1. Introduction
Owing to the swift development and rapid population growth in today's world, the substantial anthropogenic emissions of greenhouse gases have resulted in a global average temperature rise exceeding 1.2°C above pre-industrial levels [1]. In December 2015, At the UN Climate Change Conference (COP21) in Paris, France, the Paris Agreement was formally adopted by 196 parties. This agreement addresses climate change by setting and achieving incremental goals to reduce the gradual increase in the global average temperature. The ultimate aim is to minimize the increase in temperature to below 2°C compared to pre-industrial levels, becoming carbon neutral by 2050.
Extensive human activities, including the substantial consumption of fossil fuels, have resulted in excessive emissions of CO2. Much of this CO2 remains unsequestered due to ecosystem degradation from overexploitation. Addressing how to reduce carbon emissions and effectively capture and store CO2 is vital to achieving carbon neutrality.
Carbon Capture and Storage (CCS) is employed in industrial and human activities to capture and store CO2 generated during energy conversion processes, thereby preventing its release into the atmosphere. In some developing countries, fossil fuel-based electricity generation remains one of the primary methods. Compared to other carbon emission reduction methods, CCS can effectively alleviate the pressure of CO2 emissions from certain power plants. Moreover, the captured CO2 can be repurposed for secondary uses or sequestered in large-scale sites such as oceans and depleted mineral deposits. CCS technology has been demonstrated as a cost-effective and technically mature solution for reducing carbon emissions [2]. The direct application of CCS technology, based on the current energy infrastructure, can yield substantial environmental benefits. Based on data simulation analysis, achieving carbon neutrality by 2075 and 2000 would cost 12% and 71% more, respectively, without using CCS technology compared to utilizing it. This indicates that CCS technology can ensure cost-effectiveness in reducing carbon emissions while using carbon-intensive goods and energy sources [3]. This potential for high environmental value is a key reason CCS technology possesses significant application potential.
This paper, grounded in the current state of CCS technology, discusses the technical principles and application scopes of three methods: Direct Air Capture (DAC), BECCS, and Oxy-fuel combustion. The article introduces two primary methods of DAC technology and how they are employed in industrial activities. It elucidates some of the principles of oxy-fuel combustion and expands its contribution to waste incineration plants, apart from its use in industrial combustion for power generation. It also describes how BECCS technology integrates with specific fauna, flora, and ecological spaces for carbon capture in urban and natural ecosystems. Through comparative analysis, it highlights the advantages and limitations of these technologies and offers feasible recommendations.
2. Direct Air Capture
DAC is a technique that catches CO2 directly from the atmosphere and stores it afterward. DAC differs from conventional carbon capture technologies that target the source of carbon emissions, as it focuses on the excessive concentrations of CO2 present in the atmosphere. Captured from the air, the CO2 is compressed into a more concentrated stream for subsequent storage and utilization.
Currently, mainstream DAC directly separates CO2 from the atmosphere using artificial contactors. This approach has been demonstrated to be feasible and highly promising in potential net emission schemes. As a direct technical support for DAC, gas separation is becoming a mainstream research focus. Several mature gas separation technologies utilize physical processes. However, the composition of air is not constant, posing a current research challenge in ensuring the selective removal of CO2 while leaving other components. Researchers should select media with a high affinity for CO2 as artificial contactors to facilitate its separation.
2.1. Adsorption
Absorption represents a prevalent technique for DAC, akin to its established use in CO2 capture from stationary sources. The process of using an electricity blower to force air into a filter that has a hydroxide liquid absorbent result in CO2 reacting with sodium hydroxide to form sodium carbonate precipitate (Figure 1). This acid-base reaction facilitates CO2 capture, employing techniques that increase the contact area between gas and liquid to maximize reaction efficiency. The second cycle starts from the causticizer unit. The sodium carbonate produced in the previous process reacts with calcium hydroxide within this module, forming calcium carbonate. The calcium carbonate is then subjected to calcination at 900℃, during which CO2 is released and subsequently captured. The calcium oxide produced is mixed with water in a digester apparatus to regenerate calcium hydroxide. The sodium hydroxide in the reaction products is regenerated and returned to the contactor to initiate another absorption cycle.
