1. Introduction
Nanoparticles (NPs) are tiny particles with a dimensionality of less than one hundred nanometers. They are becoming increasingly popular in the cosmetic industry because of their unique properties, which include increased surface area, high reactivity, and enhanced absorption [1]. Therefore, NPs become a widely used material in various fields. For instance, in medicine, nanoparticles have shown great potential in many fields like cosmetics and the carriers of drug transport. They can be designed to track the target like some tumour cells and viruses and also be able to be used to enhance the solubility and bioavailability of drugs. Nanoparticles can also be utilized in cosmetics. Table 1 shows some examples of different kinds of metal oxide NPs applied in cosmetics.
Iron oxide can be used in pigments. Iron oxide minerals like hematite, goethite, and magnetite are heated to extremely high temperatures to create iron oxide pigments. Figure 1 shows the structure of Fe2O3. Depending on the mineral used, the heating temperature, and the length of the heating process, a variety of colours are produced in the pigments. For instance, heating hematite will yield red iron oxide, while heating magnetite will yield black iron oxide [2]. Besides, titanium oxide is a commonly used opacifier due to its high refractive. In addition, zinc oxide can be used in sunscreen and against sunlight, and thus it also can be used in anti-aging products. It gives support for preventing the skin from free radical destruction which contributes to aging by breaking down collagen and elastin fibers in the skin [3]. By preventing the development of bacteria that produce odour on the skin, zinc oxide is a common ingredient in deodorants. By killing the bacteria that cause acne, zinc oxide can aid in the reduction of inflammation and redness during acne treatments.
Among various cosmetic products, sunscreen is essential for skin care. It can conserve the skin's surface and block the UV light from the sunlight. The novel sunscreen that contains nanoscale zinc oxide and titanium oxide is very attractive for its higher efficacy compared to conventional sunscreen. However, apart from the benefits of nanoparticles, the potential risks are also should be concerned like the toxicity and environmental effect. This paper mainly illustrates the mechanism of sunscreen and the zinc oxide and titanium oxide nanoparticles of sunscreen and their potential risks.
Table. 1 The overview of metal oxide application in cosmetic.
Application in cosmetics (metal oxide) |
1.sunscreen |
2.pigments (foundation, blush, eyeshadow, etc) |
3.opacifier |
4.anti-aging (anti-aging creams and serums) |
5.antibacterial (deodorants and acne treatments) |
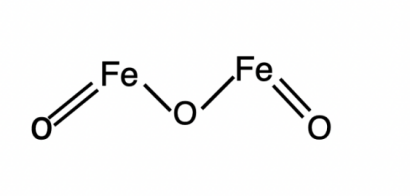
Figure 1. The structure of Fe2O3 which is called Iron (III) oxide.
2. Metal oxide application
2.1. Zinc oxide and titanium dioxide
The two common ingredients in physical sunscreens are zinc oxide and titanium dioxide because they are able to absorb and scatter solar ultraviolet (UV) radiation [4]. UV can be divided into two types which are ultraviolet A (UVA) and ultraviolet B (UVB) [5] One measure of sunscreen protection is short sun protection factor (SPF) which is a method of a sunscreen's ability to protect your skin from the sun's UV rays. The largest the SPF number, the more protection the sunscreen provides against UVB radiation, which is the dominant cause of burns from the sun and skin diseases. A sunscreen with a low protection level, such as SPF 6 and Persistent Pigment Darkening (PPD) 12, which is a method of measuring the effectiveness of sunscreen in conserving the skin and defending against UV radiation, may be suitable for those who tan easily and rarely burn. Medium protection, with an SPF of 15 and PPD 20-25, may be more appropriate for those who burn occasionally. High-protection sunscreens, with an SPF of 30, 40 or 50 and a PPD of 20-40, are recommended for those with fair or sensitive skin, and very high-protection sunscreens with SPF 50+ and PPD 30 are suitable for people with very fair or extremely sensitive skin [6]
Wurtzite and zincblende are the two most prevalent crystalline forms of zinc oxide. Ilmenite ore is the main material for titanium dioxide in nature and has 45–60% TiO2.
