1. Introduction
The concept of high-entropy is first used in the alloy, but in recent years it was introduced into ceramic, it is calculated by Eq. (1), where k is the Boltzmann constant; S is the entropy, the measure of molecular movement or disorder; Ω is the number of possible microscopic states.
\( S=kln \) Ω (1)
If the results of Eq. (1) would be more than 1.61R(R is the molar gas constant which is 8.314 J·mol-1·K-1), that would be high-entropy. And in the very near time, the implementation of high-entropy in solid electrolytes is convinced to boost some special properties such as ionic conductivity and less reliance on specific chemistries in the crystal [1]. In solid electrolytes, it is desirable to achieve the features of high ionic conductivity and low electronic conductivity simultaneously. To improve it to the next level, there are a lot of scientists that look into reframing and configuring. But, it is also very low so that it can’t be widely used in Electric Vehicles. By contrast, high-entropy is hopefully regarded as one of the most useful strategies to change that situation, so this paper reviews the recent development in high-entropy and analyses the feasibility of this method. This paper puts eyes on the high-entropy used in the LLZO garnet structures and explains the mechanism in it.
In this review, the development of high-entropy metal cation involved in Li-garnet structure is described. This paper shows the structure of Li-garnet structure first. Next, the most cutting-edge methods currently in use to create high-entropy materials are analyzed and compared. Subsequently the mechanisms behind strong ionic conductivity, including the chemical site energy, are discussed. The industrial applications of high-entropy materials are discussed in the final section. These applications span a wide variety of sectors. It can be identified that this paper can add up to unveil the mystery in this field and do a better job for introducing a whole new system in solid electrolytes.
2. Garnet structure and the transport mechanism of Li ions
2.1. Garnet-type solid-state electrolyte structure
Garnet structure Li7La3Zr2O12 (LLZO), a typical oxide lithium ion solid electrolyte, has an ionic conductivity of 10-4 ~10-3 S/cm, near to the lithium ion migration number of 1, a large electrochemical window, high stability, and thermal stability against lithium. The chemical formula of garnet structure is A3B2(XO4)3 (A = Ca, Mg, Fe, Mn; B = Al, Fe, Cr, Ti, Zr, V), where A, B and X contain 8, 6 and 4 oxygen coordination, respectively. Centered cubic (FCC) garnet has an Ia \( \overline{3} \) d space group. When the X is Li+, it has the Li+ conduction capability. The Li (X) atoms are in the void of the frame structure.
Many pure phase LLZO have lattice constants between 12.95 and 12.97 Å [2]. Cubic (c-LLZO) and tetragonal (t-LLZO) LLZO crystal structures exist. The most significant difference between the two structures is the occupation of Li. Li partially occupies the gap in the cubic phase, while Li occupies the full gap in the tetragonal phase. T-LLZO has 10–6 S/cm ionic conductivity, two orders of magnitude less than c-LLZO. Table 1 shows spatial group and ion conductivity differences between t-LLZO and c-LLZO.
Table 1. Structural fundamentals of t/c LLZO.
Index | Tetragonal phases (t-LLZO) | Cube phase(c-LLZO) |
Space Group | I41/acd (No.142) | Ia-3d (No.230) |
Cell Parameters | a = 13.07 ~ 13.12 Å,c =12.67 ~ 12.72 Å | a = 12.975 Å |
Ionic Conductivity at Room Temperature | 10-6 ~ 10-5 S/cm | 10-4 ~ 10-3 S/cm |
Activation Energy | 0.4 ~ 0.5 eV | 0.3 ~ 0.4 eV |
Lithium-ion Occupation Situation | 8°, 16f, 32g All occupy; 16e Not occupied | 24d, 96h Part of the occupy |
2.2. Li ion transport in LLZO and effect of doping on lithium ion transport
Understanding Li+ transport in the garnet structure can help increase the ionic conductivity of LLZO solid electrolyte. Lithium ion transport in the crystal lattice begins with determining their unique occupancy. In 2011, Goodenough et al. collected Li7La3Zr2O12 neutron diffraction data from room temperature in the cubic phase down to 600 ℃ (Figure 1) [3]. Maximum entropy analysis shows that lithium at 24d and 96h is delocalized, while at temperatures above 400 ℃, Li ions with different occupation sites are distributed from the discontinuous nuclear density and gradually connected to form the 3D transport channel of 24d→96h→48g→96h→24d. As temperature rises, Li occupancy at position 48g in the twisted octaheon core increases.
