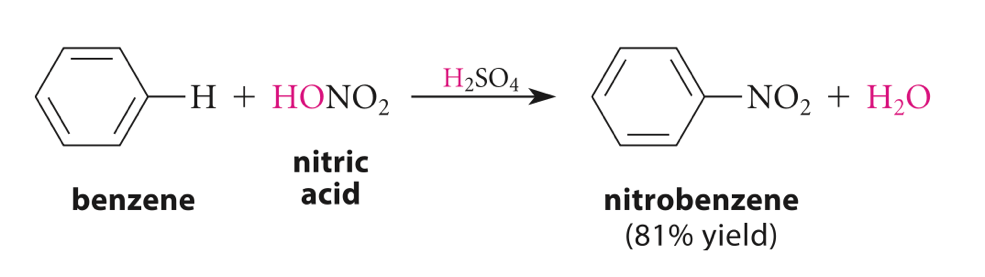
Figure 3. Nitration of Benzene [11]
The nitration reaction of benzene involves the reaction of benzene with concentrated nitric acid in the presence of a sulfuric acid catalyst to form nitrobenzene and water [11], as shown in figure 3. The mechanism of nitration of benzene involves the following steps.
First, the electrophile for the reaction is nitronium ion (NO2+), and it is generated by using a nitrating mixture, which is a mixture of concentrated nitric and concentrated sulfuric acids. As sulfuric acid is more vital (Figure 4), it will protonate the nitric acid, letting it lose a molecule of water to produce NO2+ [10].
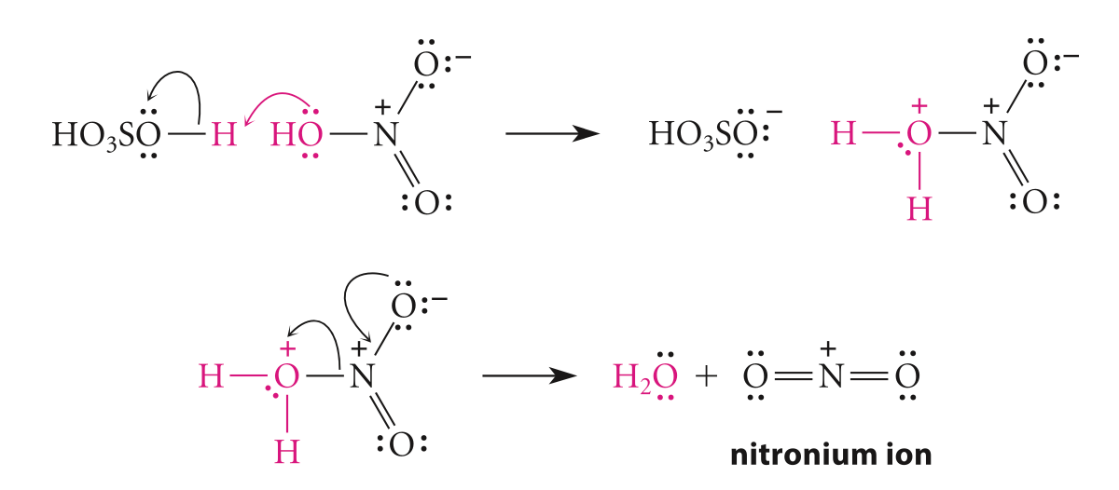
Figure 4. Step 1, generation of electrophile [11]
Second, a resonance-stabilized carbocation intermediate, arenium ion (Figure 5), is formed through the reaction of the benzene π electrons with the strong electrophile NO2+ [10,11].