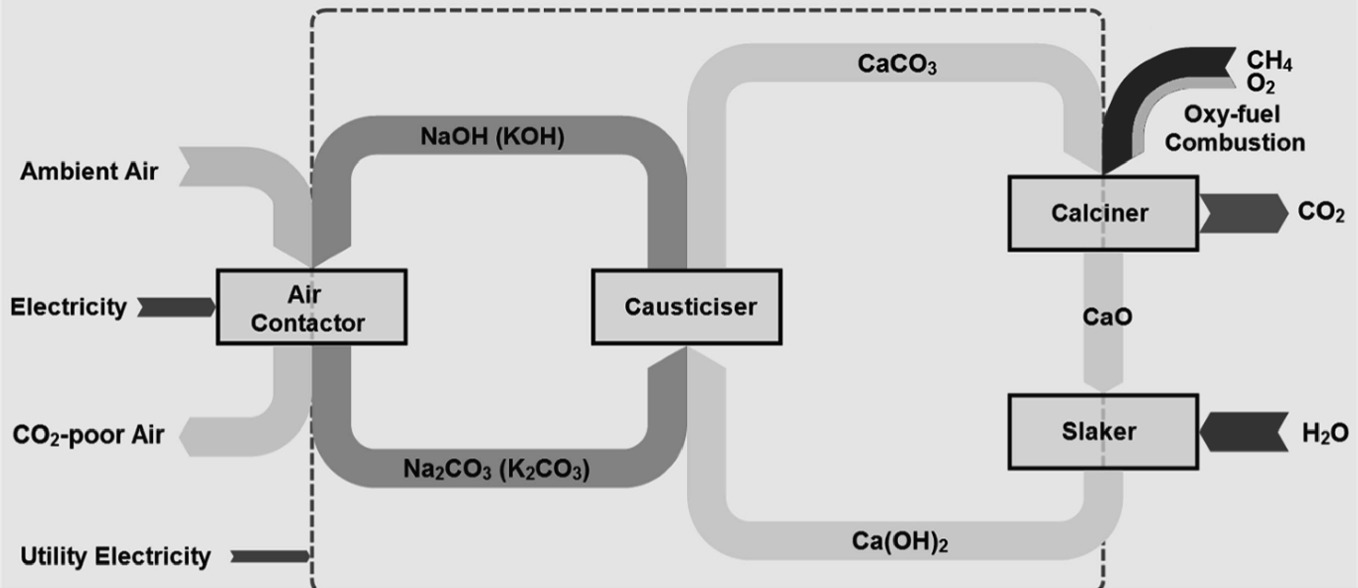
Figure 1: The theory of DAC technology [3]
2.2. Adsorption
Adsorption is a technique that employs solid sorbents instead of liquid solvents to capture CO2 from air chemically, and the CO2 captured undergoes gas-solid interaction. Initiating a chemical reaction between the surface and the adsorbate is what chemisorption involves, which creates new chemical bonds between the adsorbate and the substrate surface to ensure its immobilization. At the end of the cycle, these chemical bonds are disrupted by heating, releasing CO2 from the solid sorbent. The liberated CO2 can enter the recycling system for capture and further use. From the perspective of the operational environment and practical cost, the resin is an effective adsorbent due to its solid-state nature and inherent flexibility. This characteristic enhances its compatibility with mechanical equipment, significantly reducing initial equipment investment costs.
Additionally, this technology can utilize natural airflow instead of fans and blowers to direct air into the filter. Consequently, compared to absorption, adsorption demands less water and occupies a smaller footprint. From another perspective, using solid solvents in adsorption implies that the reactions occur in a relatively low-temperature environment, which can significantly reduce energy waste. Moisture swing adsorption (MSA) and temperature swing adsorption (TSA) are currently the two predominant methods of adsorption, and their reactions involve the moisturizing of the CO2-enriched sorbent [4]. Figure 2 presents methodologies employed by various companies and the temperatures required for DAC through absorption and adsorption processes.
