1. Introduction
The integration of molecular diagnostics into healthcare heralds a new era of personalized medicine, where treatment strategies are increasingly tailored to the individual genetic makeup of patients. This evolution in medical practice is not merely a technical advancement but a paradigm shift that promises to enhance patient outcomes, streamline therapeutic approaches, and navigate the complex ethical landscape of genetic information. Molecular diagnostics, including Polymerase Chain Reaction (PCR) and gene sequencing, have become cornerstone technologies in this shift. They offer unprecedented precision in detecting genetic mutations, understanding individual predispositions to diseases, and identifying the most effective treatments based on genetic profiles. This paper explores the implications of these advancements for nursing care, emphasizing the need for nurses to adapt to the demands of personalized medicine through continuous education and ethical practice. The application of molecular diagnostics in healthcare is transformative, allowing for the early detection of diseases, accurate risk assessment, and the development of personalized treatment plans. These technological advancements not only enhance the efficacy of treatments but also open new avenues for preventive medicine. For example, genetic screening can identify individuals at high risk for specific cancers, enabling early intervention and potentially saving lives. Similarly, disease typing and classification at the molecular level allow for the selection of targeted therapies that are more likely to be effective, reducing the trial-and-error approach often associated with traditional treatment methods. However, the transition to personalized medicine facilitated by molecular diagnostics presents significant challenges [1]. The ethical implications of genetic testing, including concerns about data privacy and genetic discrimination, require careful consideration and the development of comprehensive guidelines to protect patient rights. Furthermore, the complexity of genetic information necessitates a higher level of expertise among nursing professionals, highlighting the importance of specialized training and continuous education in genetics and molecular biology.
2. Technological Advancements in Molecular Diagnostics
2.1. Genetic Screening and Risk Assessment
The application of Polymerase Chain Reaction (PCR) and gene sequencing for genetic screening and risk assessment represents a quantum leap in our ability to foresee and manage health risks. PCR amplifies tiny segments of DNA, enabling the detailed analysis of genetic mutations associated with various diseases. Gene sequencing, on the other hand, reads the genetic code, offering a comprehensive overview of an individual’s genetic predispositions, as shown in Figure 1. For instance, the identification of BRCA1 and BRCA2 gene mutations through these methods has been pivotal in assessing breast and ovarian cancer risks among women. Quantitative analysis in this context involves comparing mutation frequencies within populations to individual genetic profiles, using statistical models to calculate individual risk scores [2]. These scores can guide healthcare professionals in recommending preventive measures, such as lifestyle changes or more rigorous screening protocols, potentially mitigating the development of severe conditions.
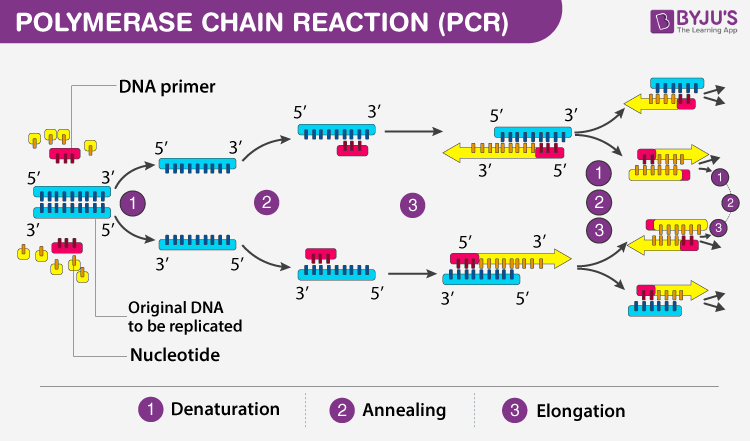
Figure 1. Principle, Steps, Types, Components and Applications Of PCR (Source: BYJU’S)
2.2. Predictive Analysis of Treatment Responses
Predictive analysis of treatment responses leverages genetic information to forecast a patient’s reaction to specific medications or treatments. This field has grown with the advent of pharmacogenomics, which studies how genes affect a person’s response to drugs. Mathematical models play a crucial role here, incorporating genetic variant data to simulate the pharmacokinetics (how the body affects a drug) and pharmacodynamics (how the drug affects the body) of therapeutic agents. One notable application is in the treatment of chronic myeloid leukemia (CML) with the drug imatinib. Genetic testing for the BCR-ABL mutation, responsible for CML, not only confirms the diagnosis but also predicts the effectiveness of imatinib. Patients with certain polymorphisms in the CYP3A5 gene, which affects drug metabolism, may require adjustments in dosing to achieve optimal outcomes. These predictive models are grounded in complex algorithms that analyze vast datasets, identifying patterns that correlate specific genetic profiles with treatment responses. Table 1 encapsulates the predictive analysis of treatment responses.
