1. Introduction
Myasthenia gravis (MG) is a prototype autoimmune disease in which the neuromuscular junctions of patients are blocked, changed, or destroyed, and lead to inhibition of muscle movement [1]. One of the features of MG is the rapid development of neuromuscular blockade during repetitive motor nerve activity [2]. The two major causes of myasthenia gravis are antibodies and thymus gland malfunction. Antibodies against the acetylcholine receptor (AChR), muscle-specific kinase (MuSK) or low-density lipoprotein receptor related protein 4 in the postsynaptic muscle membrane would lead to the blocking of the receptors, and block the movement of muscle. The most specific and reliable diagnostic test of myasthenia gravis is the elevation of AChR-antibody levels in the serum, especially in adult generalized MG [3].
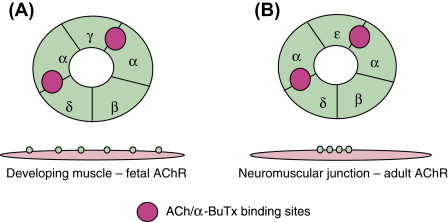
Figure 1. Diagrammatic representation of AChR.
The nicotinic AChR is a pentameric postsynaptic membrane ion channel consisting of five homologous subunits around the central pore that, when the binding between acetylcholine (ACh) released from the motor nerve terminal bind and its two α-subunits is achieved, would open central pore and allow inward flowing of cations into muscles down the electrochemical gradient [4]. This leads to an endplate potential sufficient to surpass the threshold for the opening of sodium channels that causes muscle action potential. In patients with myasthenia gravis, insufficient AChRs leads to decreasing of endplate potential and prevent it from reaching the threshold, causing defective neuromuscular transmission and characteristic fatigable muscle weakness.
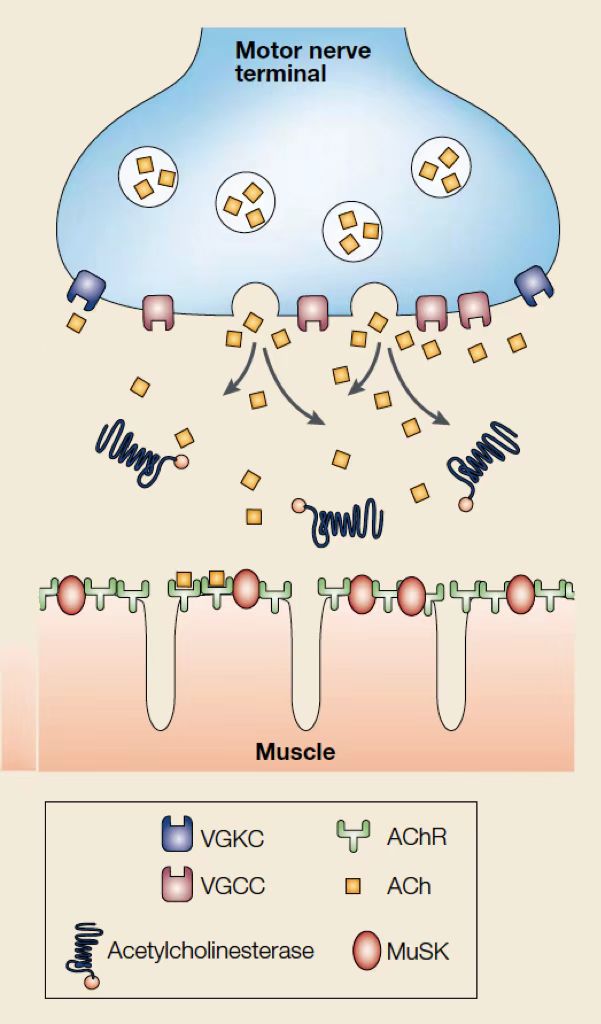
Figure 2. Neuromuscular Transmission.
Voltage-gated calcium channels (VGCCs) acts as the intermediate pathway through which calcium could enter the motor nerve terminal and generates the liberation of acetylcholine. When acetylcholine binds to postsynaptic ACh receptors (AChR), ion channels would be opened, leading to depolarization that generates an action potential, which causes muscle contraction [5]. Acetylcholinesterase (AChE), a cholinergic enzyme existing in muscles and nerves which can break down or hydrolyzes acetylcholine into acetic acid and choline immediately, stimulates the repolarization of motor nerve terminal by opening voltage-gated potassium channels (VGKCs) [6]. Extension of ACh action duration could be achieved by inhibition of acetylcholinesterase, and therefore relieve the symptoms of myasthenia gravis.
The influence of acetylcholine (ACh) liberation on neuromuscular transmission was testified by Dale through multiple experiments on muscles of mammals and frogs, with the object of testing the effect of the motor nerve fibres innervating voluntary muscle fibres stimulation on the liberation of acetylcholine in large qualities without the impact on autonomic or sensory fibres [7]. Previous studies had proved that acetylcholine would be liberated when a nerve impulse contacted the terminal of a preganglionic fibre regardless of contact between the ending and suprarenal medulla [8] or any nerve cell in ganglion [9]. It had been widely believed that mammalian muscles, under the situation which motor nerve supply is intact, would be completely insensitive to the action of acetylcholine, but Dale and his colleagues figured out that they would response by twitches or fibrillation rather than slow contracture (that is how muscles of frogs and several lower vertebrates would present). During the experiment, the mammalian muscles were perfused with Locke’s solution at 37 °C by Dale-Schuster pump, pre-oxygenated to saturation, and contained eserine. The time of perfusion was well controlled to ensure that while the blood was washed out from the vessels completely, the muscle could maintain its sensitivity to motor nerve impulses. The results of the experiment conducted by Dale demonstrated that sufficient ganglion acetylcholine would be released to stimulates ganglion cells effectively, while the excitation of the muscle would no longer occur when the release of acetylcholine lapsed due to exhaustion. Two hypotheses about acetylcholine were raised: (1). When propagation disruption in the nerve fibre reaches the nerve ending, acetylcholine momentarily attunes it to that of the nerve, and allows further propagation. (2). Acetylcholine stimulates nerve cell and muscle end plate directly, leading to generation of new disseminated excitation waves, thus ensures the alacrity of transmission by releasing chemical transmitter on the responsive structure.
