1. Introduction
Since 2010, most vehicles continue to use traditional fossil fuels, consuming substantial resources while increasing carbon emissions, statistically, the oil consumed by vehicles annually accounts for 25.5% of global oil consumption, and highway vehicle carbon emissions account for over 80% of total emissions in the transportation sector in 2021 [1]. Hence, restructuring the current energy matrix and focusing on renewable energy has become a necessary choice in today’s society. Addressing the high resource consumption and carbon emissions of the automobile industry, developing new energy vehicles, and accelerating the transition of the energy system from traditional fossil fuels to green, low-carbon new energy are universally accepted measures to tackle environmental pollution and achieve economic sustainability. Compared to internal combustion engine vehicles (ICEVs), new energy electric vehicles perform better, have a longer use-life, and produce less noise during operation. Moreover, new energy electric vehicles have higher energy conversion efficiency, resulting in less environmental pollution, making them both energy-saving and environmentally friendly. With zero emissions and zero pollution, new energy vehicles are advantageous compared to traditional energy sources like gasoline and diesel, effectively addressing the global energy scarcity issue.
The power batteries of new energy vehicles can mainly be categorized into physical, chemical, and biological batteries. Physical batteries, such as solar cells and supercapacitors, generate electricity from physical energy. Biological batteries, such as microbial and enzyme batteries, generate electricity through biochemical reactions. Chemical batteries, like lead-acid batteries (LAB), nickel-metal hydride batteries (Ni/MH), fuel cells, and lithium-ion batteries (LIB), generate electric power through chemical reactions. Chemical power batteries, characterized by environmental friendliness, high safety, and high energy density, have a vast application prospect in the field of new energy automobiles [2].
Recently, countries and regions including the United States, Europe, Japan, and South Korea have elevated the development of the power battery industry to national or regional strategies and have issued a series of specific refinement schemes and measures to accelerate the promotion of the localization and international market capture of relevant industries. In 2012, China recognized new energy vehicles as one of the seven pivotal industries in the country, leading to a period of rapid development, with electric vehicles, especially pure electric vehicles, being the current mainstream. Since 2015, China’s power battery industry has developed rapidly. Currently, it has a global leading scale, the most complete industrial chain, and prominent cost competitive advantages, forming a preliminary comprehensive competitive advantage. From 2015 to 2021, the accumulated capacity of energy storage batteries in China has been steadily increasing (the 2020 installation volume dropped due to the impact of the pandemic), and in 2021, with a 51.2% share, it firmly held the first place worldwide. According to SNE Research, the worldwide installed power battery capacities reached a scale of 296.8 GW during the initial three quarters of 2021, a year-on-year increase of 102.2%, an increase of 731.8% from the 26.7 GWh in 2015. China, the European Union, and the United States contributed to over 90% of the global new energy vehicle market, becoming the main driving force for the rapid growth of the global power battery [3].
However, China’s research and development in new energy vehicles are relatively behind compared to developed countries in Europe and America. At present, although lithium-ion power batteries occupy most of the market, continuously improving in performance and having an increasing lifespan, there are still certain indicators that need to be improved, such as electromotive force, rated voltage, battery capacity, specific energy, energy density, power, capacity efficiency, energy efficiency, charge-discharge efficiency, usage lifespan, cost, safety, etc. The battery should meet basic advantages such as elevated specific energy, increased specific power, enhanced charge-discharge efficiency, relative stability, low usage cost, and good safety. Meanwhile, with the significant increase in the number of new energy electric vehicles, the scale of retired power batteries in China is expected to exceed 100 GWh by 2025. In terms of the quantity, type, and distribution area of retired batteries, the retirement volume is positively correlated with the amount in use, and the accumulated retirement volume of ternary batteries is relatively high. This article will present an overview of the current development status and future trends of commercially available power battery development.