As shown in Figure 2 (a), the garnet structure has an eightfold coordination indicated by 24c and 16a, respectively LaO8 dodecahedral sites and sixfold coordination ZrO6 octahedral sites, where Li atoms randomly and partially occupy the backbone space or eccentric position 96h sites of the tetrahedral 24d and octahedral 48g sites.
According to the typical crystal structure and Li+ distribution of LLZO (Figure 2b), there are two pathways for Li+ migration. In one way, Li+ transfers from the octahedral site through the tetrahedral gap, while in the second way, Li+ passes through a shared triangular plane belonging to the octahedral and tetrahedral positions.
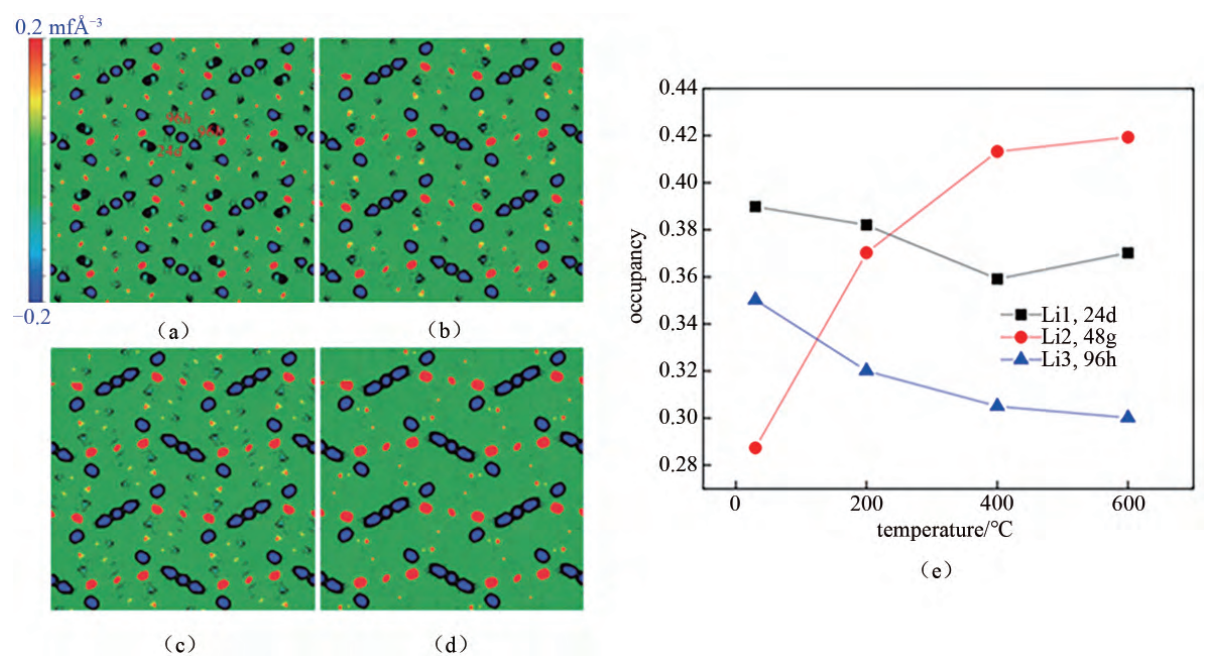
Figure 1. The color filling map of kernel density distribution of cubic phase Li7La3Zr2O12 at different temperatures along the (001) section: (a) room temperature, (b) 200 ℃, (c) 400 ℃ and (d) 600 ℃; (e) occupancy changes of different Li sites at different temperatures [3].