Using the sulphate or chloride procedures, this or an enriched derivative (known as titanium slag) can be used to produce pure TiO2. This most prevalent and reliable form of this pigment is rutile [4]. Zinc oxide and titanium dioxide are both white, powdery substances. Figure 2 and Figure 3 show the crystal structure of ZnO and TiO2.
UV is one of the most significant natural factors. They provide broad-spectrum protection and are safe for use on the skin. It is pivotal to prevent our skin from UV exposure to decrease the risk of non-melanoma skin cancer [7]. The initial sunscreen-ultraviolet B filters were invented in 1928 and the sun protection factor was produced in 1974 [8]. These UV sunscreens can be divided into two categories: chemical and physical sunscreen. Physical sunscreen is a type of sunscreen that works by physically blocking the sun's rays from reaching the skin. It contains mineral-based active ingredients, such as ZnO and TiO2, which provide protection on the skin and mirror the UV rays away from the skin [9]. Zinc oxide and titanium dioxide belong to physical sunscreen and they are superior to sunscreens with organic compounds [4]. Newman et al. indicate that before using nanoparticles in sunscreens, conventional sunscreens usually have a thick texture and are difficult to absorb by the skin. Because of the thick texture, sunscreen is not popular in customers. Traditional sunscreen has a composition of zinc oxide and titanium dioxide but not nanoparticles. By development of techniques, nanosized zinc oxide and titanium dioxide play a salient role in solving the problem of the grotesque, white film and become more transparent, absorbed easily in skin texture [4].
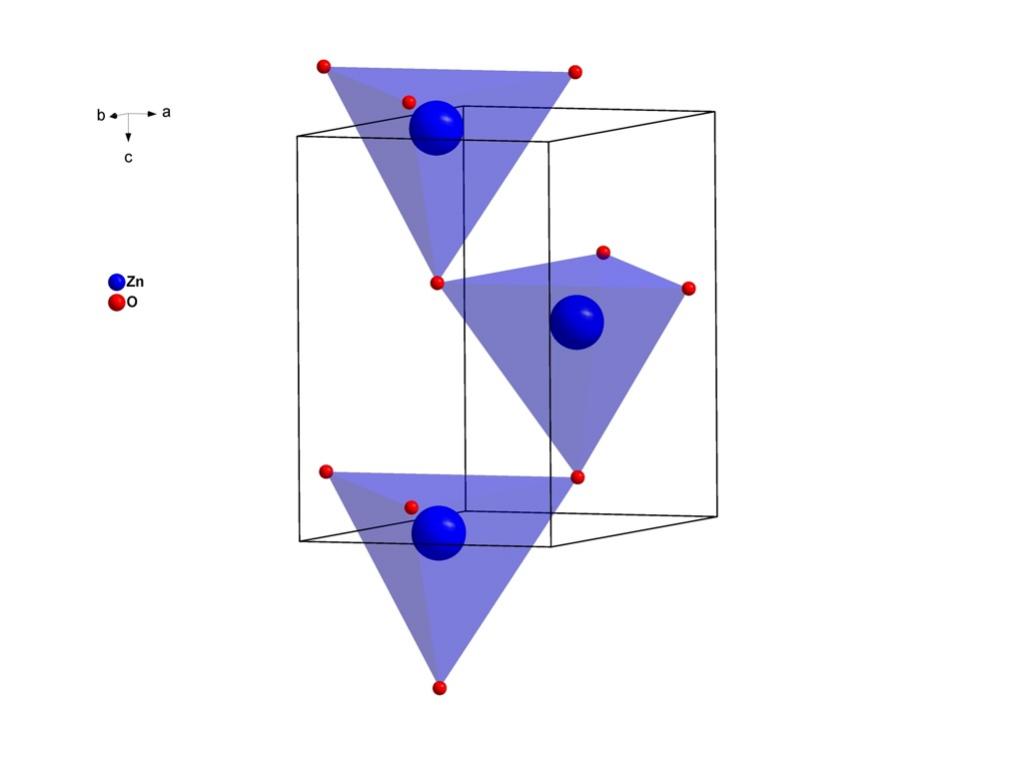
Figure 2. The crystal structure of Wurtzite. “Zn” stands for zinc and “o” stand for oxygen. “a,b,c” stand for coordinate system.