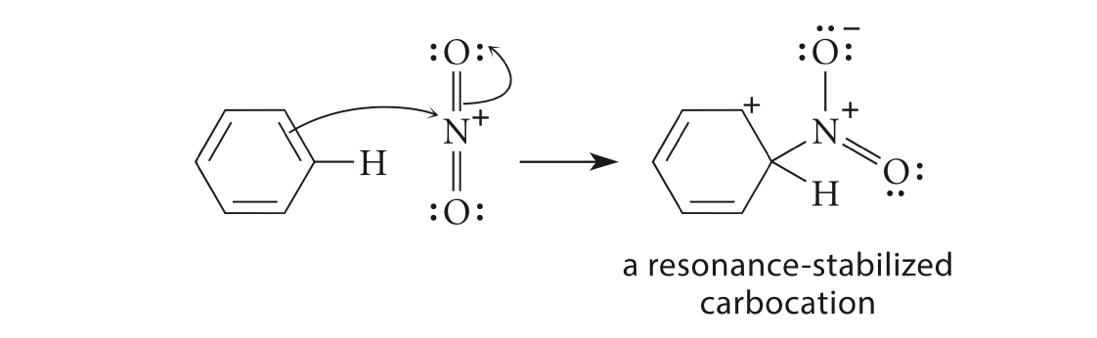
Figure 5. Step 2, formation of intermediate [11]
Third, the carbocation intermediate loses a proton and reforms the aromatic ring to give a new aromatic compound, the product nitrobenzene (Figure 6), and the hydrogen ion released will react with the base HSO4- in the electrophile generation step and reform sulfuric acid, H2SO4 [10,11].
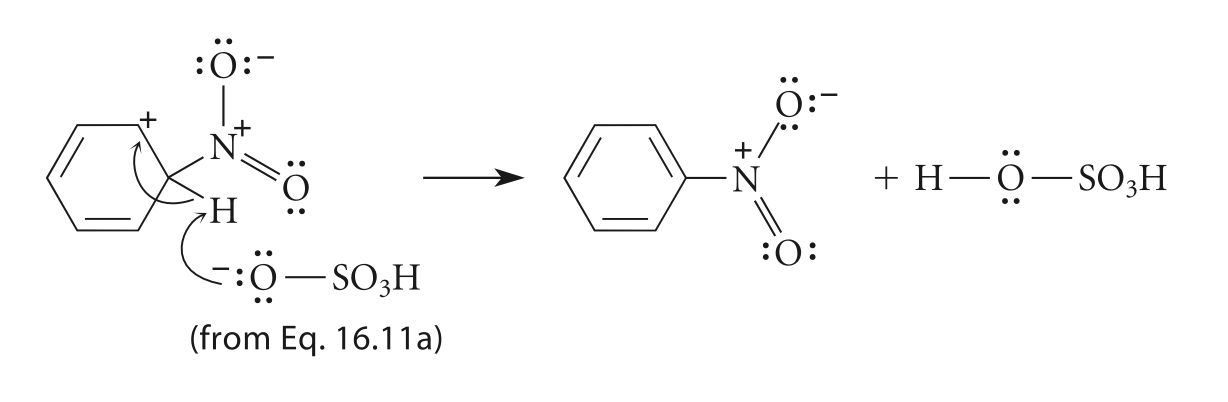
Figure 6. Step3, the reformation of the aromatic ring [11]
The mechanism of electrophilic substitution reactions will follow a similar path with different electrophiles [10].
Moreover, there can also be an electrophilic aromatic substitution reaction of mono-substituted benzene, and in this case, three possible disubstitution products can be obtained. An example is the nitration of bromobenzene, which would give ortho-, meta- or para-bromonitrobenzene (Figure 7) [11].