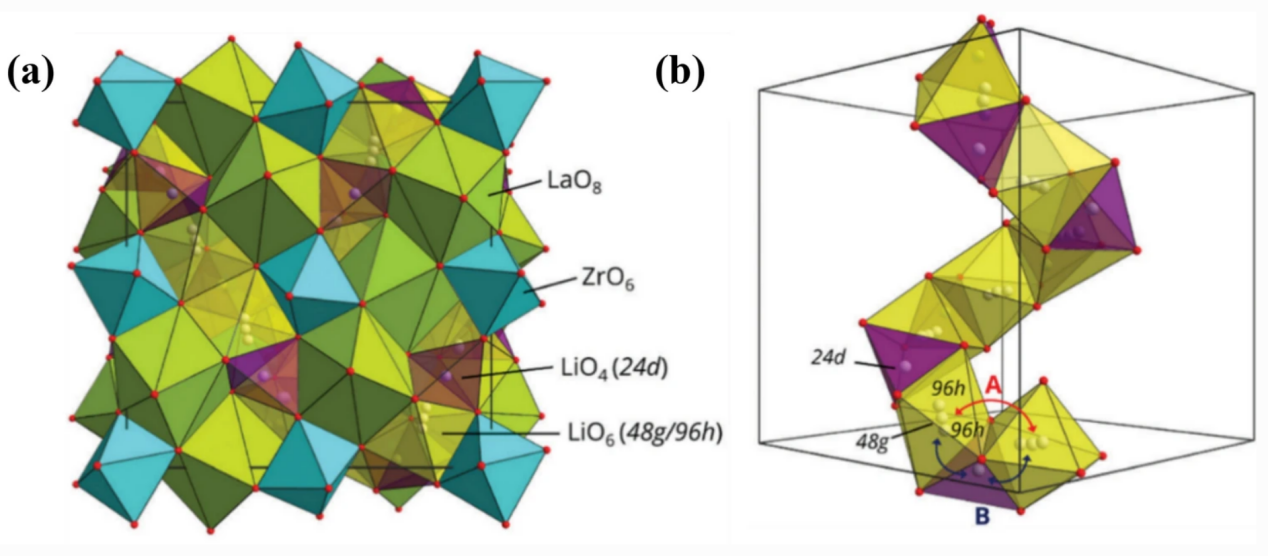
Figure 2. (a) Cubic phase, LLZO, and the crystal structure; (b) The Wyckoff position of the lithium ions in the cubic phase [4].
The centers of the tetrahedral and octahedral positions are at the 24d and 48g positions respectively, and the 96h position is slightly shifted from the 48g position. The connection of LiO6 and LiO4 and the possible migration path of Li (A and B). Path B is the most likely migration mechanism of Li in the cubic phase structure LLZO.
Doping of some Li, La or Zr elements in the LLZO crystal structure, its mechanism of action mainly has two points: First, regulating the number of lithium atoms in the lattice by a charge compensation mechanism. The formed lithium vacancy can disrupt the arrangement of lithium atoms, to generate more disordered cubic phases. At the same time, the presence of lithium vacancy can also dredge the Li+ migration channel, thus enhancing the lithium ion conductivity of LLZO; Secondly, using the radius difference of the doped ions and Li+, La3+, Zr4+ to properly adjust the bottleneck size of the Li+ migration channel, reducing the activation energy of Li+ diffusion can improve the mobility rate of Li+.
3. Feasibility of high entropy doping in LLZO garnet type solid electrolyte
3.1. The concept of high entropy
The concept of "high entropy" was originally developed from the concept of high entropy alloys (HEAs) proposed by Professor Ye Junwei, a Taiwan, China scholar. The alloy is a solid solution composed of the amount of five or nearly a single crystal structure, and the content of each element is 5% to 35% [5]. In recent years, domestic and foreign researchers have expanded the design concept of HEAs to the field of high entropy ceramics (HECs) materials, and developed a series of high entropy oxides.