In 1964, the reduction of miniature endplate potentials in amplitude in muscle biopsies of myasthenia patients were recorded by Elmqvist et. al with traditional electrophysiological techniques. The fine motor nerve branches were stimulated so that the end-plate potentials at end-plates could be recorded, and post-synaptic sensitivity was investigated with the measurement of response to acetylcholine analogues [10]. A reduction in the amount of transmitter was found in myasthenic neuromuscular junction, which indicated that the failure of the nerve-endings to form adequate acetylcholine wrapped in quanta would be a cause of neuromuscular blockade.
2. Immunoglobulin G and neonatal FC receptor
Immunoglobulin G (IgG) is the most common antibody and the main efficacious molecule of humoral immune response [11]. Comparing to other serum proteins, IgG have a longer average half-life in healthy humans [12]. Located on the cytomembrane of immune cells, IgG Fc receptors including FcRn, FcγIR, FcγRIIA, FcγRIIB, FcγRIIIA, FcγRIIIB, moFcγRIV and boFcγ2R are vital in the afferent and efferent phase of immune response [13]. If IgG antibodies binds to AChR and other muscle antigens, inflammatory responses would initiate, resulting in injury of neuromuscular junction and depletion of muscle contraction [14].
|
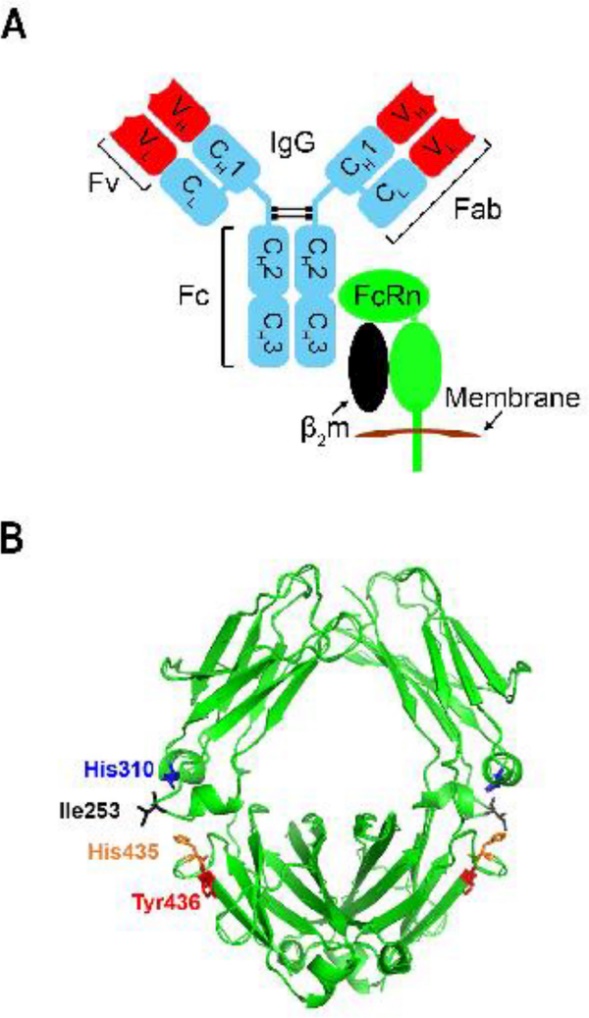
Figure 3. The Molecular Nature of the IgG-FcRn Interaction. The constant regions are colored blue, while the variable domains are colored red. |
It had been proved that the neonatal crystallizable fragment receptor (FcRn), a 52-kDa membrane-bound heterodimeric glycoprotein, could exert influence on the regulation of IgG catabolism [15]. FcRn is composed of a 15-kDa soluble light chain (β2-microglobulin) and a 50-kDa transmembrane anchored heavy chain (α-FcRn) [16]. FcRn interacts with antibodies of IgG class and binds to the constant or fragment crystallizable ((Fc) region of IgG, playing a vital role in the dynamic behavior regulation [17]. Located on the exposed loops at the CH2-CH3 domain interface of IgG1, as shown in Fig 1B, Histidine (His) 310, His 435, isoleucine (Ile) 253, and tyrosine (Tyr) 436 are crucial for human IgGs and FcRn combination [18].
Generalized myasthenia gravis (gMG) is a type of myasthenia gravis which effects multiple muscle groups throughout the body. Even though treatment of gMG is usually a combination of symptomatic drug therapy, immunosuppressive drug therapy, thymectomy, and supportive therapy, the needs of MG patient population was still unmet with limitation in daily functions, side effects of current therapy, or slow action rate of drugs. Vyvgart™ is a novel drug for severe general myasthenia gravis therapy targeting the neonatal crystallizable fragment receptor (FcRn) developed by Argenx and approved on 2021. The effective constituent of Vyvgart is efgartigimod alfa-cab, a human IgG1-derived Fc fragment with higher physiological and acidic pH affinity for FcRn [19].