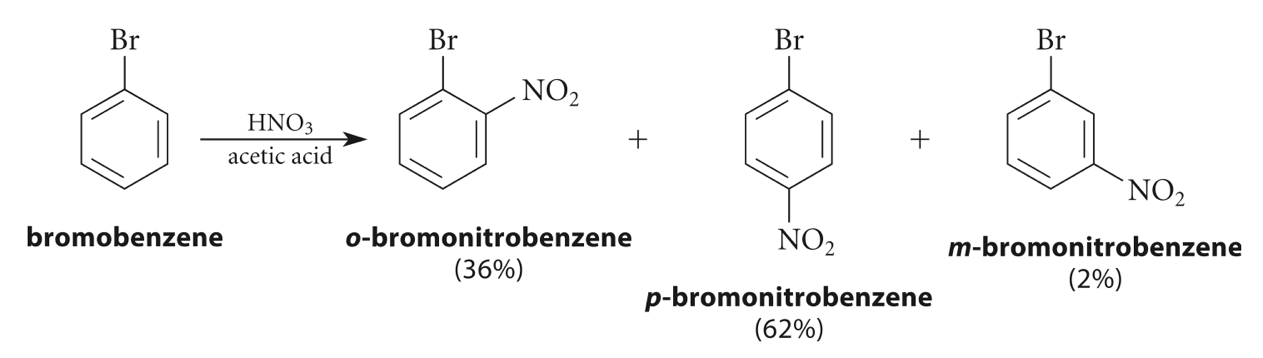
Figure 7. Nitration of bromobenzene [11]
Other examples include nitration of phenols, nitration of the cellulose hydroxyl groups to give nitrocellulose (a powerful explosive), nitration of pyrrole, nitration of pyridine, and more [11].
3 Flow chemistry
Most industrial production involving nitration utilizes batch reaction, which is less efficient, harder to scale up, and may produce multiple by-products. Continuous flow nitration enables large-scale production and increases the efficiency of the reaction. In continuous flow nitration, it is necessary to ensure that the reactants are continuously added to ensure the continuous outflow of products, usually using kettle or tubular equipment.
3.1 Conventional Batch Mode
Nitration reaction is one of the high-risk processes. The mixed acid nitration process uses corrosive concentrated nitric acid and concentrated sulfuric acid as nitrating agents. The materials are highly corrosive, the reaction releases a large amount of heat, and the products are generally flammable and explosive. The nitration process is mainly divided into batch nitration and continuous nitration. The batch nitration process adopts the method of dropwise addition of mixed acid or raw materials to the reaction kettle to ensure that the nitration reaction is carried out at a suitable temperature. During the reaction process, it is necessary to continuously add raw materials to avoid excessive temperature drops caused by untimely heat exchange. In the process, unreacted raw materials will enter the separator after many reactions and continue to react at high temperatures, which will cause the reactor to heat up and bring danger to the entire production process. In the experiment of nitration reaction, the reaction temperature is easier to control due to the small size of the reactor and the small number of raw materials. However, once industrialized, the volume of the reactor will be scaled up, the exothermic effect is obvious, and the risk is significantly increased. One of the main reasons many companies insist on using the batch nitration process is that the process route can be changed according to market conditions to produce other products with more significant sales [12].
3.2 Flow Chemistry
3.2.1 Equipment
In continuous flow nitration, the state-of-the-art instrument is the microchannel reactor. This instrument is the safest and most efficient. This instrument is made up of many pipes, which are only a few millimeters in diameter. reactor with internal diameters of several millimeters or less. Since the nitration reaction is exothermic, a huge amount of heat is released during the reaction, which will cause the reactor's temperature to increase continuously and bring safety hazards to the entire production process. The heat transfer performance of the microchannel reactor is better, and the temperature inside the microchannel reactor can be kept constant by adding an external reactor to achieve precise temperature control. Therefore, the production efficiency of the microchannel reactor is higher and safer. In addition to the microchannel reactor, a tank reactor can also be used, which can be controlled by remote control, continuously feeding, continuously reacting, separating, and feeding above the reactor to control the flow rate of the pipeline (Figure 8) [13].