The Boltzmann relation of S = kln Ω states that the higher the degree of chaos in a thermodynamic system, the higher its entropy. (where k is the Boltzmann constant; S is the macroscopic system entropy, which is the measure of molecular movement or disorder; Ω is the number of possible microscopic states). According to the Gibbs free energy formula G=H-TS, the general concept of entropy stability is based on reducing the Gibbs free energy G of the system by increasing the configurational entropy (Sconfig) of the system, so as to stabilize the single-phase crystal structure. The lattice of high entropy ceramics is composed of two different sublattices, anion and metal ion sublattice. The anion sublattice may be occupied by anions and some vacancies, while the metal ion sublattice is randomly occupied by several different metal atoms. The molar configuration entropy of the system can be calculated according to Equation (2):
Sconfig = -R \( \lbrace \frac{X}{X+Y}\sum _{i=1}^{N}{x_{i}}ln({x_{i}})+\frac{Y}{X+Y}\sum _{j}^{N}{x_{j}}ln({x_{j}})\rbrace \) (2)
Where \( {x_{i}} \) and \( {x_{j}} \) are the mole fractions of cation and anion, X and Y are their lattice numbers, and R is the molar gas constant. (8.314 J mol-1 K-1). Entropy can reach 1.61R in a five-component system with equal substance amount. Sconfig≥1.61R materials are "high entropy," 1.61R>Sconfig≥1R are "medium," and Sconfig <1R are "low". High-entropy multi-group ceramic materials have Sconfig≥1.61R and five or more metal cations in equal amounts. High-entropy materials have being redefined as research improves. The four-group quantity system is currently dubbed the high-entropy system [6].
3.2. Mechanism of action of high-entropy doping
Zeng et al. doped lithium garnet structure with La, Pr, Nd, Te, W and other elements to prepare Li3(La,Pr,Nd)3(Te,W)2O12 (LLPNTWO), and the mechanism of high entropy doping was deduced through experiments and calculations [1]. A “slow” ordered lattice can be made faster by introducing disorder, which creates fast ion percolation pathways, and by raising the charge-carrier concentration.
Locally, distortions will cause the site energy to fluctuate, resulting in a distribution of site energies. When this distribution is sufficiently massive, the energies of neighboring sites will overlap, encouraging ion hopping between them.