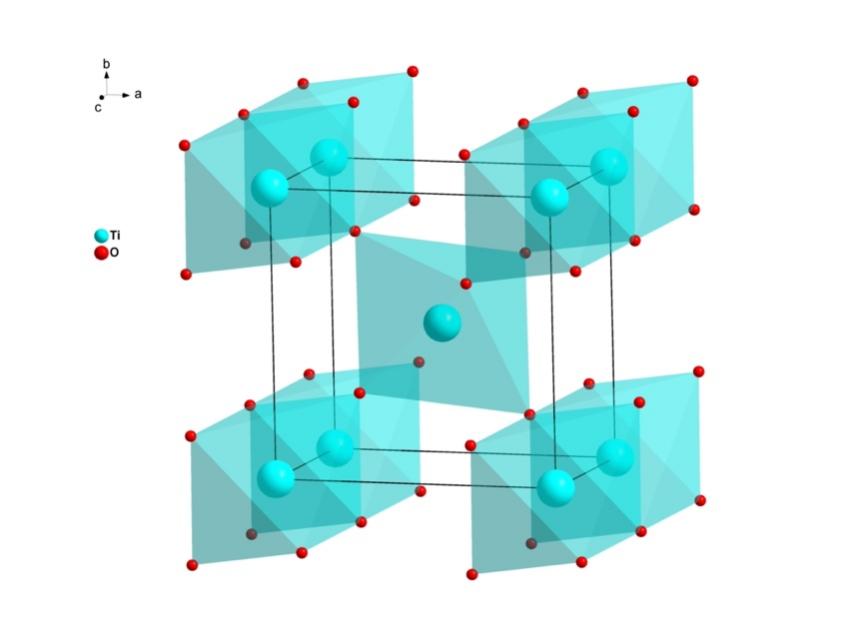
Figure 3. The crystal structure of TiO2. “Ti”stands for titanium and “o” stand for oxygen. “a,b,c” stand for coordinate system.
2.2. The mechanism of zinc oxide and titanium oxide sunscreen
Figure 4 shows the difference of organic sunscreen and physical sunscreen. Although they are both mineral or physical sunscreens, organic sunscreens and sunscreens containing zinc oxide protect against the sun in different ways thanks to their various active ingredients. Chemical (organic) sunscreens contain elements that can take in UV rays and then diffuse the energy through dissipating the heat to release the excess energy because it is unstable in high energy [5].
Physical (inorganic) sunscreens, on the other hand, offer broad-spectrum protection because they both absorb UVA and UVB radiation, preventing UV light from penetrating the skin.[10]. The film thickness, refractive index, and particle size of the sunscreen products are the main factors influencing how effective physical sunscreens are [5]. The film thickness of sunscreen refers to the quantity of sunscreen that is used on the skin and forms a protective layer to block or absorb UV radiation. The film thickness of sunscreen is important because it determines the degree of protection provided by the sunscreen. Interestingly, the frequency distribution of film thickness in various vehicles determines the effectiveness of sunscreen [11]. Myriam et al. used the pig ears which were in place of the human skin to research the amount of film thickness by wearing sunscreen to the pig ears [11]. Finally, they found that SPF was decreased by increased application pressure and a long spreading time [11].
Organic sunscreens might also include other organic components that are good for the skin, like oils or plant extracts [12]. On the other hand, zinc oxide and titanium oxide sunscreens typically only have zinc oxide and titanium oxide as their active component.