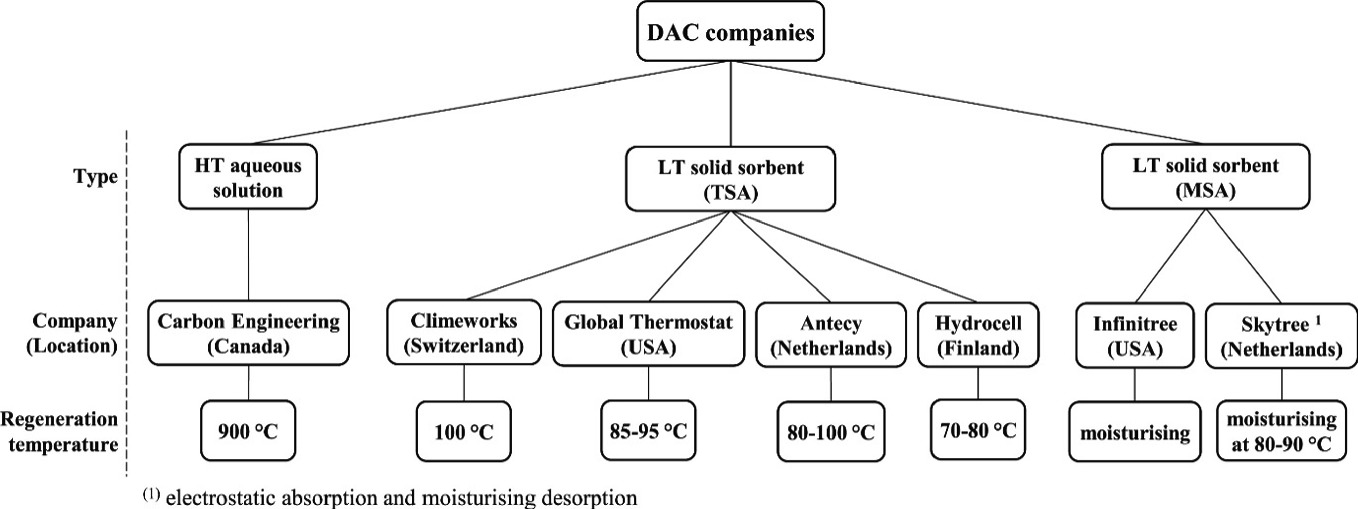
Figure 2: Methodologies employed by various companies and the temperatures required for DAC through absorption and adsorption processes [4]
DAC has a significant advantage over other carbon capture technologies because it can capture CO2 efficiently in relatively small areas. This implies a smaller area of use and higher operational efficiency. Furthermore, it has lower environmental requirements which means it can operate under harsh conditions, continuously and stably capturing CO2. However, DAC has not yet achieved large-scale commercial deployment as an emerging technology. It is constrained by the current development of contactor and adsorbent technologies, with the associated costs range from $264–1,000/tCO2. If the adsorption efficiency of the adsorbents can be enhanced and mechanical equipment improved, the widespread application of DAC technology in the future might become feasible [5].
3. Bio Energy Carbon Capture and Storage
3.1. Urban Spaces nd Natural Ecosystems
In terms of life sciences, organisms themselves can serve as mediators for carbon capture. Bio-capture and bio-sequestration are processes that enhance the ability of organisms and ecosystems to capture CO2. Compared to the aforementioned capture methods, bio-capture of greenhouse gases exhibits characteristics of sustainability and cost-effectiveness, which could potentially confer a competitive advantage leading to broader adoption.
Many ecosystems have demonstrated significant carbon capture potential. For instance, mangroves capture an average of 654 kilograms of carbon per square meter annually [6]. Tropical forests and other natural ecosystems also serve as crucial carbon sinks, effectively storing carbon for extended periods. Biomass from these ecosystems also presents a more sustainable alternative to conventional fuel sources. Therefore, promoting reforestation efforts and prioritizing conservation initiatives are essential strategies to consider. In addition to terrestrial natural ecosystems, the oceans are also significant contributors to carbon capture and storage. As the largest carbon sink on Earth, the oceans store more carbon annually than all the forests combined. Enhancing carbon capture in the oceans through ocean fertilization is an effective method. This involves stimulating the growth of single-celled microalgae such as diatoms and seagrass by releasing CO2 into the ocean. As these microalgae proliferate, they contribute significantly to carbon sequestration [7]. Nonetheless, ocean carbon capture technologies also confront several challenges. First of all, the complexity and fragility of marine ecosystems necessitate careful consideration to prevent adverse impacts on marine biodiversity and ecological balance. Secondly, the feasibility and efficacy of ocean carbon capture technologies require further research and assessment. Additionally, the costs and sustainability of these technologies must be taken into account to ensure their practicality in real-world applications.