2. The development status of the power battery
2.1. Lead storage battery (LSB)
In 1859, Gaston Planté first proposed the concept of a rechargeable lead-acid battery (Pb‖H2SO4‖PbO2). During the discharge process, the PbO2 positive electrode is reduced to form PbSO4, and the Pb negative electrode is oxidized to form the PbSO4 compound [4]. After more than 160 years of development, lead-acid battery technology has made significant strides in theoretical research, product design, production process, and capacity performance. Lead-acid batteries, due to their mature technology, low cost, excellent stability, and safety, have found widespread application in the field of small electric vehicles [5, 6]. Despite their widespread use, are constrained by a set of inherent drawbacks, which include a relatively low energy density, limited cycle life, and a modest charge/discharge rate [7]. These shortcomings have impeded the expansion of lead-acid batteries in the domain of large-scale energy storage. Particularly, concerning energy density, lead-acid batteries only achieve 30~40% of their theoretical limit, which pales in comparison to lithium batteries that realize up to 90% of their theoretical energy density limit [4].
In the past decade, the focus of lead-acid battery research has been on lead-carbon batteries. By incorporating high-activity carbon materials into the negative electrode, it is possible to effectively mitigate the rapid degradation of capacity caused by the formation of sulfate compounds on the negative electrode under certain charged states. This development can effectively enhance the cycle life of the battery and improve its fast charging and discharging capabilities. Anode additives with unique porous structures and relatively high hydrogen evolution potentials can enhance the cyclic performance of lead-acid batteries. Chen et al investigated the inclusion of vanillin in the negative lead paste results in increased water demand during paste mixing and alters the apparent density of the paste. These changes ultimately impact the battery’s cyclic performance under partial state of charge conditions. The addition of vanillin effectively reduces the water loss of the battery under 100% depth of discharge (DOD) cycles, thereby prolonging battery life.
The issue of charging lead-carbon batteries has consistently been a significant factor hindering their development. An increased charging speed leads to greater battery polarization and more severe hydrogen evolution. However, fast charging remains a trend in future market development, and a higher charging acceptance ability continues to be a crucial performance index that lead-carbon batteries strive to achieve.
2.2. Ni-MH battery (Ni-MH)
The nickel-metal hydride (Ni-MH) battery comprises an anode of nickel hydroxide and a multicomponent alloy cathode, comprised of metallic elements like vanadium, manganese, and nickel, as a mature secondary battery, which boasts numerous advantages including high safety, superior assembly properties, abuse tolerance, high recyclability, and environmental friendliness [8]. Consequently, it finds extensive application in sectors such as new energy vehicles, electric tools, consumer electronics, emergency devices, and military equipment. Nevertheless, its relatively lower specific energy puts it at a disadvantage in the market competition against Lithium-ion batteries.
In comparison to lead-acid batteries, nickel-metal hydride batteries have tripled the energy volumetric density and tenfold increased the specific power. The distinctive benefits of this technology include a higher operating voltage, superior energy and power density, enhanced tolerance to overcharge and discharge, and enhanced thermal performance. By April 2016, the cumulative sales of Hybrid Electric Vehicles (HEVs) employing nickel-metal hydride (Ni-MH) batteries globally had surpassed 11 million units. Whereas, the use of nickel-metal hydride batteries reveals several problems, which are detailed as follows:
(1) The electrolyte may freeze at lower temperatures, thereby precluding further charging and discharging activities.
(2) Over the course of prolonged usage, a steady depletion of the electrolyte and gradual evaporation of moisture occurs, leading to the phenomenon of electrolyte dry-out.
(3) During high-current charging and discharging processes, the internal pressure of the battery increases, precipitating potential alkali leakage. This, coupled with negative electrode oxidation and pulverization, exerts a detrimental effect on the battery’s lifespan.
High-density spherical Ni (OH)2 has been widely employed in nickel-metal hydride batteries due to its potential to increase the filler volume and discharge capacity of electrode materials. A significant disadvantage associated with nickel electrodes pertains to volumetric expansion during cycling. This phenomenon is attributable to phase transitions of the active material, which manifest as volumetric expansion and contraction during the transformation process. In recent years, research emphasis has been primarily concentrated on enhancing the shape, chemical composition, particle size distribution, structural defects, and surface activity of spherical Ni (OH)2 in order to further improve material attributes such as tap density, discharge capacity, and cyclic stability. It has been found that the formation of Ni1-xCox (OH)2 solid solutions via cobalt doping can effectively improve reaction reversibility, enhance mass transfer and conductivity, elevate the oxygen evolution potential, decrease internal battery pressure, and enhance material utilization.