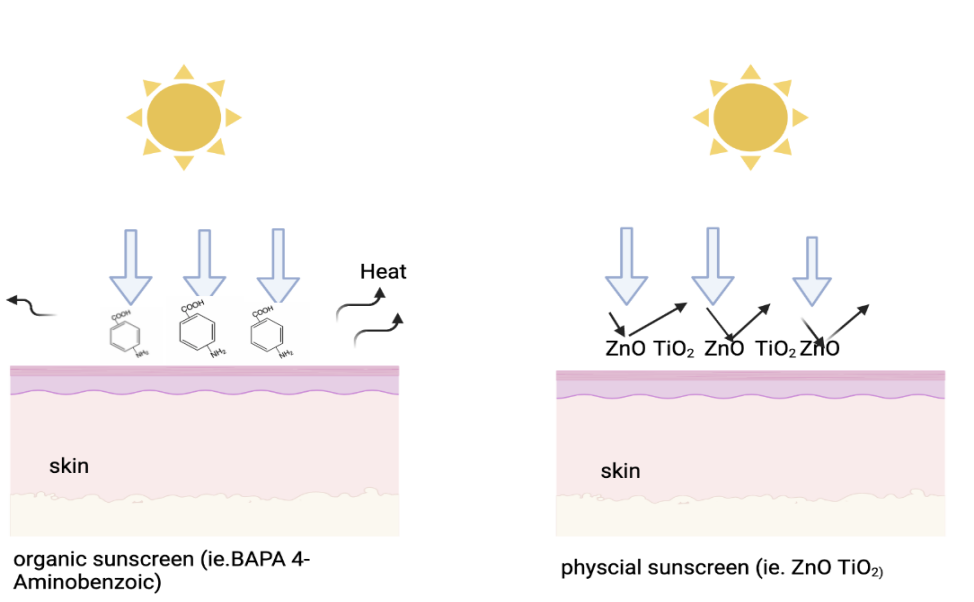
Figure 4. The difference action of different types of sunscreen.
3. Potential risks of metal oxide nanoparticles
Metal oxide nanoparticles (NPs) have attracted significant attention because of their special physical, chemical, and biological properties. However, their widespread use in various industrial and consumer products causes the concernment of their potential dangers to human health and the environment. It is suggested that metal oxide nanoparticles have potential risks associated with their use, particularly in regard to human safety and environmental protection (Figure 5).
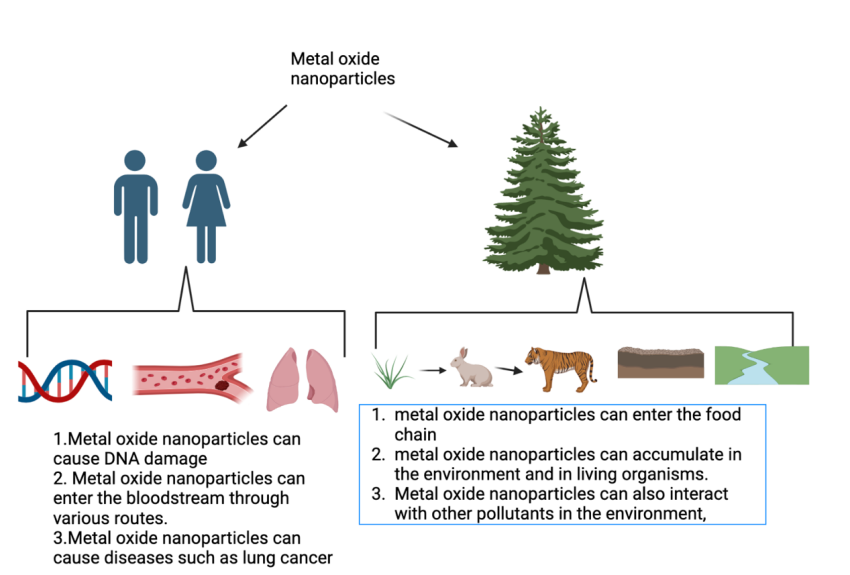
Figure 5. Risks of metal oxide nanoparticles for human health and environment.