|
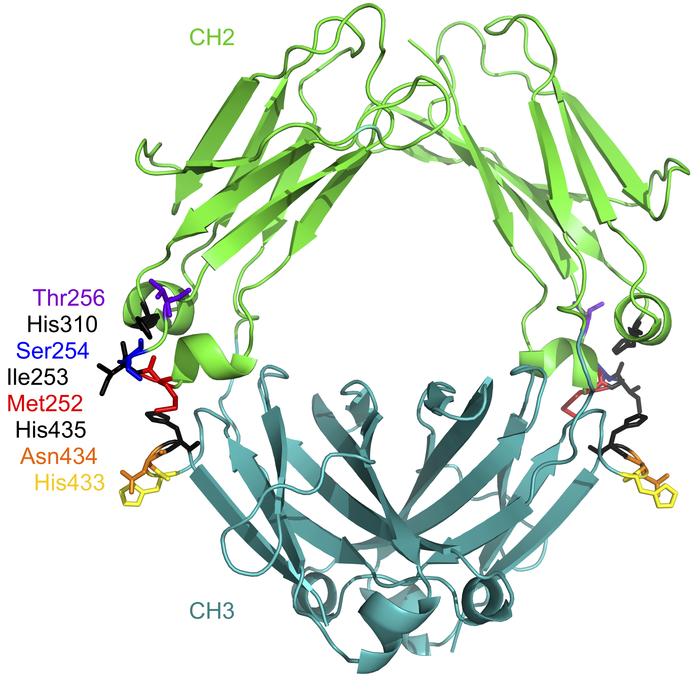
Figure 4. The location of the residues targeted for mutation in efgartigimod. |
3. Mechanism and several trials
3.1. Neonatal FcR blockade & alleviation of MG symptoms in rats
Based on previous data suggesting that FcRn blockade enhances IgG clearance, a high affinity anti-rat FcRn H chain mAb, 1G3 [20] was investigated to demonstrate the relationship [21]. AChR was purified to 90%. The soluble rat FcRn interaction with 1G3 or its isotype control mIgG1Ctrl was assessed by a biosensor system. Equilibrium dissociation constants (KD) was determined. After incubation, rat FcRn-expressing cells were washed with either pH 6.0 or pH 7.4 PBS containing 1% BSA, and stained by fluorescence. Curve-fitting analysis was conducted to calculate the concentration of mAb that inhibits binding by 50% (IC50). The spleen cells were measured and analysed for surface marker expression and antibodies in the serum. Both passive and active experimental autoimmune myasthenia gravis (EAMG) were assessed.
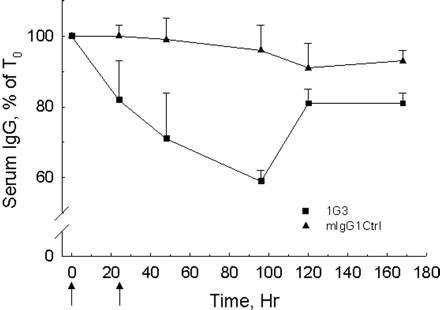
Figure 5. Effects of 1G3 on serum IgG concentration in rats.
Two groups of rats received injection with 1G3 or control mlgG1 at 0 and 24 h, and their serum IgG concentrations were measured at 0, 24, 48, 96, 120, and 168 h. As shown in Figure 5, rats with 1G3 injection experienced a reduction of the serum IgG concentration to 60% of the baseline, while the control group did not show remarkable changes in IgG concentration.
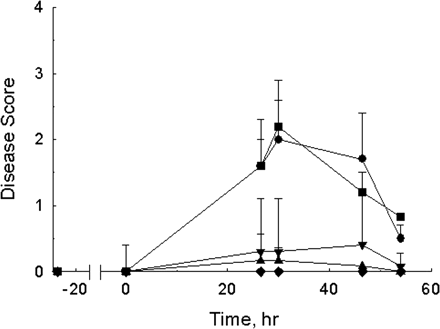
Figure 6. Effects of 1G3 on passive EAMG.
The dose response to mAb35, an anti-AChR mAb used to induce passive EAMG, was investigated by scoring the disease symptoms of different groups of rats treated with vehicle, 1G3, or mlgG1Ctrl before treatment of mAb35. Rats receiving 30 mg/kg 1G3 24 h and 2 h twice prior to mAb35 injection hardly showed any symptoms of EAMG. A single dose of 1G3 (30 mg/kg) could also inhibit the expression of EAMG symptoms. However, the other groups treated with vehicle and mlgG1Ctrl could not prevent the rats from EAMG.
Serum levels of total IgG, IgM and specific anti-AChR IgG were measured to investigate the influence of FcRn blockade exerted on the serum immunoglobulins in immunized animals. Rats immunized with AChR were treated with dexamethasone, 1G3, and mlgG1Ctrl. The total IgG level of rats in the control group increased during significantly from 1.21 ± 070 mg/ml to 17.1 ± 4.2 mg/ml. The inhibition of IgG caused by 1G3 was obvious, while that by dexamethasone was slower and less. In addition, serum IgM levels were not affected by 1G3.
The results of the experiment demonstrated that 1G3 could improve the situation of rats with passive EAMG as well as reduce the active EAMG symptoms without nonspecific depression of the immune system, conveying the signal blockade of FcRn would be efficacious in treating autoantibody-mediated autoimmune illness [22].
3.2. Properties of FcRn antagonist efgartigimod in cynomolgus monkeys
The IgG of cynomolgus monkeys are very similar to that of human, which makes them suitable for the exploration of the pharmacodynamics properties of efgartigimod [23]. As shown in Figure 7, efgartgimod showed an elevated affinity to FcRn of both human and cynomolgus monkey [24].