2.3. Fuel cell
A fuel cell is an electrochemical apparatus that transforms the chemical energy of fuel into electrical energy. Proton exchange membrane fuel cells (PEMFCs) currently represent the most prevalent form of fuel cell development, possessing distinctive advantages such as environmental benignity, high efficiency of energy conversion, superior power density, negligible thermal radiation and emission, and minimal sound pollution. Accordingly, the market prospects for PEMFCs are broad, with their applications keep growing—from portable electronics and tiny fixed-base stations to the aerospace, defence, and all-electric vehicle industries [9].
In PEMFCs, the Proton Exchange Membrane (PEM) is the critical component, providing a channel for hydrogen ions while isolating the reaction gases at both poles. Presently, the large-scale commercialization of PEMFCs is still confronted with two major challenges: high raw material costs and short battery life. Hence, addressing these two major issues constitutes the current research focus in the field of PEMFCs [10].
Currently, Nafion series perfluorosulfonic acid proton exchange membranes are the most widely used both domestically and internationally. These membranes possess several advantages, including solid mechanical strength, stable chemical performance, and high conductance under the setting of high humidity. Moreover, these membranes exhibit a significant current density at moderate temperatures, which facilitates proton conduction. The primary drawbacks are as follows:
(1) The membrane tends to undergo chemical degradation as the temperature rises, leading to diminished proton conductivity.
(2) The synthesis process for the monomer is demanding, resulting in high material costs and, consequently, a steep final product price.
(3) When utilized in methanol fuel cells, methanol is prone to permeation.
In the recent past, scholars have discovered that nitrogen-containing heterocyclic compounds like imidazole, triazoles, and tetrazoles (as shown in Figure 1) also possess commendable proton conduction capabilities. However, owing to the small molecular structure of these compounds, their direct incorporation into proton exchange membranes results in gradual seepage over the prolonged operation, leading consequently to a steady decline in the membrane's proton conductivity until it ceases to function. A common solution to this predicament involves anchoring the nitrogen-containing heterocyclic functional groups to polymer molecules or specific nanostructured frameworks through chemical bonds.
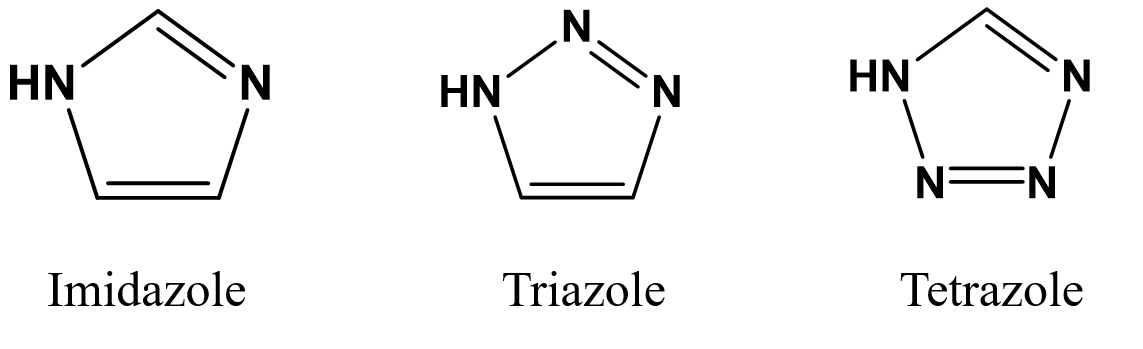
Figure 1. The molecular structure of nitrogenous heterocyclic compounds.