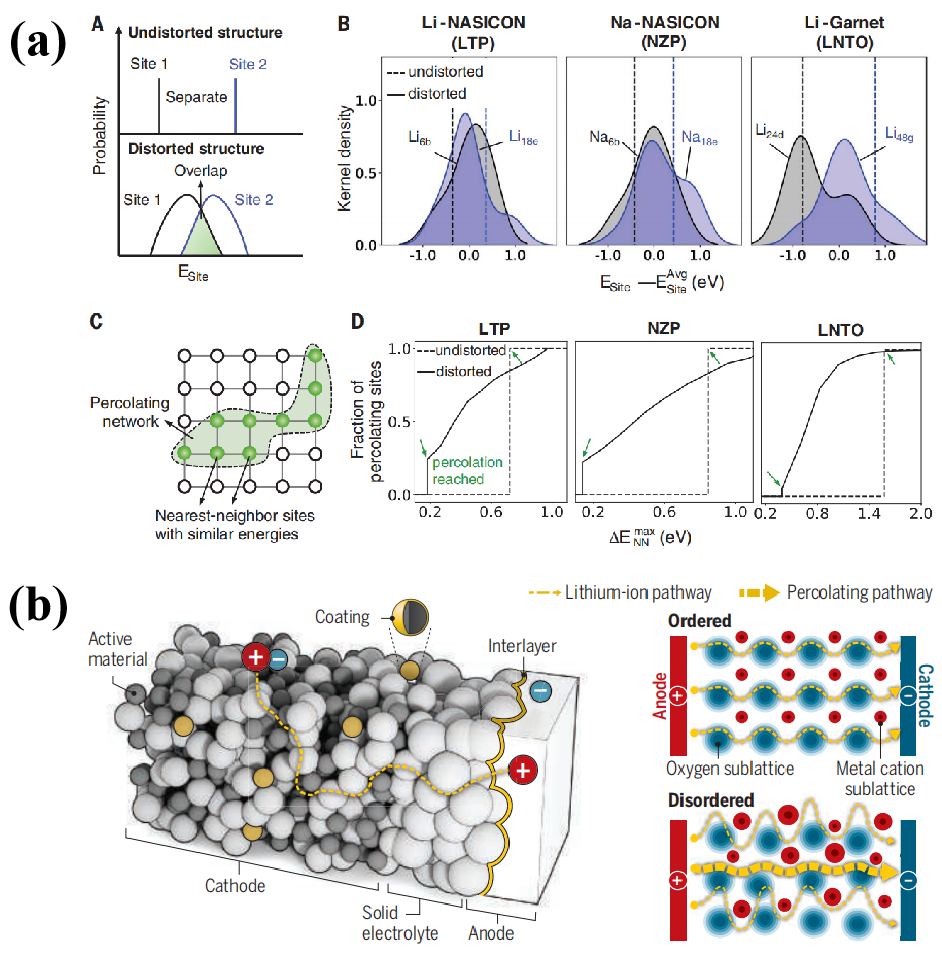
Figure 3. Structure-based distortions' impact on alkali site energies and percolation [1,7].
Disruptions to the structure have an effect on the energy and percolation of alkali sites. Figure 3 depicts the result. Overlapping site-energy distributions are formed due to local distortions, as seen in (a). In (a)B and (a)D, we see that the site energy and the fraction of percolating sites are significantly impacted by the distortion. Lithium ion mobility is enhanced by the preexisting percolating network due to the lower site energy. (b) demonstrates a precise model of ion transport along the percolation pathway.
Meanwhile, structural frameworks offer straightforward ion migration via connected, low-barrier diffusion channels. It induces percolating channels for rapid ion migration in normally low-mobility lattices. This has the potential to improve battery efficiency and conductivity.
3.3. The advantage of the high-entropy doping
According to the research of Jung et al., a rise in entropy is likely responsible for the stabilization of the cubic-phase garnet. By using an entropy-driven synthetic strategy, we can reduce the 750 °C required for cubic-phase nucleation in the solid state and get access to hitherto inaccessible chemical space in cubic-phase garnet [8].
Zeng et al. used La, Pr, Nd, Te, W doped into the lithium garnet structure, Li3(La,Pr,Nd)3(Te,W)2O12 (LLPNTWO), electrochemical impedance spectroscopy was used to determine the ionic conductivities of high-entropy materials. The ionic conductivity is 1.7×10-3 mS/cm for LLPNTWO (27% porosity) [1].
One more benefit is enhanced interface interaction. Changing the electrolyte composition has been shown to considerably cut down on Li dendrites. Li6.5La3Zr1.5Ta0.5O12, a garnet-type material, was doped with Li3PO4. Li dendrite formation can be stopped by dramatically changing the solid electrolyte interphase (SEI) composition at the Li/electrolyte interface. According to Zhou et al., the ability of Li metal to wet Li6.75La3Zr1.75Ta0.25O12 (LLZTO) can be significantly improved. Improved wettability resulted from the in-situ synthesis of the Li-inserted species Li7La3Zr1.5Ti0.25Ta0.25O12, which was produced when Li-metal was used to decrease the elemental Ti [9].