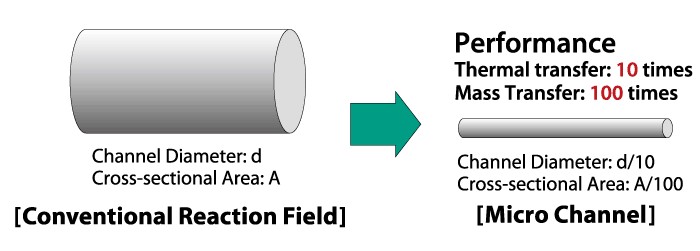
Figure 8. Microchannel in a reactor [14]
3.2.2 Nitrating Agent
In continuous flow nitration, the selection of the nitrating agent is also essential. In the nitration reaction, mixed acid is generally used, and the mixed acid is mixed with a certain proportion of sulfuric acid and nitric acid simultaneously. In the course of the reaction, there will be a large amount of sulfuric acid that needs to be treated, and it is necessary to carry out a neutralization reaction at the end and separate these sulfuric acids. As mentioned, using an acid mixture that consists of sulfuric acid will have a safety hazard due to the corrosiveness of sulfuric acid. Thus, there are several viable alternatives to overcome the disadvantages during manufacturing processes, and this includes using solid acid catalysts, other sources of NO2, organic nitrating agents, metal nitrates, and other acids to replace sulfuric acid such as inorganic acidic salts (NaHSO4-H2O, Mg(SO4), Oxone) and silica sulfuric acid, etc. [9]. Solid acid eliminates the use of sulfuric acid, avoids subsequent sulfuric acid pollution, and the recovery of solid acid is relatively easy, safe, and clean. However, the acidity of solid acids is usually very low, which is likely to reduce the efficiency of the reaction. Both methods can be used as nitrating agents. The study shows that temperature, residence time, and flow rate affect the conversion rate [15].
3.2.3 Temperature and Residency Time
Temperature and residency time are critical parameters in flow chemistry that influence the yield, and thermal management is critical in continuous flow nitration. In microchannel reactors, heating is required in its built-in piping, usually through a rectangular face for heat transfer. In addition, the reactor can also be placed in a constant temperature bath, and the temperature can be controlled by controlling the continuously circulating liquid outside. Integrating the heating system into the reactor is convenient, or it can be controlled externally using an immersion system. Both methods can be used. The modularity of the entire system and the utility costs are essential for choosing which heating mode to choose for scale-up (Figure 9) [13].
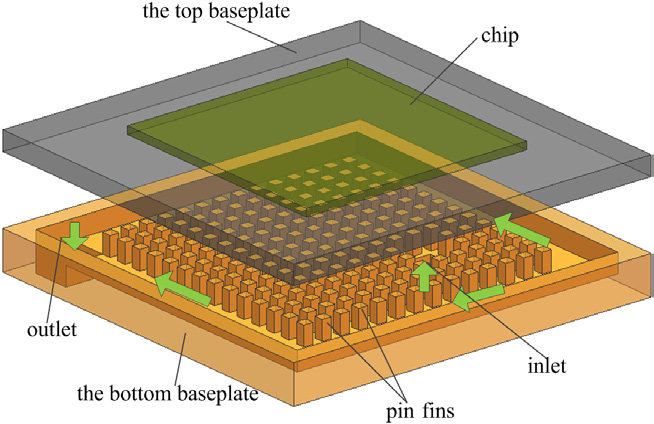
Figure 9. Microchannel heat sinks [16]
The residence time in the nitration reaction will impact the by-products. The residence time of less than 20min is likely to lead to a decrease in the conversion rate of the final product. For more than 30min, the reactant will likely continue to react with the nitro group in the production process, thereby increasing by-products. Therefore, the residency time of the reactor is significant. The nitration reaction may produce by-products that are difficult to handle, and how to deal with these by-products is very important [17].
3.2.4 Literature Examples
The following literature examples demonstrate the comparison between batch and flow chemistry.
1-Methyl-4,5-dinitroimidazole (4,5-MDN1) is a novel insensitive explosive. When the N-methylimidazole nitration reaction is carried out in the traditional kettle-type reactor, the heat released is highly exothermic. To control the reaction temperature, the material needs to be slowly added dropwise, the reaction time is long, and the production yield is low. Rapidly increasing the yield of 4,5-MDN1 by utilizing the high heat transfer properties of the microchannel reactor. In industrial production, heat can be increased by increasing the number of microchannel reactors while a constant reaction temperature is maintained. While reducing the amount of mixed acid, the output can be significantly improved, and it has broad development prospects [18].