In urban spaces, besides traditional methods such as increasing roadside vegetation, we can also introduce new types of microalgae into artificial ponds. These ponds can function as open photobioreactors, capturing CO2 and methane from the urban atmosphere. Flourishing under high CO2 concentrations, these microalgae can double their biomass within 24 hours, making them more suitable than traditional tree planting methods [8].
3.2. Blue Carbon
Blue carbon refers to a biological process that utilizes marine activities and ecosystems, such as phytoplankton and marine plant ecosystems, to capture carbon in oceanic environments. Although coastal biological systems are small, their capacity to capture CO2 is superior to other ecosystems, resulting in their size being unrestricted. At the same time, they can also store CO2 for extended periods.
Mangrove ecosystems constitute a significant and irreplaceable part of carbon capture and storage within coastal ecosystems. Mangroves primarily inhabit coastal regions, where most of their rooted soils consist of submerged anaerobic sediments, with only a small portion of oxygenated soils exposed at the surface. This results in biomass decomposition occurring at a prolonged rate, effectively preventing CO2 captured through photosynthesis from returning to the atmosphere. Additionally, mangrove soils harbor microbial communities, such as Rhizophora, which form biofilms over sediment surfaces (approximately 1-2 millimeters thick) to prevent greenhouse gases from escaping during low-tide exposure to sunlight from leaf litter and other biomass. Every year, 45-250 million tons of CO2 is captured by the sediment in forest soil and the biomass surrounding mangrove roots [9].
As depicted above, tidal salt marshes also play a crucial role in carbon capture. Unlike mangroves, salt marshes are dominated by salt-tolerant shrubs and herbaceous plants, which are the primary contributors to carbon capture. The eco-structure of tidal salt marshes is unique, allowing for the sequestration of 428 teragrams (Tg) of carbon per year. Compared to other ecosystems, such as some tropical rainforests, they can even exceed the total carbon fixation capacity of all the tropical rainforests on Earth combined.
The issues faced by mangrove ecosystems are analogous to the shortcomings of marine ecosystems; their singular ecosystem structure renders them vulnerable. In the context of climate change, these ecosystems often fail to optimally fulfill their carbon sequestration roles. In the field of biological carbon capture technologies and methods, besides the need for developing new technologies, there is a growing recognition of the critical role that certain plants within ecosystems play in the carbon capture process. This implies that these plants should be protected from environmental changes and developmental disruptions.
4. Oxy-Fuel Combustion
Oxy-fuel combustion represents a promising CCS technology aimed at addressing the excessive CO2 emissions associated with power generation. While some developed nations and regions, such as the United States and European union, are progressively phasing out traditional coal-based fossil fuels, achieving notable progress. Many developing countries, including China and India, continue to have a substantial demand for coal [10]. Fossil fuels remain a crucial component of our current energy matrix, indicating the inevitable reality of significant CO2 emissions from fossil fuel combustion.
Oxy-fuel combustion technology integrates an air separation unit into the power generation process of coal plants. An air separation unit separates oxygen and nitrogen, and the enriched oxygen is then introduced into the boiler. The generated steam is directed into a turbine for electricity generation, while the resultant high-concentration CO2 is condensed and purified, then compressed into solid form, facilitating subsequent utilization and storage. After condensation and separation, the recovered flue gas can also be used as an oxidant to re-enter combustion. This process achieves the objective of carbon capture. The advantage of oxy-fuel combustion technology lies in the preliminary filtration of nitrogen and other gases, ensuring that the exhaust gas primarily comprises water vapor (H2O) and CO2. Additionally, emissions related to nitrogen, such as NOx, are significantly reduced. Figure 3 presents the flowchart of the oxy-fuel combustion process used to generate power from fuels.