3.4. The feasibility of high entropy doping in LLZO garnet solid electrolyte
4. System feasibility. At normal temperature, doping c-LLZO with an element near to the primary ion stabilizes and improves ion conductivity. Doping or replacing ions creates charge-compensated gaps. (i. e., dopants different from the valence state of the main ion). Cation substitution stabilizes cubic phase by decreasing Li content or increasing Li vacancy concentration while maintaining oxygen chemical equilibrium. Moreover, the co-doping technology has also been widely used. Co-doping can not only stabilize the cubic structure but also effectively facilitate Li transport. In addition, density functional theory (DFT) calculates the possible positions of dopants in LLZO and provides a choice for LLZO matrix composites with excellent properties [10]. Subtions include: Al, Fe, Ge, and Ga to replace Li; Y, Sr and Ce replace La; Mg, Sc, Nb, Ti, Ta, Sb, Zn, Ru, W, Te replace Zr and other.
5. Process feasibility. High entropy electrodes and solid-state electrolytes are difficult and time-consuming to produce using conventional processes such solid-state synthesis, high-temperature sintering, mechanochemical synthesis, and wet chemical synthesis. Recent work by Hu's team has resulted in an ultrafast high-temperature sintering procedure that, in just seconds, can produce high-quality solid-state electrolytes [11]. The single phase can be kept at room temperature because of the extremely quick heating and cooling rate. Therefore, this fast synthetic process may hasten the creation of high entropy materials for battery applications.
4. Conclusion
This paper mainly discusses the latest progress of high entropy metal cation doping in lithium garnet type solid electrolyte, and its basic structure and mechanism of action. After analysis, the present paper explores the development potential of high entropy in garnet structure, which has the advantages of stabilizing LLZO cubic phase and improving ionic conductivity. The increase in ionic conductivity may be related to the possibility of random disturbance to the energy path of ion diffusion. It facilitates high-entropy battery materials to provide a solution to achieve target performance without the need for "key elements" such as cobalt or nickel-rich (in cathode materials) or germanium or titanium (in solid electrolyte), thus better solving the material supply chain problem of batteries. At the same time, a potential system advantage is to reduce the transport of electrons, prevent the formation of lithium dendrites, and improve the availability of solid-state batteries. This article is not discussed in depth here. At present, we have only put forward theoretical and experimental evidence to confirm and describe the feasibility and operation mechanism of this matter, and we still need a lot of follow-up work to truly realize industrialization and commercialization. From the application level, the study discusses the problem of ion conductivity in solids, so all the problems related to atoms and ion diffusion in the future can be discussed from this result and the next step.
References
[1]. Zeng, Y., Ouyang, B., Liu, J., Byeon, Y. W., Cai, Z., Miara, L. J., ... & Ceder, G. (2022). High-entropy mechanism to boost ionic conductivity. Science, 378(6626), 1320-1324.
[2]. Murugan, R., Thangadurai, V., & Weppner, W. (2007). Fast lithium ion conduction in garnet‐type Li7La3Zr2O12. Angewandte Chemie International Edition, 46(41), 7778-7781.
[3]. Xie, H., Alonso, J. A., Li, Y., Fernandez-Diaz, M. T., & Goodenough, J. B. (2011). Lithium distribution in aluminum-free cubic Li7La3Zr2O12. Chemistry of Materials, 23(16), 3587-3589.
[4]. Jia, M., Zhao, N., Huo, H., & Guo, X. (2020). Comprehensive investigation into garnet electrolytes toward application-oriented solid lithium batteries. Electrochemical Energy Reviews, 3, 656-689.
[5]. Qin Y, Liu J X, Li F, et al. A high entropy silicide by reactive spark plasma sintering[J]. Journal of Advanced Ceramics, 2019, 8: 148-152.
[6]. Jiang S, Shao L, Fan T W, et al. Elastic and thermodynamic properties of high entropy carbide (HfTaZrTi) C and (HfTaZrNb) C from ab initio investigation[J]. Ceramics International, 2020, 46(10): 15104-15112.
[7]. Botros, M., & Janek, J. (2022). Embracing disorder in solid-state batteries. Science, 378(6626), 1273-1274.