Secondly, nitration of 2-amino-4-bromo-benzoic acid methyl ester, as shown by number 3 in the image, in batch and under continuous flow was compared by Brocklehurst et al. The results suggested that the nitration in batch forms by-products that are hard to purify or be used for further reactions. Moreover, the quality of the product cannot be assured as it is challenging to precisely control the parameters such as temperature for nitration in batch. Such inaccuracies of parameters will lead to by-product formation numbered 5, which need to be removed by crystallization and lead to a significant loss in yield. In addition, the largest exotherm observed had an onset at 140°C and 641 Jg-1 energy released, and thus this reaction cannot be scaled up in batch as it is too risky (Figure 10) [8].
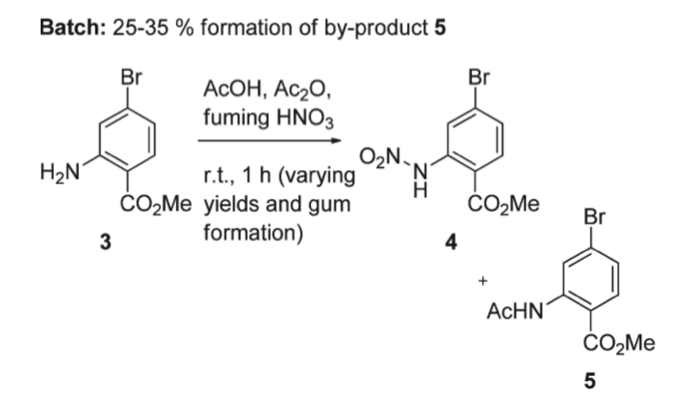
Figure 10. Nitration of 2-amino-4-bromo-benzoic acid methyl ester in batch [8]
In contrast, the reaction is run under continuous flow and several screening experiments are done to determine the optimum condition for flow reaction which only consumed a few hours, including independent variables of residency time, temperature, and stoichiometric ratio to HNO3. The reaction was performed using 3 inlet streams, as shown in the diagram. The diagram showed the scaled-up reaction under continuous flow. The reaction begins with 0.8M 2-amino-4-bromo-benzoic acid methyl ester in acetic acid and fuming nitric acid. After 1.3 min of residency time, the outlet stream will be mixed with acetic anhydride in acetic acid and give out an outlet stream of 10 mL/min. Afterward, it will flow into stirred ice water to be quenched. The temperature of the water is critical as if room temperature water is used, gum formation is observed, which does not happen if ice is used. Overall, a fine crystalline solid with 84% yield is obtained with no by-product and high purity, and in 105 min of reaction, 123g of material is produced. The production rate is 70g/h (Figure 11) [8].
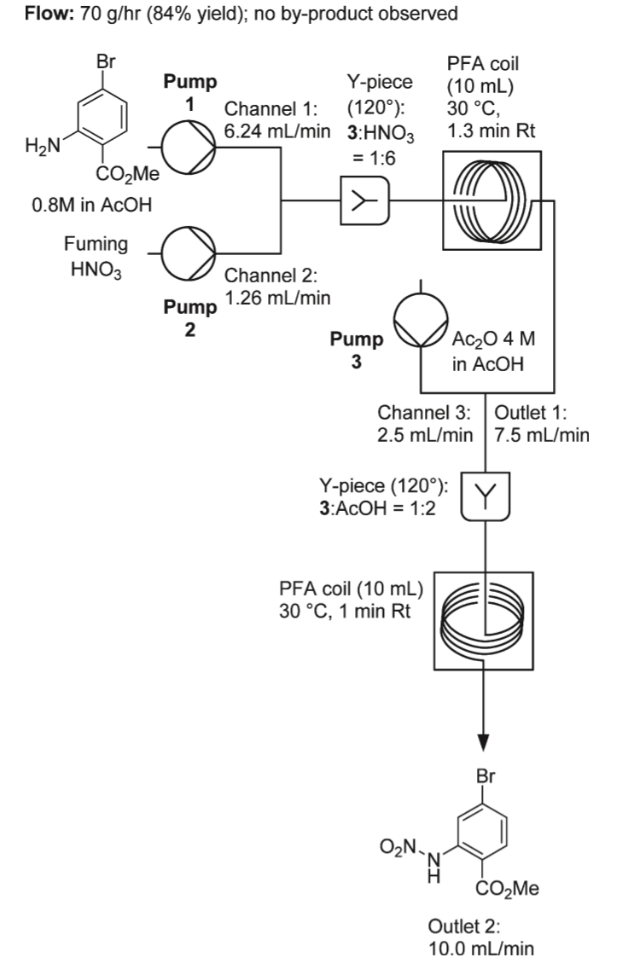
Figure 11. Nitration of 2-amino-4-bromo-benzoic acid methyl ester in flow [8]
Another example is the Synthesis of 2,4,6-Trinitrotoluene (TNT) by Kyprianou et al. In traditional synthesis, anhydrous nitric acid (100% concentration) and oleum (sulfuric acid contains up to 60% SO3) are used to achieve a high conversion rate to 98%. However, the acids are dangerous to handle and a safer process for manufacturing high purity trinitrotoluene needs to be developed. Such improvements are made in batch reactions by performing the nitration of dinitrotoluene in batch mode using N2O5/H2SO4 (98%) as a nitrating mixture to investigate a higher purity, faster reaction time, and more environmentally friendly approach [6].