3.1. For human health
Studies have shown that some metal oxide nanoparticles (MONPs) can induce oxidative stress, inflammation, and DNA damage in human cells, leading to various diseases such as cancer, respiratory disorders, and neurodegenerative diseases.
One of the major concerns is the toxicity of MONPs, which can be influenced by their size, shape, surface charge, and chemical composition. Metal oxide nanoparticles may interact with cellular structures and biomolecules, such as proteins and DNA. The free radicals produce by MONPs products like sunscreen may damage the DNA [13]. There are two possible reasons for the DNA destruction which are the formation of (ROS) and the penetration of zinc oxide and titanium oxide to the skin [13]. ROS are highly reactive molecules that contain oxygen and are generated as natural byproducts of various cellular metabolic processes. These include superoxide anion (O2•-), hydrogen peroxide (H2O2), hydroxyl radical (OH•), and singlet oxygen (1O2), among others and it plays a crucial role in various physiological and pathological processes. According to the experiments by Dunford, the different species have different penetration of nanoparticles and in normal circumstances, in rabbits, rats, pigs and humans, the penetration of nanoparticles of human skin is the worst [14]. Therefore, whether the formation of ROS can damage human health depends on the degree of penetration. The scientist should focus on the risk of nanoparticles is decided by the inherent toxicity (hazard) and the exposure [15].
The metal oxide nanoparticles may lead to inflammation. Cho et al. designed an experiment to research the unique inflammation in the lung by different metal oxide nanoparticles. According to the findings, only CeO2 nanoparticles, NiO nanoparticles, ZnO nanoparticles, and CuO nanoparticles—but only at high doses—can cause lung inflammation [16]. Small amounts of metal oxide nanoparticles do not trigger an immune response in the lung.
In conclusion, the potential risks of metal oxide nanoparticles on human health depend on the properties of the nanoparticles, the route of exposure, and the duration and frequency of exposure. Further research is needed to fully understand the mechanisms of toxicity and to develop strategies to mitigate the risks associated with their use.
3.2 For environment
The nanoparticles can accumulate in the environment and living organisms. nanoparticles, which are particles with dimensions between 1 and 100 nanometers, can be released into the environment through various sources such as industrial processes, consumer products, and natural phenomena like volcanic eruptions [17].
Overall, more research and analysis are needed to fully understand the potential dangers of metal oxide nanoparticles in the human body. In order to ensure their safety and reduce any possible risks, it is crucial to continue researching their characteristics and impacts on human health.
4. Conclusion
In conclusion, Due to their ability to absorb and scatter UV rays, metal oxide nanoparticles such as titanium dioxide and zinc oxide are commonly found in sunscreens. These nanoparticles have several advantages over conventional organic sunscreen agents, including better stability, a broader UV absorption spectrum, and lower skin penetration. However, concerns have been raised about the possible risks associated with the use of metal oxide nanoparticles in sunscreens, despite their benefits. Some studies have indicated that these nanoparticles may be harmful to the environment and may pose health risks, including skin irritation and even some cancer. Furthermore, they may have a negative impact on the environment. Some research demonstrates that these nanoparticles may can accumulate along the food chain. At present, the potential risk of nanoparticles has been paid much attention prevalently. More time and research are needed to better learn about the risks associated with metal oxide nanoparticles and to promote the development of safe and effective sunscreen products.
References
[1]. Polshettiwar, V., & Varma, R. S. 2010 Green Chemistry 12 743-754.
[2]. Mastrotheodoros, G., Beltsios, K. G., & Zacharias, N. 2010 Mediterranean Archaeology and Archaeometry 10 37-59.
[3]. Salavkar, S. M., Tamanekar, R. A., & Athawale, R. B. 2011 International Journal of Green Pharmacy (IJGP), 5 3
[4]. Smijs, T. G., & Pavel, S. 2011 Nanotechnology, Science and Applications, 4 95-112.