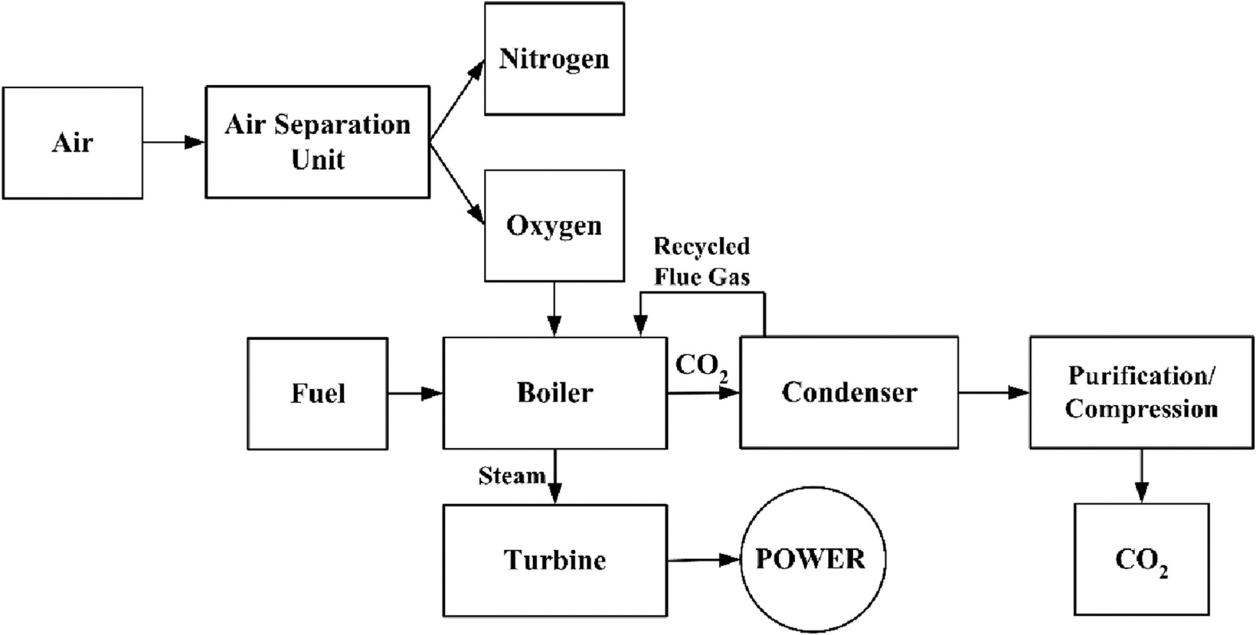
Figure 3: The flowchart of the process for oxy-fuel combustion to generate electricity from fuel [10].
In the oxy-fuel combustion process at coal-fired power plants, a novel system has been introduced that merges the condensation of CO2 with the purification of flue gases. By increasing the condensation pressure to 3.0-4.0 MPa, experiments have yielded a 99.9% pure CO2 gas stream. Additionally, the system has demonstrated a removal efficiency of over 99% for both SO2 and NO. Regarding carbon capture efficiency, this system achieves a rate of 92.61%, considered high within the current technological sphere [11]. Given the present energy infrastructure, coal-based fuel power plants still constitute a significant portion, necessitating many oxy-fuel combustion systems. This underscores the vast potential for application in the CCS domain.
Oxy-fuel combustion technology is not solely confined to industrial applications; it can also substantially contribute to carbon capture in urban space. The discussed studies suggest that oxy-fuel combustion is a promising technology due to its direct application to existing power plants and large-scale CO2 -emitting combustion facilities, including biomass.
According to data from the World Bank, biomass accounts for over 50% of global annual waste [12]. Biomass waste, characterized by its high calorific value and abundant availability, can be utilized effectively for power generation or energy production, which supports sustainable environmental practices and circular economic growth. However, the combustion of biomass inevitably results in significant CO2 emissions due to its primary composition of organic materials. In this context, the application of carbon capture technologies becomes essential to mitigate carbon emissions.
In Klemetsrud CHP, Norway, a carbon capture system is fitted into the waste-to-energy plant, which processes roughly 400,000 tons of non-recyclable waste annually. This facility produces 55 MW of thermal energy for district heating and 10.5 MW of electricity. Utilizing oxy-fuel combustion technology, it captures 400,000 tons of CO2 each year, which is then permanently sequestered in the North Sea [13]. In Zorran City, Japan, the waste incineration facility employs a Toshiba-engineered alkaline water amine carbon capture technique, which secures 10 tons of CO2 each day. This CO2 is subsequently utilized in the cultivation of local crops and the growth of algae.