On the other hand, flow chemistry was also chosen as a safer alternative for the conventional synthesis to prepare trinitrotoluene. The optimum condition for nitration of dinitrotoluene is HNO3 65%:H2SO4 98% = 3:1, 130°C, 20 min. The same conditions were used in batch mode to compare the reaction rate. As the conditions were not optimized for batch mode, there was a low conversion rate of less than 58%. In contrast, within the time of 20min, the conversion rate of 2,4-DNT to TNT (>99%) is achieved (Figure 12) [6].

Figure 12. TNT is produced under continuous flow and in batch mode [6]
The conventional batch process and flow synthesis for nitration for TNT synthesis is compared with the performed batch reaction and analysis of other literature examples. As shown in figure 12, TNT produced in batch showed a yellow color due to impurities. Also, due to better heat dissipation, the reaction can be performed safely up to 150°C. Moreover, HNO3 65%:H2SO4 98% for flow chemistry is less hazardous than fuming HNO3 (greater than) 98% and oleum used in batch mode. Scaling up for batch exists in commercial productions and scaling up in flow can be done by increasing the running time of the process. Automation, reduced reagent costs (for laboratory-scale production), and accurate control of parameters cannot be ignored [6].
The examples discussed above demonstrate the advantage of flow chemistry in performing nitration. First, comparing reaction in batch and reaction under continuous flow, a significant advantage is the comparable yields as it is challenging to scale up in batch reaction. Secondly, production in a flow reactor is usually timesaving as the process is automated and optimum conditions can be easily found [8]. From screening experiments to production, a total of one to two weeks is needed, shorter than the batch. Finally, all the literature examples mentioned are dangerous exothermic reactions, and flow chemistry enables such dangerous exothermic reactions to run safely and efficiently.
In addition, the literature examples used alternatives to replace traditional nitration mixtures. For example, in the synthesis of TNT under continuous flow, HNO3 65% and H2SO4 98% are used instead of anhydrous nitric acid and oleum. Likewise, in the nitration of 2-amino-4-bromo-benzoic acid methyl ester, acetic acid/acetic anhydride with fuming nitric acid can replace the nitrating mixtures of nitric and sulfuric acid for flow reactions, as those acids are less corrosive, making it feasible with stainless steel reactions.
4 Conclusion
The progress of investigation in new technologies for organic molecule synthesis is significant, and flow chemistry development is undoubtedly an important aspect. Overall, its various advantages over the conventional batch include a high degree of safety, simplicity due to automation, and achieving a high conversion rate and enhanced selectivity for specific reactions [6,8], flow chemistry certainly is an ideal method that enables a fastened process of organic molecule synthesis from bench to production. It not only tackled the issues of safety hazards involved in high exothermic reactions such as nitration, which leads to significant time and cost savings but also contributed to an easier way for large-scale production, as it is convenient to achieve automation and determine optimum conditions. Although novel eco-friendly synthesis methods are developed to obtain some high-energy materials, a hazardous traditional approach is applied in certain industrial productions. Nevertheless, flow chemistry is a production mode with bright prospects.