Table 1. Predictive Analysis of Pharmacogenomic Responses and Optimal Dosing Strategies
Gene Variant | Condition | Medication | Predicted Response | Optimal Outcome Strategy |
BCR-ABL Positive | Chronic Myeloid Leukemia | Imatinib | Highly Effective Treatment | Standard dosing for BCR-ABL positive patients |
CYP3A5*1/*1 (Expresser) | Varied Drug Metabolism | Various | Increased Drug Metabolism - May require lower dosage | Adjust dosing downwards to avoid toxicity |
CYP3A5*3/*3 (Non-expresser) | Varied Drug Metabolism | Various | Decreased Drug Metabolism - May require higher dosage | Adjust dosing upwards to achieve therapeutic effect |
3. Personalized Treatment Planning
3.1. Customized Therapeutic Approaches
Integrating genetic analysis into the treatment planning process enables healthcare practitioners to tailor therapeutic strategies specifically to an individual’s genetic profile. This customization is grounded in the principle that genetic variations among individuals can significantly impact the efficacy and safety of various treatments. For instance, pharmacogenomics, the study of how genes affect a person’s response to drugs, provides a scientific basis for predicting therapeutic outcomes based on genetic markers. A quantitative analysis incorporating genetic data can enhance treatment effectiveness by identifying the optimal drug and dosage for each patient, thereby minimizing the risk of adverse drug reactions, as shown in Table 2. One illustrative example is the use of targeted therapy in oncology, where treatments are designed to interfere with specific molecular targets associated with cancer cell growth and progression. Genetic testing can identify mutations in cancer cells that may be targeted by specific drugs, enabling a personalized approach to cancer treatment. For example, the presence of the BRCA1 or BRCA2 gene mutation in breast cancer patients can guide the use of PARP inhibitors, a class of drugs that are particularly effective in tumors with these mutations [3]. The decision-making process in this context relies on a mathematical model that assesses the likelihood of treatment success, taking into account the presence of specific genetic markers and the historical efficacy of targeted therapies in similar genetic contexts.
Table 2. Quantitative Analysis of Personalized Therapeutic Approaches Based on Genetic Mutations
Gene Mutation | Cancer Type | Recommended Drug | Effectiveness Rating (1-10) | Risk of Adverse Reactions | Personalization Strategy |
BRCA1 | Breast Cancer | PARP Inhibitors | 9 | Medium | Use PARP inhibitors for patients with BRCA mutations, adjusting dosage based on patient’s genetic profile and tolerance. |
BRCA2 | Breast Cancer | PARP Inhibitors | 9 | Medium | Use PARP inhibitors for patients with BRCA mutations, adjusting dosage based on patient’s genetic profile and tolerance. |
EGFR Mutation | Lung Cancer | EGFR Inhibitors | 8 | Low | Employ EGFR inhibitors in patients with EGFR mutations, considering genetic variation in dosage planning. |
ALK Rearrangement | Lung Cancer | ALK Inhibitors | 8 | Low | Administer ALK inhibitors for patients with ALK rearrangements, tailoring treatment to individual genetic and health profile. |
3.2. Molecular Biomarkers in Monitoring
The identification and continuous monitoring of molecular biomarkers play a critical role in personalized treatment planning. Molecular biomarkers are biological molecules found in blood, other body fluids, or tissues that are a sign of a normal or abnormal process, or of a condition or disease. They can be used to see how well the body responds to a treatment for a disease or condition. For example, the measurement of tumor markers, which are substances often produced by cancer cells, can provide insights into the effectiveness of a treatment regimen and indicate the presence of cancer, including its type, progression, and response to treatment. In personalized medicine, molecular biomarkers are utilized not only for initial diagnosis but also for ongoing monitoring of disease progression and treatment response. This approach enables timely adjustments to treatment plans based on quantitative measures of biomarker levels. For instance, a decrease in the level of a specific tumor marker might indicate that the cancer is responding to treatment, whereas an increase could signal resistance to the current therapy. By employing statistical models to analyze changes in biomarker levels over time, healthcare providers can make data-driven decisions about whether to continue, modify, or discontinue a treatment.