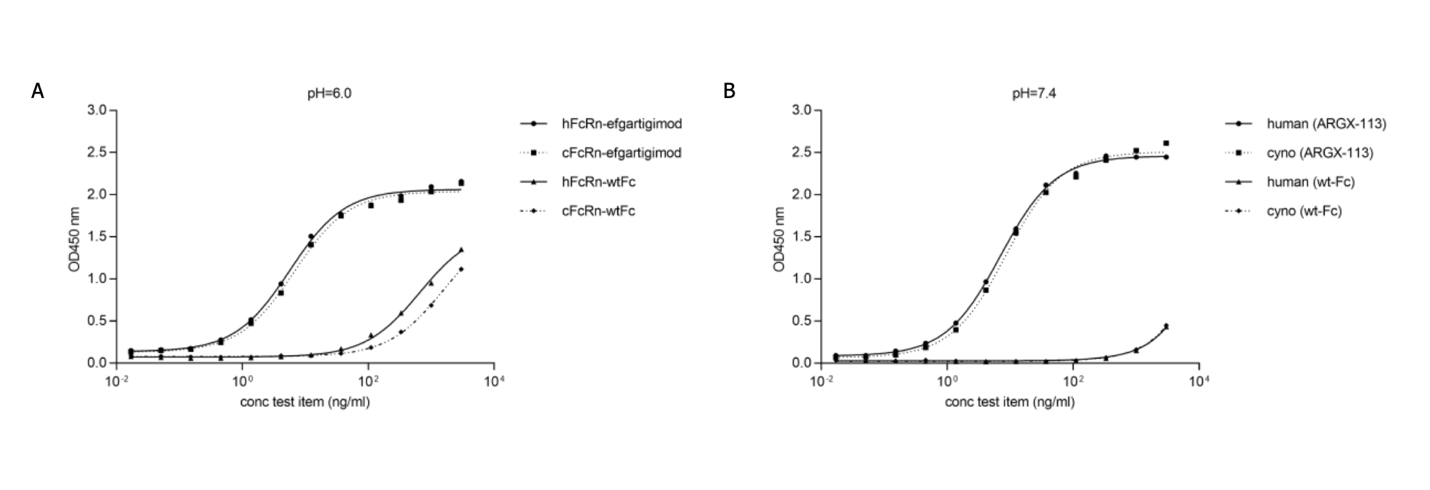
Figure 7. Cross-species FcRn binding.
Cynomolgus monkys received injection with a non-target-bound hIgG1 tracer Ab (human FR70-hIgG1 tracer Ab) which only interfere mouse CD70 but not human or monkey targets. Two days later, 70 mg/kg efgartigimod was injected. The control group was given 2g/kg IVIg, since it was the standard therapy for some autoimmune diseases, and would accelerate the clearance of IgG.
|
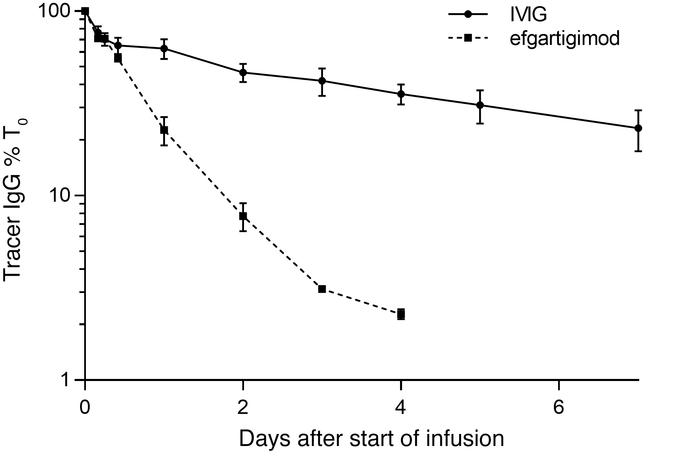
Figure 8. Effect of efgartigimod and IVIg on tracer IgG levels in cynomolgus monkeys. |
By observation of the folded lines in Figure 8, the monkeys received infusion of 70 mg/kg efgartigimod had cleared tracer Ab within 4 days, with a decrease for nearly 95%, which was more rapidly and efficiently than the group receiving IVIg did. The data demonstrated that the inhibitory effect of efgartigimod on FcRn was motivated by its superior competitiveness for endogenous IgG binding, instead of the increasing IgG level to saturate the FcRn receptor [24].
|
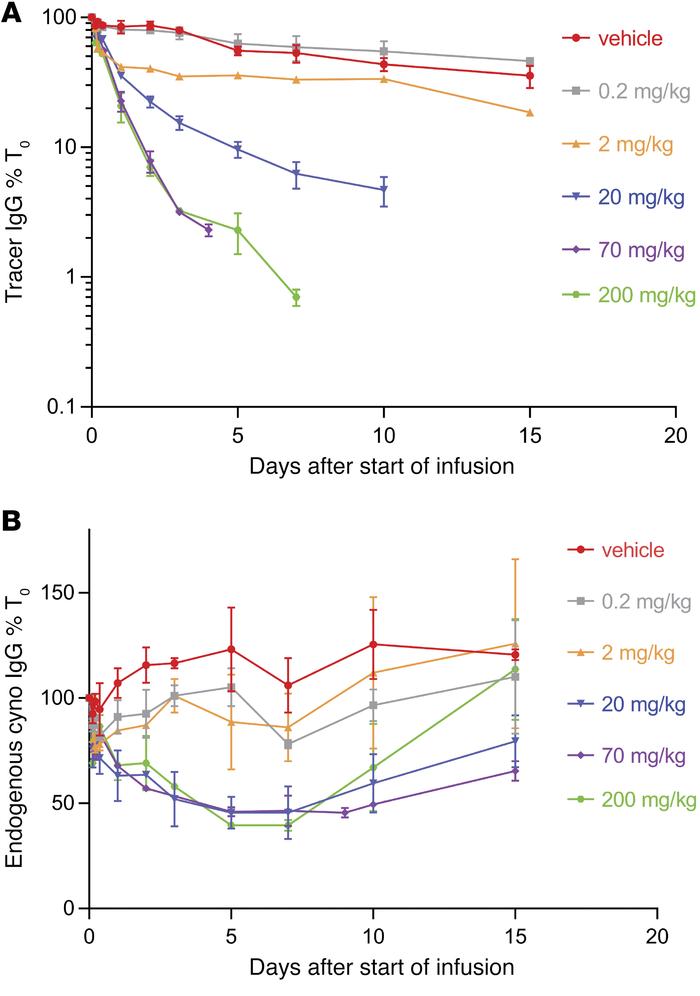
Figure 9. Efgartigimod dose-escalation study in cynomolgus monkeys. |
Dose-escalation study was conducted with the same experimental setup as described above. Tracer levels were measured with murine CD70-binding ELISA, while endogenous cynomolgus IgG levels were plotted relative to predose levels [24]. Cynomolgus monkeys received efgartigimod infusion with 0.2 to 200 mg/kg. The levels of trailer IgG and interior cynomolgus monkeys IgG were measured. Although single dose of 0.2 mg/kg efgartigimod exert no influence on both the human FR70-hIgG1 tracer level and the endogenous IgG level when comparing to the vehicle group, the other trials with 2, 20 or 200 mg/kg (including data from the previous study where 70 mg/kg efgartigimod were given) showed a maximum reduction of 55% for endogenous IgG level which plateaus after doses reached 20 mg/kg. Based on the result and several other data, the saturation dose was considered to be close to 20 mg/kg, and the half-life of efgartigimod was considered as 38 hours.