References
[1]. Clayden, Jonathan, et al. Organic Chemistry. Oxford; New York, Oxford University Press, 2012.
[2]. Smith, Keith, et al. “A Novel Method for the Nitration of Deactivated Aromatic Compounds.” Journal of the Chemical Society, no. 16, 19 July 2000, pp. 2753–2758, 10.1039/B002158J.
[3]. Hoggett, J. G., et al. Nitration and Aromatic Reactivity. Cambridge University Press, 1971.
[4]. Marsh, Neville, and Alexander Marsh. “A Short History of Nitroglycerine and Nitric Oxide in Pharmacology and Physiology.” Clinical and Experimental Pharmacology and Physiology, vol. 27, no. 4, 2000, pp. 313–319, 10.1046/j.1440-1681.2000.03240.x.
[5]. Thompson, Mike, and Charlie Thompson. “Glycerol - Molecule of the Month - January 2018 (HTML Version).” Figshare, 2017, doi.org/10.6084/m9.figshare.5567872.v1.
[6]. Kyprianou, Dimitris, et al. “Synthesis of 2,4,6-Trinitrotoluene (TNT) Using Flow Chemistry.” Molecules, vol. 25, no. 16, 6 Aug. 2020, 10.3390/molecules25163586. Accessed 8 Feb. 2021.
[7]. Jensen, Klavs. “Flow Chemistry-Microreaction Technology Comes of Age.” AIChE Journal, vol. 63, no. 3, 2017, pp. 858–869, 10.1002/aic.15642.
[8]. Brocklehurst, Cara E., et al. “Nitration Chemistry in Continuous Flow Using Fuming Nitric Acid in a Commercially Available Flow Reactor.” Organic Process Research & Development, vol. 15, no. 6, 2011, pp. 1447–1453, 10.1021/op200055r.
[9]. Zolfigol, Mohammad Ali, et al. “Nitration of Aromatic Compounds on Silica Sulfuric Acid.” Bulletin of the Korean Chemical Society, vol. 25, no. 9, 2004, pp. 1414–1416.
[10]. Brown, Catrin, and Mike Ford. Higher Level Chemistry. 2nd ed., Harlow, Essex, Pearson Education, 2014.
[11]. Loudon, Marc, and Jim Parise. Organic Chemistry. Ben Roberts, 2016.
[12]. “Research status and technological progress of nitration process.” n.p. 01 Apr 2022 https://www.sohu.com/a/534518295_121117454
[13]. Amol A. Kulkarni. ”Continuous flow nitration in miniaturized devices.” Beilstein J. Org. Chem. 2014, 10, 405–424. https://doi.org/10.3762/bjoc.10.38
[14]. “What Is a Micro Channel Reactor & Details || KOBE STEEL, LTD.” Kobelco, www.kobelco.co.jp/english/products/ecmachinery/smcr/overview.html.
[15]. Wen, Zhenghui, et al. “Process Development and Scale-up of the Continuous Flow Nitration of Trifluoromethoxybenzene.” Organic Process Research & Development, vol. 21, no. 11, Nov. 2017, pp. 1843–50, https://doi.org/10.1021/acs.oprd.7b00291.
[16]. Zhao, Junhong & Wang, Yan & Ding, Guifu & Sun, Cecilia & Wang, Guilian. (2014). Design, fabrication and measurement of a microchannel heat sink with a pin-fin array and optimal inlet position for alleviating the hot spot effect. Journal of Micromechanics and Microengineering. 24. 10.1088/0960-1317/24/11/115013.
[17]. Mándity, István M et al. “Strategic Application of Residence-Time Control in Continuous-Flow Reactors.” ChemistryOpen vol. 4,3 (2015): 212-23. doi:10.1002/open.201500018
[18]. Corning Reactor Technologies, Inc. “Review l Progress in the application of continuous nitration of aromatic compounds” n.d. https://www.yiqi.com/zt2721/article_24786.html
Cite this article
Lu,J.;He,C. (2023). Nitration and flow chemistry. Applied and Computational Engineering,7,390-399.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Clayden, Jonathan, et al. Organic Chemistry. Oxford; New York, Oxford University Press, 2012.