Perfluorosulfonic acid membranes exhibit several drawbacks, including high raw material costs, inoperability at elevated temperatures, and environmental harm. Therefore, the future ought to be committed to the development of cost-effective and high-performance non-fluorinated proton exchange membranes. Proton-conductive and chemically stable nitrogen heterocycle-based proton exchange membranes, including those from imidazole, triazole, and tetrazole, exhibit exceptional performance even under dehydrated or dry circumstances. These advantages herald promising application prospects within the realm of proton exchange membranes. Consequently, the advancement and exploration of nitrogen heterocyclic compounds need to be vigorously pursued.
2.4. Lithium-ion battery(LIB)
A crucial turning point in the evolution of lithium secondary batteries was marked by Professor Goodenough’s 1980 report regarding layered lithium cobalt oxide (LiCoO2) as a cathode material for lithium batteries. The discovery of lithium cobalt oxide as a cathode material not only enhanced the working voltage and energy density of batteries but also paved the way for an anode devoid of metallic lithium. In 1985, Japanese scientist Yoshino proposed a lithium secondary battery that utilizes lithium cobalt oxide as a cathode and petroleum coke capable of reversibly intercalating lithium ions as an anode, which was commercialized in collaboration with Sony in 1991. Subsequently, graphite replaced petroleum coke as the battery anode, creating what is commonly referred to as the “lithium-ion battery”. Considered among the most important inventions in human history, lithium-ion batteries significantly improved energy utilization efficiency and catalyzed a revolution in information and electronic technologies.
Lithium-ion batteries are primarily made up of components such as cathode materials, anode materials, and electrolytes. From the perspective of the working principle of lithium-ion batteries, enhancing the insertion and extraction capacity of lithium ions serves as the principal approach to improving battery capacity. Notably, the cathode material constitutes the main lithium-ion source, and it decisively impacts the overall electrochemical performance, safety, and cost of the battery. Therefore, to make a breakthrough in the energy density of power batteries, the research on cathode materials becomes exceedingly significant [11].
Cathode materials, based on structural types, primarily fall into three categories: layered oxides, spinel oxides, and polyanion compounds. Figure 2 provides an overview of the developmental trajectory of cathode materials [12].
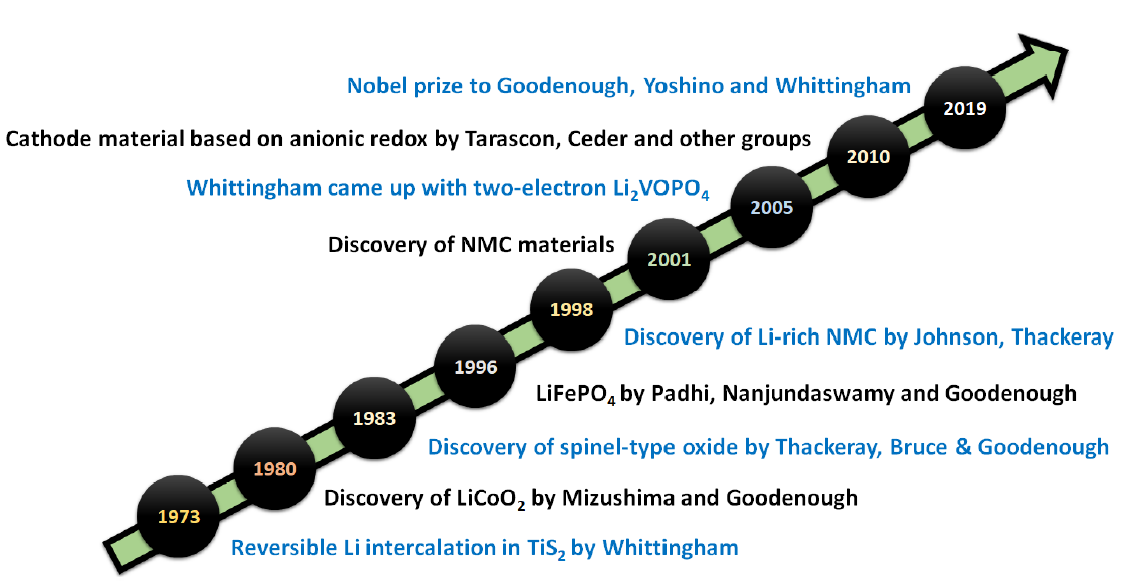
Figure 2. The evolution of cathode materials in lithium-ion battery technology [12].