3.3. The Role of Nursing in Personalized Care
Nurses are pivotal in the operationalization of personalized medicine, acting as intermediaries between the complex world of genetic information and patient care. Their responsibilities extend from the collection and handling of genetic samples, which must be done with precision to ensure the integrity of the data, to the interpretation and communication of genetic test results to patients and their families. Furthermore, nurses monitor patients’ responses to treatments, paying close attention to both physiological reactions and the expression of specific molecular biomarkers indicative of treatment efficacy or adverse reactions. The role of nursing in personalized care requires a deep understanding of genetic principles and the ability to apply this knowledge in a clinical setting. For example, in monitoring the efficacy of a personalized treatment plan, nurses must be adept at recognizing the significance of changes in molecular biomarkers and understanding the potential need for treatment adjustments [4]. This requires not only technical expertise in the procedures involved in monitoring these biomarkers but also the ability to integrate this information with other clinical data to form a comprehensive view of the patient’s condition. Nurses must also possess the communication skills necessary to explain complex genetic information in understandable terms, ensuring that patients and their families are well-informed about their care.
In conclusion, the integration of personalized treatment planning into nursing care, through customized therapeutic approaches, the monitoring of molecular biomarkers, and the central role of nursing in personalized medicine, represents a significant advance in healthcare. This approach necessitates a combination of sophisticated genetic analysis, continuous monitoring and adjustment of treatment plans based on quantitative data, and a skilled nursing workforce capable of bridging the gap between molecular biology and patient care [5].
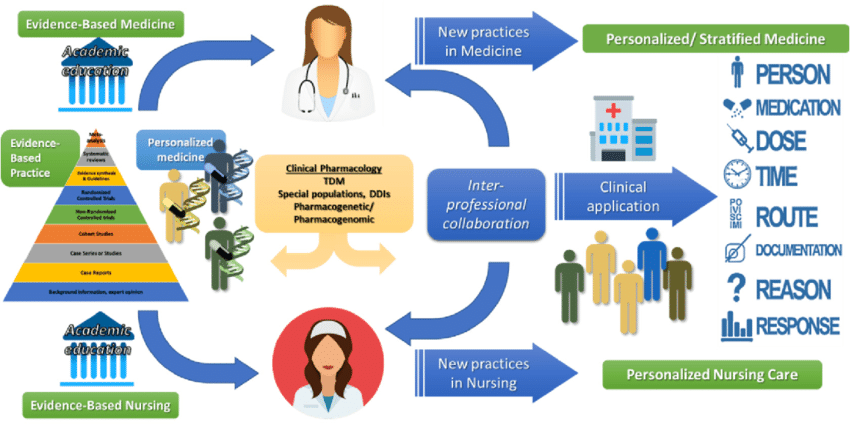
Figure 2. NP’s Role in Personalized Medicine and Nursing Care (Source: ResearchGate)
4. Challenges and Ethical Considerations
4.1. Data Privacy and Genetic Discrimination
The incorporation of genetic information into nursing diagnostics brings to the fore significant concerns regarding data privacy and the potential for genetic discrimination. With the increasing reliance on genetic data, there’s a paramount need for stringent ethical guidelines and robust privacy protections. The genetic information of an individual is not only sensitive but also deeply personal, carrying implications not just for the individual but potentially for their relatives as well. This necessitates the development of comprehensive data protection laws that specifically address the nuances of genetic information, ensuring that such data is not misused for discriminatory practices in employment, insurance, and beyond [6]. Moreover, there’s a critical need for secure data storage and handling protocols that prevent unauthorized access to genetic data, safeguarding patient confidentiality at every stage of the diagnostic and treatment process. The ethical framework guiding the use of genetic data must also include provisions for informed consent, where patients are fully aware of how their genetic information will be used, the potential risks, and the benefits, thereby empowering them to make informed decisions about their healthcare [7].
4.2. The Complexity of Genetic Information
The analysis and interpretation of genetic data entail a complex array of challenges, demanding a high level of expertise and continuous learning. Unlike traditional diagnostic methods, genetic testing can reveal a vast amount of information, some of which may have uncertain implications for the patient’s health. This complexity requires that nursing professionals possess a sophisticated understanding of genetic principles, the significance of specific genetic variations, and their potential impact on disease and treatment. The need for specialized knowledge underscores the importance of integrating genetics into the nursing curriculum and providing ongoing education and training opportunities. This education should not only cover the technical aspects of genetic testing and analysis but also ethical considerations, counseling skills, and the ability to communicate complex genetic information to patients in an understandable manner. Additionally, the interdisciplinary nature of genetic diagnostics necessitates collaboration among nursing professionals, geneticists, counselors, and other healthcare providers to ensure that the interpretation and application of genetic data are accurate and beneficial to patient care.