4. About efgartigimod alfa-facb: clinical trails
4.1. Safety, tolerability, pharmacokinetics, pharmacodynamics, and immunogenicity assessment (clinicaltrials.gov identifier: NCT03457649)
A randomized, double-blind, placebo-controlled phase I study investigating the safety, tolerability, pharmacokinetic, pharmacodynamics, and immunogenicity of efgartigimod was conducted on healthy volunteers. In a duration about a year, 62 healthy participants were assigned into either a single ascending doses (SAD) group with a capacity of 30 subjects or a multiple ascending doses (MAD) group with a capacity of 32 subjects. Participants in the SAD group were divided into 5 consecutive cohorts (cohorts 1-5) of 6 subjects, and received efgartigimod or placebo in a 4:2 ratio. Each of them received 0.2, 2, 10, 25, or 50 mg/kg of efgartigimod or placebo administered intravenously within 2 hours. Volunteers in MAD group were divided into 4 cohorts (cohorts 7-10) containing 8 people, and received efgartigimod or placebo in a 6:2 ratio. Six doses of 10 mg/kg efgartigimod or placebo q4d, four doses of 25 mg/kg efgartigimod of placebo q7d, four doses of 10 mg/kg egartigimod or placebo q7d, or four doses of 25 mg/kg egartigimod or placebo q7d were given to the four cohorts separately.
The demographics and benchmark characteristics of the SAD and MAD crops were shown in table 2, 3, and 4. The range of ages of participants in the SAD group was from 22 to 55, with a median of 46.3 years. 13.3% of the participants in SAD group were female, and 86.7% of those participants were male. For MAD participants among the q7d regimen, the median average age was 46.5 years, with a range from 22 to 55. All of the participants in the q7d regimen were men. For the q4d regimen of MAD, the average median age was 42.0, with a range from 18 to 53. All of the participants in q4d regimen were male.
For SAD participants, the increase of the average maximum concentration was scaled above the dose in the 0.2 mg/kg and 2 mg/kg groups. The total dose increased proportionally until the concentration reached a maximum value of 1,175 μg/ml between between 10 mg/kg and 50 mg/kg, as shown in Figure 10. 2.0 hours was the median time duration required to achieve Cmax for all groups, which was also the time required for the completion of infusion. The mean clearance (CL) values, half-life, and average volume of delivery (Vz) values of efgartigimod were all at the high end of biologics, indicating that efgartigimod is effectively distributed in tissues. The urinary existence of efgartigimod within 72 hours after infusion was also investigated, but the average overall amount of efgartigimod that was urinary excreted between 0 h and 72 h was minimal. The pharmacokinetic parameters measured in the MAD group were consistent with that in the SAD group, as described previously.
4.2. Comparison of route of administration (clinicaltrials.gov identifier: NCT04564066)
A phase I study comparing the safeness and potency of intravenous infusion and subcutaneous injection of efgartigimod in healthy volunteers was conducted by Argenx on August 18, 2020. The trial was randomized, open-label, parallel-group, with the investigational products efgartigimod IV for intravenous infusions and efgartigimod PH20 SC for subcutaneous injections. The percentage reduction in total IgG levels compared to baseline 7 days after the fourth IV or SC administration of efgartigimod were measured as the primary outcome. The secondary outcomes of the study included the percentage reduction in total IgG levels at other time points; the percentage reduction in levels of 4 IgG subtypes; the AUEC for percentage reduction in total IgG levels and each IgG subtypes per weekly interval; the serum levels, cmax, and ctrough of efgartimod. No result was available for the trial.
4.3. Safety and tolerability study (clinicaltrials.gov identifier: NCT03770403)
A long-term, single-arm, open-label, multicenter phase 3 clinical trial was generated by Argenx on March 1, 2019 with 151 participants to evaluate the safety and tolerability of efgartigimod in patients with gMG [25]. The primary aim of the study was to measure the safety and tolerability by measuring the emergent (serious) adverse events caused by the treatment in AChR-positive population, and the secondary outcome measures those in the overall population (both AChR-Ab seropositive and AChR-Ab seronegative patients). The trial was completed on June 30, 2022, while no result was published.