[8]. Jung, S. K., Gwon, H., Kim, H., Yoon, G., Shin, D., Hong, J., ... & Kim, J. S. (2022). Unlocking the hidden chemical space in cubic-phase garnet solid electrolyte for efficient quasi-all-solid-state lithium batteries. Nature Communications, 13(1), 7638.
[9]. J. Zhu, X. Li, C. Wu, J. Gao, H. Xu, Y. Li, X. Guo, H. Li, W. Zhou, A multilayer ceramic electrolyte for all-solid-state Li batteries, Angew. Chem. Int. Ed. Engl. 60 (2021) 3781–3790.
[10]. Miara, L. J., Richards, W. D., Wang, Y. E., & Ceder, G. (2015). First-principles studies on cation dopants and electrolyte| cathode interphases for lithium garnets. Chemistry of Materials, 27(11), 4040-4047.
[11]. Wang, C., Ping, W., Bai, Q., Cui, H., Hensleigh, R., Wang, R., ... & Hu, L. (2020). A general method to synthesize and sinter bulk ceramics in seconds. Science, 368(6490), 521-526.
Cite this article
Mei,X. (2023). Advances in high entropy doping of Li7La3Zr2O12 (LLZO) garnet solid electrolyte: Properties and feasibility analysis. Applied and Computational Engineering,23,102-108.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zeng, Y., Ouyang, B., Liu, J., Byeon, Y. W., Cai, Z., Miara, L. J., ... & Ceder, G. (2022). High-entropy mechanism to boost ionic conductivity. Science, 378(6626), 1320-1324.
[2]. Murugan, R., Thangadurai, V., & Weppner, W. (2007). Fast lithium ion conduction in garnet‐type Li7La3Zr2O12. Angewandte Chemie International Edition, 46(41), 7778-7781.
[3]. Xie, H., Alonso, J. A., Li, Y., Fernandez-Diaz, M. T., & Goodenough, J. B. (2011). Lithium distribution in aluminum-free cubic Li7La3Zr2O12. Chemistry of Materials, 23(16), 3587-3589.
[4]. Jia, M., Zhao, N., Huo, H., & Guo, X. (2020). Comprehensive investigation into garnet electrolytes toward application-oriented solid lithium batteries. Electrochemical Energy Reviews, 3, 656-689.
[5]. Qin Y, Liu J X, Li F, et al. A high entropy silicide by reactive spark plasma sintering[J]. Journal of Advanced Ceramics, 2019, 8: 148-152.
[6]. Jiang S, Shao L, Fan T W, et al. Elastic and thermodynamic properties of high entropy carbide (HfTaZrTi) C and (HfTaZrNb) C from ab initio investigation[J]. Ceramics International, 2020, 46(10): 15104-15112.
[7]. Botros, M., & Janek, J. (2022). Embracing disorder in solid-state batteries. Science, 378(6626), 1273-1274.
[8]. Jung, S. K., Gwon, H., Kim, H., Yoon, G., Shin, D., Hong, J., ... & Kim, J. S. (2022). Unlocking the hidden chemical space in cubic-phase garnet solid electrolyte for efficient quasi-all-solid-state lithium batteries. Nature Communications, 13(1), 7638.
[9]. J. Zhu, X. Li, C. Wu, J. Gao, H. Xu, Y. Li, X. Guo, H. Li, W. Zhou, A multilayer ceramic electrolyte for all-solid-state Li batteries, Angew. Chem. Int. Ed. Engl. 60 (2021) 3781–3790.
[10]. Miara, L. J., Richards, W. D., Wang, Y. E., & Ceder, G. (2015). First-principles studies on cation dopants and electrolyte| cathode interphases for lithium garnets. Chemistry of Materials, 27(11), 4040-4047.
[11]. Wang, C., Ping, W., Bai, Q., Cui, H., Hensleigh, R., Wang, R., ... & Hu, L. (2020). A general method to synthesize and sinter bulk ceramics in seconds. Science, 368(6490), 521-526.