[2]. Smith, Keith, et al. “A Novel Method for the Nitration of Deactivated Aromatic Compounds.” Journal of the Chemical Society, no. 16, 19 July 2000, pp. 2753–2758, 10.1039/B002158J.
[3]. Hoggett, J. G., et al. Nitration and Aromatic Reactivity. Cambridge University Press, 1971.
[4]. Marsh, Neville, and Alexander Marsh. “A Short History of Nitroglycerine and Nitric Oxide in Pharmacology and Physiology.” Clinical and Experimental Pharmacology and Physiology, vol. 27, no. 4, 2000, pp. 313–319, 10.1046/j.1440-1681.2000.03240.x.
[5]. Thompson, Mike, and Charlie Thompson. “Glycerol - Molecule of the Month - January 2018 (HTML Version).” Figshare, 2017, doi.org/10.6084/m9.figshare.5567872.v1.
[6]. Kyprianou, Dimitris, et al. “Synthesis of 2,4,6-Trinitrotoluene (TNT) Using Flow Chemistry.” Molecules, vol. 25, no. 16, 6 Aug. 2020, 10.3390/molecules25163586. Accessed 8 Feb. 2021.
[7]. Jensen, Klavs. “Flow Chemistry-Microreaction Technology Comes of Age.” AIChE Journal, vol. 63, no. 3, 2017, pp. 858–869, 10.1002/aic.15642.
[8]. Brocklehurst, Cara E., et al. “Nitration Chemistry in Continuous Flow Using Fuming Nitric Acid in a Commercially Available Flow Reactor.” Organic Process Research & Development, vol. 15, no. 6, 2011, pp. 1447–1453, 10.1021/op200055r.
[9]. Zolfigol, Mohammad Ali, et al. “Nitration of Aromatic Compounds on Silica Sulfuric Acid.” Bulletin of the Korean Chemical Society, vol. 25, no. 9, 2004, pp. 1414–1416.
[10]. Brown, Catrin, and Mike Ford. Higher Level Chemistry. 2nd ed., Harlow, Essex, Pearson Education, 2014.
[11]. Loudon, Marc, and Jim Parise. Organic Chemistry. Ben Roberts, 2016.
[12]. “Research status and technological progress of nitration process.” n.p. 01 Apr 2022 https://www.sohu.com/a/534518295_121117454
[13]. Amol A. Kulkarni. ”Continuous flow nitration in miniaturized devices.” Beilstein J. Org. Chem. 2014, 10, 405–424. https://doi.org/10.3762/bjoc.10.38
[14]. “What Is a Micro Channel Reactor & Details || KOBE STEEL, LTD.” Kobelco, www.kobelco.co.jp/english/products/ecmachinery/smcr/overview.html.
[15]. Wen, Zhenghui, et al. “Process Development and Scale-up of the Continuous Flow Nitration of Trifluoromethoxybenzene.” Organic Process Research & Development, vol. 21, no. 11, Nov. 2017, pp. 1843–50, https://doi.org/10.1021/acs.oprd.7b00291.
[16]. Zhao, Junhong & Wang, Yan & Ding, Guifu & Sun, Cecilia & Wang, Guilian. (2014). Design, fabrication and measurement of a microchannel heat sink with a pin-fin array and optimal inlet position for alleviating the hot spot effect. Journal of Micromechanics and Microengineering. 24. 10.1088/0960-1317/24/11/115013.
[17]. Mándity, István M et al. “Strategic Application of Residence-Time Control in Continuous-Flow Reactors.” ChemistryOpen vol. 4,3 (2015): 212-23. doi:10.1002/open.201500018
[18]. Corning Reactor Technologies, Inc. “Review l Progress in the application of continuous nitration of aromatic compounds” n.d. https://www.yiqi.com/zt2721/article_24786.html