4.4. Efficacy and safety Study (clinicaltrials.gov identifier NCT03669588)
To assess the effectives and security of Vyvgart (efgartigimod alfa-fcab), Argenx conducted a randomized, double-blind, placebo controlled Phase 3 study on August 22, 2018 at 56 sites worldwide, which lasted for a maximum of 28 weeks, comprising a screening period of 2 weeks. 167 participants were divided 1:1 within each stratum to receive efgartigimod (ARGX-113) intravenous (IV) 10 milligrams/kilogram (mg/kg) or placebo. Pregnant and lactating women, MGFA Class I and V patients, patients with worsening muscle weakness secondary to concurrent infections or medications, and patients with known seropositivity were excluded for the experiment. In addition, participants should be on a stable dose of standard of care, and did not receive immunoglobulins by IV, subcutaneous or intramuscular route or plasma exchange 1 month prior to screening. The total study duration was divided into a 2-week screening period, an initial 8-week treatment cycle (TC) and an inter-treatment cycle (ITC) without destined length and could be adjusted according to the patient. On Day1, 8, 15, and 22 in each cycle, patients received efgartigimod 10 mg/kg administered as a 1-hour infusion, or matching placebo administered as a 1-hour infusion according to the group they were assigned to. Following each TC, ITC visits evaluated retreatment criteria to determine whether a patient was qualified to proceed to the next cycle on the basis of assessed clinical response. Myasthenia Gravis Activities of Daily Living (MG-ADL) scale (an 8-item patient-reported scale to assess MG symptoms and their effects on daily life) was regarded as the tool of evaluation. The range of MG-ADL total score was 0-24, in which a higher score meant higher severity of the disease. If the MG-ADL total score of a patient experience a reduction of ≥ 2 points from the baseline of C1 for ≥ 4 weeks successively, while the first reduction happened within a week after the final injection of investigational medical product, the patient would be considered as an MG-ADL responder.
Among the 167 patients enrolled in the experiment, 80 participants in efgartigimod group and 76 participants in placebo group completed the entire trial. The reasons why participants had to drop out of either group included adverse event (1 of 84), physician decision (1 of 83), protocol violation (1 of 84), rescue therapy needed (1 of 83), sponsor decision (1 of 84 and 1 of 83), and withdrawal by subject (1 of 84 and 4 of 83). The primary outcome was to measure the proportion of MG-ADL responders during cycle 1(C1) in the AChR-Ab seropositive population with baseline of time up to day 63 (end of TC1). The result demonstrated that 67.7% of the efgartigimod group were MG-ADL responders during cycle 1 (C1), while 29.7% of the placebo group were MG-ADL responders. The experimental result means that comparing to patients receiving placebo, the patients who obtained efgartigimod were more likely to have an improvement in their daily living quality, from which researchers could infer that efgartigimod is effective in gMG treatment.
Serious adverse events of the trial included thrombocytosis, rectal adenocarcinoma, myasthenia gravis and depression, and participants also shown adverse events containing diarrhea, nausea, bronchitis, nasopharyngitis, upper respiratory tract infection, urinary tract infection, myalgia, dizziness, headache, cough, oropharyngeal pain, and hypertension. It should be highlighted that upper respiratory tract infection was an adverse event that happened in patients receiving efgartigimod more frequently than those in the placebo group (10.71% to 4.82%). Another adverse event that would be brought by efgartigimod was urinary tract infection, with a possibility of 9.52% in the efgartigimod group and a possibility of 4.82% on the placebo group.
The experimental results demonstrated that efgartigimod was well tolerated and effective among individuals with generalized myasthenia gravis.
4.5. Additional trials (clinicalTrials.gov identifier: NCT04777734, NCT04833894, NCT05374590)
Before regulatory approval of efgartigimod, an expanded access protocol was applied to patients with gMG who were not enrolled in an ongoing clinical trial. The ages eligible for study were 18 years and older, while patients should guarantee that they would use an acceptable method of contraception, do not have autoimmune disease that would disturbe the precise evaluation of gMG symptoms, or have previously received rituximab within 6 months before the first dose of efgartigimod. Further information of the trial was not published (NCT04777734).
With the purpose of investigating the PK, PD, safety and activity of efgartigimod IV in children and adolescents with gMG, Argenx had launched a phase 2 & 3 study which would last for maximum 28 weeks. The estimated enrollment were 12 participants, and all of the participants would receive intravenous infusion efgartigimod IV. On the first 8 weeks, the dose would be confirmed, and the next 18 weeks would be used for treatment response-confirmatory. The primary outcomes of the study were to measure efgartigimod concentrations to determine removal rates and distributional volume, total immunoglobulin G (IgG) levels to modeling analyze pharmacokinetics and pharmacodynamics, and Anti-acetylcholine receptors antibodies (AChR-Ab) for pharmacokinetics and pharmacodynamics modeling analysis. The secondary outcome measurements including adverse events, efgartigimod serum concentrations in blood samples, absolute values of levels of IgG, and many other data. No result or further information was posted as the experiment was not completed (NCT04833894).
For evaluation of long-term security of efgartigimod for intravenous administration in children with gMG, Argenx had designed another phase 2 & 3 study with efgartigimod IV. The estimated enrollment was 12 participants with a range of age from 2 years to 18 years who had already completed a previous trial ARGX-113-2006. The primary measurement of the trial were the probability and seriousness of adverse events and issues of particular concern; changes in height and weight from baseline; and electrocardiogram of heart rate and QTcF (ms). Since the previous study had not been completed, this trial was not yet recruiting (NCT05374590).
4.6. Current Status of Vyvgart in generalized myasthenia gravis treatment
Before the approval of Vyvgart (efagartigimod alfa-fcab), the drugs frequently used for gMG treatment were prednisone, CellCept, azathioprine, Ultomiris and Soliris.
Prednisone is a synthetic, anti-inflammatory glucocorticoid; an immunosuppressive agent considered as one of the most effective oral immunosuppressive agent for MG therapy. After entering the liver, prednisone is converted into prednisolone. As a steroid drug, prednisone suppresses the activity of the immune system to alleviate certain immune-related symptoms by inhibiting the migration of polymorphonuclear leukocytes and decreasing capillary permeability. High-dose predniosone would lead to cushingoid features, cutaneous changes, hypertension, and long-term side effects such as avascular necrosis and osteopenia. Patients with diabetes would have limitations when receiving prednisone therapy since diabetes was a strong contraindication to steroids [26].