5. Conclusion
The integration of molecular diagnostics into healthcare practice marks a significant leap towards the realization of personalized medicine, offering profound benefits for patient care and treatment outcomes. This paper has explored the crucial role of molecular diagnostics in enhancing personalized medicine, particularly through genetic screening, disease typing, and the predictive analysis of treatment responses. As these technological advancements redefine the landscape of healthcare, they necessitate a corresponding evolution in nursing practice, emphasizing the importance of specialized knowledge, ethical considerations, and a commitment to continuous learning. The challenges identified, including the ethical dilemmas posed by genetic data, the complexity of genetic information, and issues related to cost and accessibility, underscore the multifaceted nature of this transition. Addressing these challenges requires a concerted effort from healthcare professionals, educators, policymakers, and the broader society to ensure that the benefits of personalized medicine are realized equitably and responsibly. In the era of personalized medicine, the role of nursing professionals is more critical than ever. Nurses are at the forefront of implementing these advancements, necessitating an interdisciplinary approach that blends technical expertise with compassionate care. As we navigate the complexities and opportunities presented by molecular diagnostics, the nursing profession must embrace innovation, advocate for ethical standards, and commit to lifelong learning to fully realize the promise of personalized medicine for improving patient outcomes.
References
[1]. Serrano, Dolores R., et al. “3D printing technologies in personalized medicine, nanomedicines, and biopharmaceuticals.” Pharmaceutics 15.2 (2023): 313.
[2]. Sadee, Wolfgang, et al. “Pharmacogenomics: driving personalized medicine.” Pharmacological reviews 75.4 (2023): 789-814.
[3]. Oliveira, Beatriz B., et al. “Engineering gold nanoparticles for molecular diagnostics and biosensing.” Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 15.1 (2023): e1836.
[4]. Patel, Jena, Joshua Klopper, and Elizabeth E. Cottrill. “Molecular diagnostics in the evaluation of thyroid nodules: Current use and prospective opportunities.” Frontiers in endocrinology 14 (2023): 1101410.
[5]. Khan, Zafran, et al. “Molecular diagnostics and potential therapeutic options for Mycobacterium tuberculosis: Where we stand.” Medicine in Omics (2023): 100022.
[6]. Karagoz, Aysel, et al. “Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis.” Journal of infection and public health 16.4 (2023): 531-541.
[7]. Sahm, Felix, et al. “Molecular diagnostic tools for the World Health Organization (WHO) 2021 classification of gliomas, glioneuronal and neuronal tumors; an EANO guideline.” Neuro-oncology 25.10 (2023): 1731-1749.
Cite this article
Hong,P.;Tan,Y. (2024). Enhancing personalized medicine through molecular diagnostics: Implications for nursing care. Theoretical and Natural Science,37,283-288.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Environmental Geoscience and Earth Ecology
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Serrano, Dolores R., et al. “3D printing technologies in personalized medicine, nanomedicines, and biopharmaceuticals.” Pharmaceutics 15.2 (2023): 313.
[2]. Sadee, Wolfgang, et al. “Pharmacogenomics: driving personalized medicine.” Pharmacological reviews 75.4 (2023): 789-814.
[3]. Oliveira, Beatriz B., et al. “Engineering gold nanoparticles for molecular diagnostics and biosensing.” Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 15.1 (2023): e1836.
[4]. Patel, Jena, Joshua Klopper, and Elizabeth E. Cottrill. “Molecular diagnostics in the evaluation of thyroid nodules: Current use and prospective opportunities.” Frontiers in endocrinology 14 (2023): 1101410.
[5]. Khan, Zafran, et al. “Molecular diagnostics and potential therapeutic options for Mycobacterium tuberculosis: Where we stand.” Medicine in Omics (2023): 100022.
[6]. Karagoz, Aysel, et al. “Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis.” Journal of infection and public health 16.4 (2023): 531-541.
[7]. Sahm, Felix, et al. “Molecular diagnostic tools for the World Health Organization (WHO) 2021 classification of gliomas, glioneuronal and neuronal tumors; an EANO guideline.” Neuro-oncology 25.10 (2023): 1731-1749.