2.4.1. Layered oxide cathode materials. Representative layered oxide cathodes encompass LiMO2 (M = Co, Ni, Mn), ternary materials such as LiNi1−y−zMnyCozO2 (NMC), LiNi1−y−zCoyAlzO2 (NCA), as well as lithium-rich manganese-based materials of the form (1−y)Li2MnO3·yLiMO2 (M = Mn, Co, Ni, Ru, etc.). The predominant advantage of layered oxide materials lies in their high energy density.
Despite the dominance of LiCoO2 as a cathode material, its market share is progressively diminishing due to inherent drawbacks, including high cost, substantial toxicity, and poor thermal stability. In NMC cathode materials, cobalt is employed to stabilize the structure, nickel enhances capacity, and manganese reduces cost and improves thermal stability. The contemporary demand in the electric vehicle market for high energy density has stimulated the development of high-nickel ternary materials (mol% Ni > 0.6). As the nickel’s proportion increases, the specific capacity of the material amplifies, but the capacity retention rate and heat resistance correspondingly decrease. To augment electrochemical performance and safety, it is requisite to employ strategies such as bulk doping, surface coating, microscopic structure design, and single crystallization. The Layered Li-rich Manganese (LLRM) cathode material exhibits several notable advantages. First, it offers excellent stability, and its manganese element is non-toxic, thereby minimally polluting the environment. Second, it is cost-effective, with manganese being more affordable than nickel and abundant in reserves within our country. Utilizing lithium manganese as the cathode material significantly reduces the cost of batteries. Third, it possesses a high specific capacity. The theoretical specific capacity of lithium manganese material can reach 283 mAh·g–1, while its actual values also attain a high range of 160 to 190 mAh·g–1. However, LLRM encounters challenges such as poor starting Coulombic efficiency, inadequate rate capability, and serious depreciation of voltage and capacity [13].
2.4.2. Spinel-type cathode materials. The representative spinel-type oxide cathodes mainly include LiMn2O4 (4 V vs. Li+/Li0) and LiNi0.5Mn1.5O4 (4.7 V vs. Li+/Li0). These types of cathode materials exhibit advantages such as low cost, relatively high voltage, and environmental friendliness. However, the reversible specific capacity of LMO is relatively low (120 mAh·g−1), and it is affected by significant capacity fading after multiple cycles. The substitution of Mn in LMO with Ni results in the fabrication of LiNi0.5Mn1.5O4 cathode materials, possessing a theoretical capacity of 147 mAh·g−1 and a high voltage reaching 4.8 V. The high voltage of 4.7 V presents a challenge to the existing electrolyte systems. Concurrently, lithium nickel manganese oxide exhibits susceptibility to corrosion by hydrofluoric acid. These factors contribute to problems in LiNi0.5Mn1.5O4 such as poor high-temperature cycling, low coulombic efficiency, and decomposition of the electrolyte under high voltage. Common modifications to LiNi0.5Mn1.5O4 encompass microscopic morphology regulation, doping, coating, and matching with high-voltage electrolytes [14].
2.4.3. Polyanion-type cathode materials. Polyanion-type cathode materials are typically denoted by the chemical formula LixMy(XOn)z, where ‘M’ represents transition metal elements such as Fe, Ni, Mn, and ‘X’ denotes elements like S, P, Si, and B. In comparison to oxides, the chemical composition and crystal structures of polyanion cathodes are rather diverse, encompassing sulfates, phosphates, silicates, borates, and others. Lithium iron phosphate (LFP), bearing an olivine structure, has been commercialized owing to its stable charge-discharge platform, environmental friendliness, and elevated safety [14]. Efforts to improve its conductivity and energy density primarily involve modifications such as ion doping, surface coating, morphological control, and the addition of lithium-enriched materials. Present explorations into modification techniques, such as sulfate coating or carbon coating, are less extensive than those for phosphates, indicating the future potential for performance enhancement and commercialization. Moreover, research into low-cost mixed polyanion cathode materials is presently scant, representing a valuable direction for future study.