CellCept contains mycophenolate mofetil which would be converted into mycophenolic acid and block the inosine monophosphate dehydrogenase (IMPDH) enzyme after entering the body. Inosine monophosphate dehydrogenase enzyme was significant for DNA formation in cells, as it supported guanosine nucleotide synthesis required for B and T lymphocytes. The multiplication of lymphocytes and other white blood cells would be suppressed without IMPDH [27], and the immunoreaction would therefore be inhibited. The most serious risks of CellCept are gastrointestinal discomfort, leukopenia and infection.
5. Conclusion
In conclusion, Vyvgart™ (efgartigimod alfa-fcab) is a promising, effective, well tolerated new approved drug which could be used in various patients. By targeting and inhibiting FcRn, Vyvgart™ leads to the catabolism of IgG, and thus reduces pathological autoantibody levels. Although the experimental data about the application of efgartigimod to children with general myasthenia gravis was not yet collected, and some of the clinical trials were not finished, Vyvgart™ is still with sufficient potential, and its investigation would pave the path for the development of other drugs for general myasthenia gravis treatment.
References
[1]. Gilhus, N. and Lindroos, J., 2022. Myasthenia Gravis. Comprehensive Pharmacology, pp.461- 478.
[2]. Dale, H., Feldberg, W. and Vogt, M., 1936. Release of acetylcholine at voluntary motor nerve endings. The Journal of Physiology, 86(4), pp.353-380.
[3]. Silvestri, N., Barohn, R. and Wolfe, G., 2017. Acquired Disorders of the Neuromuscular Junction. Swaiman's Pediatric Neurology, pp.1098-1105.
[4]. Gilhus, N., Tzartos, S., Evoli, A., Palace, J., Burns, T. and Verschuuren, J., 2019. Myasthenia gravis. Nature Reviews Disease Primers, 5(1).
[5]. Vincent, A., 2022. Unravelling the pathogenesis of myasthenia gravis.
[6]. Trang, A. and Khandhar, P., 2022. Physiology, Acetylcholinesterase.
[7]. Clinicaltrials.gov. 2022. A Safety and Tolerability Study of ARGX-113 in Patients With Myasthenia Gravis Who Have Generalized Muscle Weakness.
[8]. Feldberg, W., Minz, B. and Tsudzimura, H., 1934. The mechanism of the nervous discharge of adrenaline. The Journal of Physiology, 81(3), pp.286-304
[9]. Gaddum, J. and Schild, H., 1934. Depressor substances in extracts of intestine. The Journal of Physiology, 83(1), pp.1-14.
[10]. Elmqvist, D., Hofmann, W., Kugelberg, J. and Quastel, D., 1964. An electrophysiological investigation of neuro-muscular transmission in myasthenia gravis. The Journal of Physiology, 174(3), pp.417-434.
[11]. Spiegelberg, H., 1974. Biological Activities of Immunoglobulins of Different Classes and Subclasses. Advances in Immunology Volume 19, pp.259-294.
[12]. Wang, W., Lu, P., Fang, Y., Hamuro, L., Pittman, T., Carr, B., Hochman, J. and Prueksaritanont, T., 2011. Monoclonal Antibodies with Identical Fc Sequences Can Bind to FcRn Differentially with Pharmacokinetic Consequences. Drug Metabolism and Disposition, 39(9), pp.1469-1477.
[13]. Wang, Y., Tian, Z., Thirumalai, D. and Zhang, X., 2014. Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering. Journal of Drug Targeting, 22(4), pp.269-278.
[14]. Nicolle, M., 2002. MYASTHENIA GRAVIS. The Neurologist, 8(1), pp.2-21.
[15]. Low, S. and Mezo, A., 2009. Inhibitors of the FcRn:IgG Protein–Protein Interaction. The AAPS Journal, 11(3).
[16]. Simister, N. and Mostov, K., 1989. An Fc receptor structurally related to MHC class I antigens. Nature, 337(6203), pp.184-187.
[17]. Ward, E. and Ober, R., 2018. Targeting FcRn to Generate Antibody-Based Therapeutics. Trends in Pharmacological Sciences, 39(10), pp.892-904.
[18]. Clinicaltrials.gov. 2022. An Efficacy and Safety Study of ARGX-113 in Patients With Myasthenia Gravis Who Have Generalized Muscle Weakness - Study Results - ClinicalTrials.gov
[19]. Vaccaro, C., Zhou, J., Ober, R. and Ward, E., 2005. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nature Biotechnology, 23(10), pp.1283-1288.
[20]. Raghavan, M., Chen, M., Gastinel, L. and Bjorkman, P., 1994. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity, 1(4), pp.303-315.
[21]. Liu, L., Garcia, A., Santoro, H., Zhang, Y., McDonnell, K., Dumont, J. and Bitonti, A., 2007. Amelioration of Experimental Autoimmune Myasthenia Gravis in Rats by Neonatal FcR Blockade. The Journal of Immunology, 178(8), pp.5390-5398.
[22]. Huda, S. and Vincent, A., 2014. Acetylcholine Receptor and Muscle-Specific Kinase Autoantibodies. Autoantibodies, pp.575-580.
[23]. Abdiche, Y., Yeung, Y., Chaparro-Riggers, J., Barman, I., Strop, P., Chin, S., Pham, A., Bolton, G., McDonough, D., Lindquist, K., Pons, J. and Rajpal, A., 2015. The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity. mAbs, 7(2), pp.331-343.
[24]. Ulrichts, P., Guglietta, A., Dreier, T., van Bragt, T., Hanssens, V., Hofman, E., Vankerckhoven, B., Verheesen, P., Ongenae, N., Lykhopiy, V., Enriquez, F., Cho, J., Ober, R., Ward, E., de Haard, H. and Leupin, N., 2018. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. Journal of Clinical Investigation, 128(10), pp.4372- 4386.