Overall, the application of oxy-fuel combustion technology in power generation is advantageous. Oxy-fuel combustion occurs in an environment with a higher oxygen concentration, leading to increased reaction temperatures that enhance combustion efficiency and further reduce energy consumption. Additionally, the technology can significantly decrease the volume of flue gases to around one-fifth, as approximately 80% of flue gases in air combustion are nitrogen, which results in an increase in boiler efficiency [14].
However, the technological advancement of oxy-fuel combustion is still in its initial stages, requiring extensive testing and pilot operations to prove its feasibility. From another perspective, as clean energy generation increasingly replaces traditional combustion-based power generation, the progress of oxy-fuel combustion technology may be challenging due to the reduced demand for combustion-based power generation.
5. Conclusions
This article provides an overview and discussion of carbon capture technologies across various sectors. Three distinct carbon capture methodologies are delved into: DAC, BECCS, and Oxy-fuel combustion. The feasibility of carbon capture technologies as a means to mitigate environmental change and facilitate carbon neutrality is substantiated by it. In addition, the strengths and limitations of these carbon sequestration techniques have been identified, offering a foundation for feasible suggestions concerning their prospective avenues of advancement in this review. The key conclusions are as follows:
DAC, as a method utilizing chemical reactions to capture CO2 offers several advantages. It imposes minimal requirements on operational environments and can be deployed virtually anywhere due to its direct extraction of CO2 from the atmosphere, making it highly adaptable for densely populated urban areas with high CO2 densities. However, its limitations include the current high cost of the contactor and the need to enhance the adsorption efficiency of sorbents.
Oxy-fuel combustion is primarily applied in the field of power generation from fossil fuels, which still dominate the global energy mix. This implies that widespread adoption of oxy-fuel combustion could yield significant carbon capture benefits in the current scenario. Additionally, oxy-fuel combustion, by utilizing enriched oxygen as the combustion gas, can achieve CO2 concentrations exceeding 90%. Limitations accompany its advantages, as its applicability is relatively narrow and requires extensive deployment to demonstrate its efficiency.
BECCS, compared to Oxy-fuel combustion and DAC, requires less human intervention and research effort. As a carbon capture technology playing a significant role in ecosystems, BECCS can capture substantial amounts of CO2 through processes such as photosynthesis in ecosystems like mangrove ecosystems. Correspondingly, it provides insights into carbon sequestration in urban spaces. Nevertheless, these ecosystems are more fragile compared to others due to their unique ecological structures. Therefore, greater attention is needed on how to protect these vulnerable ecosystems.
Considering the severity of current environmental change issues, CCS technology is a reliable countermeasure. If existing CCS technologies can be improved and applied, they can alleviate climate change issues to a certain extent. However, CCS technology also has certain limitations. For instance, some ecosystems are relatively fragile and cannot sustain high-efficiency CO2 capture. Therefore, integrating CCS technology with other technologies and applying them scientifically and rationally across various fields to ensure environmental sustainability and recyclability is a key factor in addressing climate change. For humanity, while continuously advancing carbon capture technologies, it is essential to minimize greenhouse gas emissions as much as possible, as this is the essence of achieving carbon neutrality.
References
[1]. Jia, Zhijie, and Boqiang Lin. (2021) How to achieve the first step of the carbon-neutrality 2060 target in China: The coal substitution perspective. Energy, 233, 121179.
[2]. Wilberforce, Tabbi, et al. (2019) Outlook of carbon capture technology and challenges. Science of the total environment, 657, 56-72.
[3]. Sen, G. (2021) Research & Development Pathways/Challenges in Direct Air Capture of CO2. Climate Change and Green Chemistry of CO2 Sequestration, 181-194.
[4]. Lackner, Klaus S. (2009) Capture of carbon dioxide from ambient air. The European Physical Journal Special Topics, 176.1 93-106.
[5]. Ozkan, Mihrimah, et al. (2009) Current status and pillars of direct air capture technologies. Iscience, 25.4
[6]. Ray, Raghab, and Tapan Kumar Jana. (2017) Carbon sequestration by mangrove forest One approach for managing carbon dioxide emission from coal-based power plant. Atmospheric environment, 171, 149-154.
[7]. Sethi, Deepak, et al. (2020) Diatoms for carbon sequestration and bio-based manufacturing. Biology, 9.8 217.