3. Development trends of power batteries
3.1. Sodium-ion battery (SIB)
Sodium’s abundance in the Earth’s subsoil is as high as 2.64%, far exceeding lithium’s 0.002%, exhibiting a balanced and extensive global distribution. Correspondingly, the price of related raw materials is low, and the environmental impact is benign. Importantly, both sodium and lithium ions, belonging to the first main group of the periodic table, possess electrochemical potentials of –2.71 V and –3.05 V, respectively. Assembled batteries from both elements display nearly identical charging and discharging platform potentials, demonstrating very similar physical and chemical properties. Consequently, sodium-ion batteries have emerged as a highly prospective energy storage technology potentially replacing lithium-ion batteries [15].
The future direction of sodium-ion batteries is directly correlated with their characteristics. Considering energy density, the cells of sodium-ion batteries typically offer 105~150 Wh/kg. In contrast, the lithium-ion battery cells’ energy density is generally over 190 Wh/kg, even exceeding 230 Wh/kg for ternary systems with high nickel content. It is clear that, at present, sodium-ion batteries fall short when compared to ternary lithium batteries. However, in comparison to the energy density of lithium iron phosphate batteries, which ranges between 120~200 Wh/kg, and lead-acid batteries, which ranges between 35~45 Wh/kg, sodium-ion batteries are already capable of partially overlapping or even superseding these batteries in their application domains. This indicates a potential avenue for the advancement of sodium-ion battery technology and its broader applications.
Future advancements in material improvements will enhance the maturation of materials and the scalability of industrial production, thereby bolstering the energy density of sodium-ion batteries. Furthermore, these developments will result in a substantial decrease in the expense of anode and cathode materials.
3.2. Solid-state Li-ion battery (SLIB)
In traditional lithium-ion batteries, an inverse relationship exists between energy density and stability, wherein a higher energy density often correlates with poorer stability and enhanced potential safety hazards. An increased battery energy density frequently necessitates the use of high-nickel cathode materials. Nickel, however, demonstrates a potent catalytic effect on the decomposition of liquid electrolytes. As nickel content rises, the likelihood of unwanted side reactions in the electrolytic solution also increases, leading to greater heat release. This subsequently induces further side reactions, ultimately culminating in thermal runaway. This phenomenon is a fundamental cause underlying the majority of safety incidents associated with lithium-ion batteries in power applications. In contrast to traditional lithium-ion batteries, all-solid-state lithium batteries employ a solid electrolyte to substitute the conventional liquid electrolyte system and separator, a modification which is anticipated to significantly enhance battery safety, energy density, and lifespan [16].
As a crucial component of all-solid-state lithium batteries, the rational application of solid electrolytes is contingent upon meeting a range of criteria, which encompasses achieving elevated ionic conductivity, excellent mechanical properties, and compatibility with electrode materials. That is, in terms of the overall development of solid-state batteries, the stability of the electrolyte/electrode interface remains a significant challenge for lithium-ion transmission. Thus, in the prospect of future research on solid-state batteries, it is pertinent to continue placing emphasis on improving the ionic conductivity of materials and enhancing the stability of interfaces. Such a focus facilitates the targeted design of high-performance solid-state electrolyte systems, which are instrumental in the development of lithium batteries with high safety and high energy density [17].
4. Conclusion
The propulsion in electric vehicles is derived from their power batteries. The characteristic of low energy density in these batteries often results in a short driving range for electric vehicles, thereby posing a hindrance to the development of both power batteries and electric vehicles. Consequently, the development trajectory for power batteries should focus on traits such as rapid charging, comprehensive supporting infrastructure, compact size and light-weight, high energy storage per charge with sufficient range, stable performance, high safety, ease of maintenance, lifespan exceeding other vehicle components, ample raw material availability, cost-effectiveness, and environmental friendliness. The major development trends of five key types of batteries are as follows.
(1) The development of novel anode active material additives is pivotal to enhancing the actual energy density of lead-storage batteries and prolonging their cycle life, thus representing significant research value and practical implications.