[25]. Challa, D., Velmurugan, R., Ober, R. and Sally Ward, E., 2014. FcRn: From Molecular Interactions to Regulation of IgG Pharmacokinetics and Functions. Fc Receptors, pp.249- 272.
[26]. Sanders, D. and Evoli, A., 2010. Immunosuppressive therapies in myasthenia gravis. Autoimmunity, 43(5-6), pp.428-435.
[27]. European Medicines Agency. 2022. CellCept - European Medicines Agency
Cite this article
Li,S. (2023). Development of Vyvgart (Efgartigimod Alfa-Fcab) for Generalized Myasthenia Gravis (gMG) Therapy. Theoretical and Natural Science,3,487-498.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Part I
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Gilhus, N. and Lindroos, J., 2022. Myasthenia Gravis. Comprehensive Pharmacology, pp.461- 478.
[2]. Dale, H., Feldberg, W. and Vogt, M., 1936. Release of acetylcholine at voluntary motor nerve endings. The Journal of Physiology, 86(4), pp.353-380.
[3]. Silvestri, N., Barohn, R. and Wolfe, G., 2017. Acquired Disorders of the Neuromuscular Junction. Swaiman's Pediatric Neurology, pp.1098-1105.
[4]. Gilhus, N., Tzartos, S., Evoli, A., Palace, J., Burns, T. and Verschuuren, J., 2019. Myasthenia gravis. Nature Reviews Disease Primers, 5(1).
[5]. Vincent, A., 2022. Unravelling the pathogenesis of myasthenia gravis.
[6]. Trang, A. and Khandhar, P., 2022. Physiology, Acetylcholinesterase.
[7]. Clinicaltrials.gov. 2022. A Safety and Tolerability Study of ARGX-113 in Patients With Myasthenia Gravis Who Have Generalized Muscle Weakness.
[8]. Feldberg, W., Minz, B. and Tsudzimura, H., 1934. The mechanism of the nervous discharge of adrenaline. The Journal of Physiology, 81(3), pp.286-304
[9]. Gaddum, J. and Schild, H., 1934. Depressor substances in extracts of intestine. The Journal of Physiology, 83(1), pp.1-14.
[10]. Elmqvist, D., Hofmann, W., Kugelberg, J. and Quastel, D., 1964. An electrophysiological investigation of neuro-muscular transmission in myasthenia gravis. The Journal of Physiology, 174(3), pp.417-434.
[11]. Spiegelberg, H., 1974. Biological Activities of Immunoglobulins of Different Classes and Subclasses. Advances in Immunology Volume 19, pp.259-294.
[12]. Wang, W., Lu, P., Fang, Y., Hamuro, L., Pittman, T., Carr, B., Hochman, J. and Prueksaritanont, T., 2011. Monoclonal Antibodies with Identical Fc Sequences Can Bind to FcRn Differentially with Pharmacokinetic Consequences. Drug Metabolism and Disposition, 39(9), pp.1469-1477.
[13]. Wang, Y., Tian, Z., Thirumalai, D. and Zhang, X., 2014. Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering. Journal of Drug Targeting, 22(4), pp.269-278.
[14]. Nicolle, M., 2002. MYASTHENIA GRAVIS. The Neurologist, 8(1), pp.2-21.
[15]. Low, S. and Mezo, A., 2009. Inhibitors of the FcRn:IgG Protein–Protein Interaction. The AAPS Journal, 11(3).
[16]. Simister, N. and Mostov, K., 1989. An Fc receptor structurally related to MHC class I antigens. Nature, 337(6203), pp.184-187.
[17]. Ward, E. and Ober, R., 2018. Targeting FcRn to Generate Antibody-Based Therapeutics. Trends in Pharmacological Sciences, 39(10), pp.892-904.
[18]. Clinicaltrials.gov. 2022. An Efficacy and Safety Study of ARGX-113 in Patients With Myasthenia Gravis Who Have Generalized Muscle Weakness - Study Results - ClinicalTrials.gov
[19]. Vaccaro, C., Zhou, J., Ober, R. and Ward, E., 2005. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nature Biotechnology, 23(10), pp.1283-1288.
[20]. Raghavan, M., Chen, M., Gastinel, L. and Bjorkman, P., 1994. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity, 1(4), pp.303-315.
[21]. Liu, L., Garcia, A., Santoro, H., Zhang, Y., McDonnell, K., Dumont, J. and Bitonti, A., 2007. Amelioration of Experimental Autoimmune Myasthenia Gravis in Rats by Neonatal FcR Blockade. The Journal of Immunology, 178(8), pp.5390-5398.
[22]. Huda, S. and Vincent, A., 2014. Acetylcholine Receptor and Muscle-Specific Kinase Autoantibodies. Autoantibodies, pp.575-580.
[23]. Abdiche, Y., Yeung, Y., Chaparro-Riggers, J., Barman, I., Strop, P., Chin, S., Pham, A., Bolton, G., McDonough, D., Lindquist, K., Pons, J. and Rajpal, A., 2015. The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity. mAbs, 7(2), pp.331-343.
[24]. Ulrichts, P., Guglietta, A., Dreier, T., van Bragt, T., Hanssens, V., Hofman, E., Vankerckhoven, B., Verheesen, P., Ongenae, N., Lykhopiy, V., Enriquez, F., Cho, J., Ober, R., Ward, E., de Haard, H. and Leupin, N., 2018. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. Journal of Clinical Investigation, 128(10), pp.4372- 4386.
[25]. Challa, D., Velmurugan, R., Ober, R. and Sally Ward, E., 2014. FcRn: From Molecular Interactions to Regulation of IgG Pharmacokinetics and Functions. Fc Receptors, pp.249- 272.
[26]. Sanders, D. and Evoli, A., 2010. Immunosuppressive therapies in myasthenia gravis. Autoimmunity, 43(5-6), pp.428-435.
[27]. European Medicines Agency. 2022. CellCept - European Medicines Agency