[8]. López-Pacheco, Itzel Y., et al. (2021) Phycocapture of CO2 as an option to reduce greenhouse gases in cities Carbon sinks in urban spaces. Journal of CO2 Utilization, 53, 101704.
[9]. Jennerjahn, Tim C. (2020) Relevance and magnitude of'Blue Carbon'storage in mangrove sediments Carbon accumulation rates vs. stocks, sources vs. sinks. Estuarine, Coastal and Shelf Science, 247, 107027.
[10]. Finkelman, Robert B., Amy Wolfe, and Michael S. Hendryx. (2021) The future environmental and health impacts of coal. Energy Geoscience, 2.2, 99-112.
[11]. Xu, Ming-Xin, et al. (2021) Design and evaluation of a novel system for the flue gas compression and purification from the oxy-fuel combustion process. Applied Energy, 285, 116388.
[12]. Kaza, Silpa, et al. (2018) What a waste 2.0 a global snapshot of solid waste management to 2050. World Bank Publications.
[13]. Svalestuen, Jørild, Svein G. Bekken, and Lars Ingolf Eide. (2017) CO2 capture technologies for energy intensive industries. Energy Procedia, 114, 6316-6330.
[14]. Tang, YuTing, et al. (2013) Energy analysis and environmental impacts of a MSW oxy-fuel incineration power plant in China. Energy policy, 60, 132-141.
Cite this article
Chen,Q. (2024). Application Status and Development Trends of Carbon Capture and Storage Technologies. Advances in Economics, Management and Political Sciences,123,110-117.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICEMGD 2024 Workshop: Policies to Enhance Sustainable Development through the Green Economy
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Jia, Zhijie, and Boqiang Lin. (2021) How to achieve the first step of the carbon-neutrality 2060 target in China: The coal substitution perspective. Energy, 233, 121179.
[2]. Wilberforce, Tabbi, et al. (2019) Outlook of carbon capture technology and challenges. Science of the total environment, 657, 56-72.
[3]. Sen, G. (2021) Research & Development Pathways/Challenges in Direct Air Capture of CO2. Climate Change and Green Chemistry of CO2 Sequestration, 181-194.
[4]. Lackner, Klaus S. (2009) Capture of carbon dioxide from ambient air. The European Physical Journal Special Topics, 176.1 93-106.
[5]. Ozkan, Mihrimah, et al. (2009) Current status and pillars of direct air capture technologies. Iscience, 25.4
[6]. Ray, Raghab, and Tapan Kumar Jana. (2017) Carbon sequestration by mangrove forest One approach for managing carbon dioxide emission from coal-based power plant. Atmospheric environment, 171, 149-154.
[7]. Sethi, Deepak, et al. (2020) Diatoms for carbon sequestration and bio-based manufacturing. Biology, 9.8 217.
[8]. López-Pacheco, Itzel Y., et al. (2021) Phycocapture of CO2 as an option to reduce greenhouse gases in cities Carbon sinks in urban spaces. Journal of CO2 Utilization, 53, 101704.
[9]. Jennerjahn, Tim C. (2020) Relevance and magnitude of'Blue Carbon'storage in mangrove sediments Carbon accumulation rates vs. stocks, sources vs. sinks. Estuarine, Coastal and Shelf Science, 247, 107027.
[10]. Finkelman, Robert B., Amy Wolfe, and Michael S. Hendryx. (2021) The future environmental and health impacts of coal. Energy Geoscience, 2.2, 99-112.
[11]. Xu, Ming-Xin, et al. (2021) Design and evaluation of a novel system for the flue gas compression and purification from the oxy-fuel combustion process. Applied Energy, 285, 116388.
[12]. Kaza, Silpa, et al. (2018) What a waste 2.0 a global snapshot of solid waste management to 2050. World Bank Publications.
[13]. Svalestuen, Jørild, Svein G. Bekken, and Lars Ingolf Eide. (2017) CO2 capture technologies for energy intensive industries. Energy Procedia, 114, 6316-6330.
[14]. Tang, YuTing, et al. (2013) Energy analysis and environmental impacts of a MSW oxy-fuel incineration power plant in China. Energy policy, 60, 132-141.