(2) The pursuit of high-energy, high-power output, wide-temperature performance, and low self-discharge Ni-MH batteries at low costs is instrumental in bolstering their market position, particularly in the domains of power batteries and energy storage.
(3) Currently, the research and development level of fuel cells is relatively low, and the cost is considerably high. The overall industry chain is not well developed, encountering issues such as immature pure hydrogen preparation technology, the need for breakthroughs in large-scale seawater hydrogen production technology, and excessive costs of hydrogen storage and transportation. Continual optimization and perfection are required for their effective application in new energy vehicles.
(4) As the application of lithium-ion batteries becomes increasingly widespread, higher performance requirements are set in terms of capacity, cost, cyclic performance, voltage, solid electrolytes, and environmental friendliness. Given the vital role of cathode materials in lithium-ion batteries, intensified research and development efforts targeting these materials are inevitable.
(5) Despite significant research on the material structure and system of sodium-ion batteries and their demonstrated decent electrochemical performance, there are still limited electrode material systems available for large-scale sodium-ion batteries. The capacity and rate performance remain key constraints to their development. With ongoing in-depth research, issues related to the batteries' specific capacity and cycle stability are expected to be further resolved and improved, thus promising large-scale application of sodium-ion batteries in energy storage systems in the future.
References
[1]. Kempton W. (2016) Electric vehicles: Driving range. Nature Energy. 1(9): 16131.
[2]. Yu Y, Song Y, Mao J. (2019) Quantitative analysis of the material, energy and value flows of a lead-acid battery system and its external performance. Science of the Total Environment. 688: 103-111.
[3]. Yan J, Tseng F-M, Lu L Y Y. (2018) Developmental trajectories of new energy vehicle research in economic management: Main path analysis. Technological Forecasting and Social Change. 137: 168-181.
[4]. Lopes P P, Stamenkovic V R. (2020) Past, present, and future of lead–acid batteries. Science. 369(6506): 923-924.
[5]. Shapira R, Nessim G, Zimrin T, Aurbach D. (2013) Towards promising electrochemical technology for load leveling applications: extending cycle life of lead acid batteries by the use of carbon nano-tubes (CNTs). Energy & Environmental Science. 6: 587.
[6]. Ding L X, Zheng F L, Wang J W, et al. (2012) Super-large dendrites composed of trigonal PbO2 nanoplates with enhanced performances for electrochemical devices. Chemical Communications. 48(9): 1275-1277.
[7]. Jiang X, Wang Y, Herricks T, Xia Y. (2004) Ethylene glycol-mediated synthesis of metal oxide nanowires. Journal of Materials Chemistry. 14(4): 695.
[8]. Yang C C, Wang C C, Li M M, Jiang Q. (2017) A start of the renaissance for nickel metal hydride batteries: a hydrogen storage alloy series with an ultra-long cycle life. Journal of Materials Chemistry A. 5(3): 1145-1152.
[9]. Steele B C H, Heinzel A. (2001) Materials for fuel-cell technologies. Nature. 414(6861): 345-352.
[10]. Tian H, Cui X, Shi J. (2021) Emerging electrocatalysts for PEMFCs applications: Tungsten oxide as an example. Chemical Engineering Journal. 421: 129430.
[11]. Li M, Lu J, Chen Z, Amine K. (2018) 30 Years of Lithium-Ion Batteries. Advanced Materials. 1800561.
[12]. Ghosh S, Charjee U B, Bhowmik S, et al. (2021) A Review on High-Capacity and High-Voltage Cathodes for Next-Generation Lithium-ion Batteries. Journal of Energy and Power Technology. 4(1): 1-59.
[13]. Park N-Y, Park G-T, Kim S-B, et al. (2022) Degradation Mechanism of Ni-Rich Cathode Materials: Focusing on Particle Interior. ACS Energy Letters. 7(7): 2362-2369.
[14]. Zhou L, Kwok C Y, Shyamsunder A, et al. (2020) A new halospinel superionic conductor for high-voltage all solid state lithium batteries. Energy & Environmental Science. 13(7): 2056-2063.