[5]. Gubitosa, J., Rizzi, V., Fini, P., & Cosma, P. 2020 Nanocosmetics 18 349-373
[6]. Bovero, A. 2011 Dermocosmetologia: dall’inestetismo al trattamento cosmetico (Milano: Tecniche Nuove)
[7]. Lee, C. C., Lin, Y. H., Hou, W. C., et al. 2020 International Journal of Environmental Research and Public Health, 17 6088.
[8]. Ma, Y., Yoo, J., 2021. J Cosmet Dermatol 20 1044–1049.
[9]. Kim, T. H., Park, S.H., Lee, S., Bharadwaj, A.V.S.L.S., et al. 2023 Energies 16 2231.
[10]. Jose, J., Netto, G. 2019 J Cosmet Dermatol 18 315–321.
[11]. Sohn M, Hêche A, Herzog B, Imanidis G. 2014 J Biomed Opt. 19 115005-115005
[12]. Pandika, M. 2018 ACS Cent. Sci. 4 788–790.
[13]. Newman, M.D., Stotland, M., Ellis, J.I. 2009 Journal of the American Academy of Dermatology 61 685–692.
[14]. Nohynek, G.J., Lademann, J., Ribaud, C., Roberts, M.S 2007 Critical Reviews in Toxicology 37 251–277.
[15]. Karlsson, H.L., Toprak, M.S., Fadeel, B. 2015 Handbook on the Toxicology of Metals, 4th ed. vol.1 p.75–112.
[16]. Cho, W. S., Duffin, R., Poland, C. A., et al. 2012 Environmental Health Perspectives, 118 1699-1706.
[17]. Buzea, C., Pacheco, I. I., & Robbie, K. 2007 Biointerphases 2 17-71.
Cite this article
Wu,H. (2023). The Application of Metal Oxide Nanoparticles in Sunscreen and Their Potential Risks. Applied and Computational Engineering,24,210-215.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Polshettiwar, V., & Varma, R. S. 2010 Green Chemistry 12 743-754.
[2]. Mastrotheodoros, G., Beltsios, K. G., & Zacharias, N. 2010 Mediterranean Archaeology and Archaeometry 10 37-59.
[3]. Salavkar, S. M., Tamanekar, R. A., & Athawale, R. B. 2011 International Journal of Green Pharmacy (IJGP), 5 3
[4]. Smijs, T. G., & Pavel, S. 2011 Nanotechnology, Science and Applications, 4 95-112.
[5]. Gubitosa, J., Rizzi, V., Fini, P., & Cosma, P. 2020 Nanocosmetics 18 349-373
[6]. Bovero, A. 2011 Dermocosmetologia: dall’inestetismo al trattamento cosmetico (Milano: Tecniche Nuove)
[7]. Lee, C. C., Lin, Y. H., Hou, W. C., et al. 2020 International Journal of Environmental Research and Public Health, 17 6088.
[8]. Ma, Y., Yoo, J., 2021. J Cosmet Dermatol 20 1044–1049.
[9]. Kim, T. H., Park, S.H., Lee, S., Bharadwaj, A.V.S.L.S., et al. 2023 Energies 16 2231.
[10]. Jose, J., Netto, G. 2019 J Cosmet Dermatol 18 315–321.
[11]. Sohn M, Hêche A, Herzog B, Imanidis G. 2014 J Biomed Opt. 19 115005-115005
[12]. Pandika, M. 2018 ACS Cent. Sci. 4 788–790.
[13]. Newman, M.D., Stotland, M., Ellis, J.I. 2009 Journal of the American Academy of Dermatology 61 685–692.
[14]. Nohynek, G.J., Lademann, J., Ribaud, C., Roberts, M.S 2007 Critical Reviews in Toxicology 37 251–277.
[15]. Karlsson, H.L., Toprak, M.S., Fadeel, B. 2015 Handbook on the Toxicology of Metals, 4th ed. vol.1 p.75–112.
[16]. Cho, W. S., Duffin, R., Poland, C. A., et al. 2012 Environmental Health Perspectives, 118 1699-1706.
[17]. Buzea, C., Pacheco, I. I., & Robbie, K. 2007 Biointerphases 2 17-71.