[15]. Rojo T, Hu Y-S, Forsyth M, Li X. (2018) Sodium-Ion Batteries. Advanced Energy Materials. 8(17): 1800880.
[16]. Yu X, Chen R, Gan L, Li H, Chen L. (2023) Battery Safety: From Lithium-Ion to Solid-State Batteries. Engineering. 21: 9-14.
[17]. Chang X, Zhao Y-M, Yuan B, et al. (2023) Solid-state lithium-ion batteries for grid energy storage: opportunities and challenges. Science China Chemistry. (in press).
Cite this article
Zhou,Z. (2023). Current state and future trends of power batteries in new energy vehicles. Applied and Computational Engineering,26,35-42.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Kempton W. (2016) Electric vehicles: Driving range. Nature Energy. 1(9): 16131.
[2]. Yu Y, Song Y, Mao J. (2019) Quantitative analysis of the material, energy and value flows of a lead-acid battery system and its external performance. Science of the Total Environment. 688: 103-111.
[3]. Yan J, Tseng F-M, Lu L Y Y. (2018) Developmental trajectories of new energy vehicle research in economic management: Main path analysis. Technological Forecasting and Social Change. 137: 168-181.
[4]. Lopes P P, Stamenkovic V R. (2020) Past, present, and future of lead–acid batteries. Science. 369(6506): 923-924.
[5]. Shapira R, Nessim G, Zimrin T, Aurbach D. (2013) Towards promising electrochemical technology for load leveling applications: extending cycle life of lead acid batteries by the use of carbon nano-tubes (CNTs). Energy & Environmental Science. 6: 587.
[6]. Ding L X, Zheng F L, Wang J W, et al. (2012) Super-large dendrites composed of trigonal PbO2 nanoplates with enhanced performances for electrochemical devices. Chemical Communications. 48(9): 1275-1277.
[7]. Jiang X, Wang Y, Herricks T, Xia Y. (2004) Ethylene glycol-mediated synthesis of metal oxide nanowires. Journal of Materials Chemistry. 14(4): 695.
[8]. Yang C C, Wang C C, Li M M, Jiang Q. (2017) A start of the renaissance for nickel metal hydride batteries: a hydrogen storage alloy series with an ultra-long cycle life. Journal of Materials Chemistry A. 5(3): 1145-1152.
[9]. Steele B C H, Heinzel A. (2001) Materials for fuel-cell technologies. Nature. 414(6861): 345-352.
[10]. Tian H, Cui X, Shi J. (2021) Emerging electrocatalysts for PEMFCs applications: Tungsten oxide as an example. Chemical Engineering Journal. 421: 129430.
[11]. Li M, Lu J, Chen Z, Amine K. (2018) 30 Years of Lithium-Ion Batteries. Advanced Materials. 1800561.
[12]. Ghosh S, Charjee U B, Bhowmik S, et al. (2021) A Review on High-Capacity and High-Voltage Cathodes for Next-Generation Lithium-ion Batteries. Journal of Energy and Power Technology. 4(1): 1-59.
[13]. Park N-Y, Park G-T, Kim S-B, et al. (2022) Degradation Mechanism of Ni-Rich Cathode Materials: Focusing on Particle Interior. ACS Energy Letters. 7(7): 2362-2369.
[14]. Zhou L, Kwok C Y, Shyamsunder A, et al. (2020) A new halospinel superionic conductor for high-voltage all solid state lithium batteries. Energy & Environmental Science. 13(7): 2056-2063.
[15]. Rojo T, Hu Y-S, Forsyth M, Li X. (2018) Sodium-Ion Batteries. Advanced Energy Materials. 8(17): 1800880.
[16]. Yu X, Chen R, Gan L, Li H, Chen L. (2023) Battery Safety: From Lithium-Ion to Solid-State Batteries. Engineering. 21: 9-14.
[17]. Chang X, Zhao Y-M, Yuan B, et al. (2023) Solid-state lithium-ion batteries for grid energy storage: opportunities and challenges. Science China Chemistry. (in press).









